ABSTRACT
Introduction
Pregnant and breastfeeding women are at an increased risk of severe illness from COVID-19. Despite this, low vaccination coverages are reported in this population sub-group.
Areas covered
The purpose of this study is to estimate the proportion of pregnant and breastfeeding women expressing hesitation to the COVID-19 vaccine worldwide. Forty-six studies were included, selected from scientific articles available in three scientific databases between 1 January 2020 and 6 February 2022. The vaccine hesitation rate among pregnant and breastfeeding women was 48.4% (95%CI=43.4–53.4%). In a sub analysis by study period, it was 40.0% (95%CI=31.6–46.6%) considering surveys administered in 2020, 58.0% (95%CI=48.9–66.9%) considering surveys administered in the first semester of 2021, and 38.1% (95%CI=25.9–51.2%) considering surveys administered in the second semester of 2021. The main reasons for vaccine hesitation were lack of information about vaccination, opinion that the vaccine is unsafe, and fear of adverse events.
Expert opinion
Available evidence in the literature has shown that fighting vaccine resistance is harsh and too slow as a process, considering the rapidity and unpredictability of a pandemic. Health education should be provided in order to improve the willingness of the community, especially for those with lower levels of education.
1. Introduction
Pregnancy is a physiological process and as such it should not be considered a disease; nevertheless, pregnant women and newborns are considered a vulnerable subgroup with an increased risk of several infectious diseases, some of which are vaccine-preventable [Citation1,Citation2].
As reported by the Center for Disease Control and Prevention (CDC), the strongly recommended vaccinations in pregnancy are the diphtheria-tetanus-pertussis (Tdpa) vaccine (27th to 36th week of gestation, although Tdap may be given at any time during pregnancy) and the influenza vaccine (in any trimester of pregnancy) [Citation3]. Pregnant and breastfeeding women are at increased risk for severe complications from COVID-19 compared with people who are not pregnant, as well as being at increased risk for preterm and stillbirth and other pregnancy complications [Citation4,Citation5]. So, the CDC recommends COVID-19 vaccination for people who are pregnant, breastfeeding, and trying to become pregnant now or who may become pregnant in the future, as well as their partners [Citation6].
Although no study of the COVID-19 vaccine has enrolled pregnant women, creating an ethical issue, phase IV studies have shown a high efficacy and safety profile for both women and the fetus/infant [Citation7,Citation8]. Furthermore, efficient transfer of SARS-CoV-2 IgG across the placenta in vaccinated women to their neonates has been demonstrated in 2021 [Citation9], as has secretion of SARS-CoV-2 specific IgA and IgG antibodies into breast milk for 6 weeks after vaccination [Citation10]; thus, in addition to maternal protection, the vaccine may also provide neonatal humoral immunity against SARS-CoV-2. Nevertheless, low vaccine coverage (VCs) has been reported in this population sub-group [Citation11]; whole-population data from a 2022 prospective cohort study in Scotland [Citation12] showed lower VC in pregnant women (32%) than in the general female population aged 18 − 44 years (77%). The authors focused on vaccine hesitation as one of the main determinants of the success (or otherwise) of the COVID-19 vaccination campaign in this high-risk group.
Vaccine hesitation is an already known phenomenon in pregnant and lactating women; in fact, many studies in the literature have reported low VCs of influenza and Tdap vaccines and high levels of vaccine hesitancy in this population [Citation13–19]; the main reasons for low vaccination uptake were vaccine safety, belief that the vaccine is not necessary or effective, not recommended by health care professionals, low knowledge of vaccines, access issues, cost, and conflicting advice [Citation20].
To estimate the proportion of pregnant and breastfeeding women expressing COVID-19 vaccine hesitancy worldwide, we conducted a systematic review of relevant literature and meta-analysis. Determinants of vaccine compliance and options suggested by these studies to deal with vaccine hesitancy were also analyzed.
2. Methods
2.1. Search strategy and selection criteria
Scopus, MEDLINE/PubMed and Google Scholar databases (up to page 10) were systematically searched. Research articles, brief reports, commentaries, and letters published between 1 January 2019 and 6 February 2022, were included in our search. The following terms were used for the search strategy: (adherence OR hesitancy OR compliance OR attitude) AND (covid* OR SARS*) AND (vaccin* OR immun*) AND (pregnan* OR post-partum OR breastfeeding OR lactating). Full-text English studies were included. Abstracts without full text, systematic reviews, meta- analyses and all studies focusing on issues unrelated to the purpose of this review (vaccine knowledge, adverse vaccine reactions, etc.) were excluded. When necessary, study authors were contacted for additional information. References of all articles were reviewed for further study. The list of papers was independently screened by title and/or abstract by two reviewers who applied the predefined inclusion/exclusion criteria. Discrepancies were recorded and resolved by consensus.
Data extracted included year, sample size, number of hesitant women, country, timing of the investigation (before or during the anti-COVID-19 vaccination campaign) and options for managing hesitant subjects.
2.2. Quality assessment
The quality of selected studies was assessed according to the STROBE checklist, which includes 22 methodological questions [Citation21]. Eligible short reports, commentaries, and letters described cross-sectional studies and their quality was therefore also evaluated using the STROBE checklist. Quality assessment was not performed for studies without full text. Studies assessed according to STROBE had a minimum and maximum possible score of 0 and 44, respectively, and were classified as low quality (<15.5), moderate quality (15.5–29.5) or high quality (30–44).
The risk of bias for each study was assessed independently by two researchers. Discrepancies were recorded and resolved by consensus.
2.3. Pooled analysis
Several different groups of meta-analysis were performed: the first included all women, the second compared hesitancy between pregnant and breastfeeding women, the third evaluated hesitancy according to different survey administration periods (2020 vs. first semester 2021 vs. second semester 2021) and the fourth by country; finally, vaccine hesitancy was compared according to different determinants, calculating the risk ratio (RR) and 95% confidence interval (95%CI). In addition, for each of the meta-analyses, a separate analysis was conducted using only high-quality papers.
The pooled proportion in the meta-analysis was calculated using the Freeman-Tukey double arcsine transformation to stabilize variances, and DerSimonian-Laird weights for random effects models, with the heterogeneity estimate obtained from the fixed-effects inverse-variance model. The pooled prevalence and associated 95% Wald confidence interval were plotted, and a forest plot was drawn. The I2 statistic was calculated as a measure of the proportion of the overall variance attributable to heterogeneity between studies rather than to chance. Heterogeneity between studies in different groups was also assessed. A value of p < 0.10 was considered to indicate statistical significance of heterogeneity.
A sensitivity analysis was conducted to evaluate stability; among the studies included in this systematic review, one study at a time was excluded, and the subsequent conclusion based on the others was then reevaluated to avoid severe distortions.
Statistical analysis was conducted using STATA MP17.
Strategies to increase vaccination compliance among pregnant and breastfeeding women and suggested strategies to address vaccination hesitancy were collected from all available studies and their respective results were compared, with particular attention to the evidence presented in several of the included papers.
3. Results
3.1. Identification of relevant studies
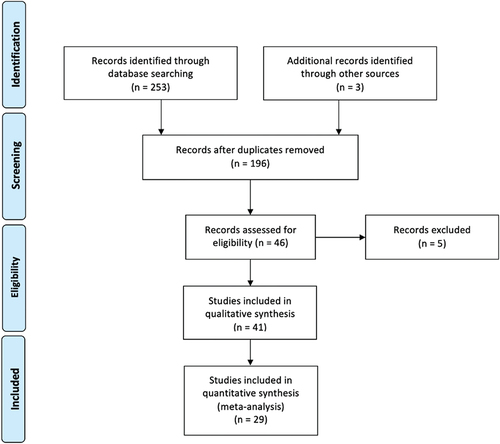
The flow-chart, constructed following the PRISMA guide [Citation22] (), shows the process of article selection. According to the aforementioned inclusion criteria, 41 articles were identified in Google Scholar, 13 in Scopus and 34 in MEDLINE/PubMed. After exclusion of duplicate articles in the two databases, there were 46 eligible studies [Citation23–68]. Of these, one [Citation64] was excluded because it evaluated the same phenomenon in a more recent, comprehensive article already included in the meta-analysis, one [Citation65] because additional information was requested from the authors but they did not respond, and three [Citation66–68] because the full text was not available; two studies [Citation62,Citation63] were excluded from the quantitative analysis because additional information was requested from the authors but they did not respond, but they were included in the qualitative analysis. Thus, in total, 41 studies were eligible [Citation23–63], of which 29 were quantitative [Citation23–51] and 12 were qualitative [Citation52–63] (). The remaining 150 studies did not meet the inclusion criteria.
Table 1. Characteristics of the selected studies included in meta-analysis and systematic review.
3.2. Quality assessment
The STROBE checklist was applied appropriately to the included studies; 75.0% of eligible papers were determined to be of high quality (). The impact of study quality was assessed in a sub-analysis.
3.3. Pooled analysis
Meta-analysis of all HCWs showed that the prevalence of vaccine hesitation was 48.4% (95%CI: 43.4–53.4%; I2 = 98.7%; p-value for heterogeneity <0.0001). Based on high-quality articles only, the pooled prevalence was 48.4% (95%CI = 43.0–53.8%; I2 = 98.6%; p < 0.0001).
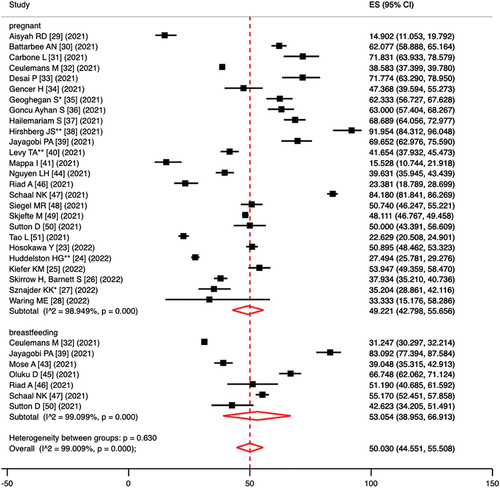
Comparing pregnancy vs. breastfeeding hesitation, the pooled prevalence of vaccine hesitancy was 49.2% (95%CI = 42.8–55.7%; I2 = 98.9%; p < 0.0001) in pregnant women and 53.1% (95%CI = 39.0–66.9%; I2 = 99.1%; p < 0.0001) in lactating women; the p-value in the test for heterogeneity between sub-groups was 0.630 (). Based on high-quality articles only, the pooled prevalence of vaccine hesitancy was 50.5% (95%CI = 43.4–57.5%; I2 = 98.9%; p < 0.0001) in pregnant women and 50.6% (95%CI = 36.2–65.0%; I2 = 99.0%; p < 0.0001) in lactating women; the p-value in the test for heterogeneity between sub-groups was 0.984.
Figure 2. Forest plot of pooled prevalence of vaccine hesitancy, per sub-populations (pregnant vs. breastfeeding women).
In a sub analysis by continents, the pooled prevalence of vaccine hesitancy was 52.2% (95%CI = 40.8–64.1%; I2 = 98.5%; p < 0.0001) in North America (ten studies), 47.7% (95%CI = 34.4–61.1%; I2 = 99.0%; p < 0.0001) in Asia (nine studies), 42.4% (95%CI = 34.1–50.9%; I2 = 98.5%; p < 0.0001) in Europe (seven studies) and 50.9% (95%CI = 47.9–53.9%; I2 = -; p = -) in Africa (two studies); the p-value in the test for heterogeneity between sub-groups was 0.299. Based on high-quality articles only, the pooled prevalence of vaccine hesitancy was 47.0% (95%CI = 38.2–55.9%; I2 = 98.6%; p < 0.0001) in North America, 47.9% (95%CI = 32.5–63.5%; I2 = 99.0%; p < 0.0001) in Asia, 47.0% (95%CI = 38.2–55.9%; I2 = 98.6%; p < 0.0001) in Europe and 50.9% (95%CI = 47.9–53.9%; I2 = -; p = -) in Africa; the p-value in the test for heterogeneity between sub-groups was 0.838.
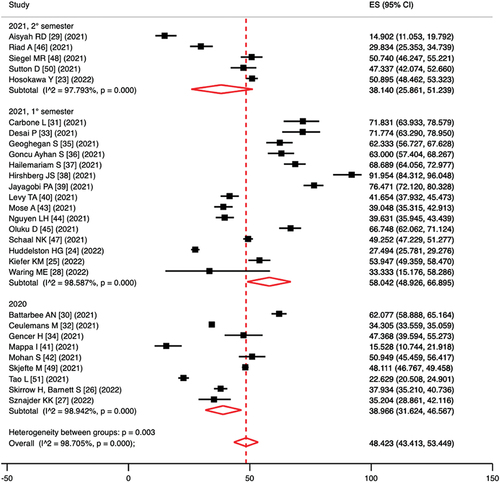
In a sub analysis according to study period, the pooled prevalence of vaccine hesitancy was 40.0% (95%CI = 31.6–46.6%; I2 = 98.9%; p < 0.0001) considering surveys administered in 2020, 58.0% (95%CI = 48.9–66.9%; I2 = 98.6%; p < 0.0001) considering surveys administered in the first semester of 2021 and 38.1% (95%CI = 25.9–51.2%; I2 = 97.8%; p < 0.0001) considering surveys administered in the second semester of 2021; the p-value in the test for heterogeneity between sub-groups was 0.003 (). Based on high-quality articles only, the pooled prevalence of vaccine hesitancy was 42.0% (95%CI = 34.3–50.0%; I2 = 99.0%; p < 0.0001) considering surveys administered in 2020, 56.3% (95%CI = 47.3–65.1%; I2 = 97.2%; p < 0.0001) considering surveys administered in the first semester of 2021 and 44.7% (95%CI = 35.8–53.8%; I2 = 94.8%; p < 0.0001) considering surveys administered in the second semester of 2021; the p-value in the test for heterogeneity between sub-groups was 0.053.
Estimates of Risk Ratio (RR) values when comparing vaccine hesitation for different determinants are described in . In the analysis based only on high-quality studies, RR values did not differ significantly. Sensitivity analysis did not show severe distortion by a specific study. In the publication bias analysis, there was no obvious asymmetry in the funnel plots and no strong evidence of publication bias (Figures S1-S12).
Table 2. Estimation of the risk ratio in a comparison of vaccine hesitancy with respect to several determinants.
3.4. Determinants of vaccination compliance and suggested strategies to address vaccination hesitancy
Many determinants of hesitancy have been investigated; most studies reported that the main reasons are lack of information about vaccination, the opinion that the vaccine is unsafe (for both mother and fetus), and fear of adverse events (including potentially long-term adverse events, especially for children of breastfeeding women) [Citation23,Citation25,Citation26,Citation30–33,Citation35,Citation36,Citation39–42,Citation44–51,Citation62,Citation63]. Moreover, the role of pharmaceutical companies in influencing vaccine policy decisions and the uncertainty associated with the rapid COVID-19 vaccine development process [Citation26,Citation31,Citation35,Citation37,Citation46,Citation48,Citation50] were also determinants of attitude. Minor factors of a negative attitude toward the vaccine were reporting a history of chronic disease or allergies [Citation25,Citation50] (although Sznajder KK et al. [Citation27] did not find a role of this topic with vaccine uptake, while Schaal NK et al. [Citation47] reported that women who breastfed at higher risk were more compliant), substance use [Citation25], lower monthly household income [Citation14,Citation26], unavailability of vaccines or not having time to receive them [Citation42] and not having private health insurance [Citation25,Citation44,Citation48]. A major role in vaccination compliance is played by family members and friends of pregnant or breastfeeding women [Citation25,Citation41] or at higher risk for COVID-19 [Citation27,Citation50,Citation62], as well as family members’ opinions [Citation36,Citation46] and having previous sons and daughters [Citation36,Citation45,Citation46,Citation50]; Indeed, personal stories are often reasons why people choose to be vaccinated [Citation27]. Concern about the risk associated with COVID-19 disease and its impact on their health and pregnancy is a determinant of a better attitude, as is adherence to infection prevention measures (social distancing, masks, hand hygiene, etc.) and knowledge of the mechanism of action of the vaccine and COVID-19 disease [Citation24,Citation25,Citation37,Citation39,Citation43,Citation47,Citation49–51]. To a lesser extent, women reported that the safety and protection of themselves, and their family members was one of the main reasons for vaccination uptake [Citation25,Citation46,Citation50]; as if most studies reported that higher education and information from scientific sources were associated with better acceptance [Citation23,Citation25,Citation29,Citation32–34,Citation37,Citation40,Citation43–46], two studies [Citation39,Citation51] reported that a low level of education was associated with higher vaccine acceptance in Chinese and Singaporean women [Citation51]. The role of social media is discussed, too; Gencer H et al. [Citation34] reported that women who used mass media or the Internet as their main source of information showed higher levels of hesitancy, while Riad A et al. [Citation46] reported that the impact of media/social media on Czech women’s attitudes was significantly positive.
Overall, another important determinant of vaccination compliance was having received previous vaccinations, either during or before/after pregnancy/breastfeeding, especially the anti-influenza vaccine [Citation25–27,Citation30,Citation33,Citation40,Citation45,Citation48].
Regarding age, higher levels of compliance were reported in younger women [Citation25,Citation29,Citation51], even though Levy TA et al. [Citation40] reported less hesitation in older subjects. Social determinants have also been reported; indeed, trust in local government and public health agencies [Citation23,Citation41,Citation46,Citation49], not belonging to an ethnic minority [Citation25,Citation26,Citation30,Citation40,Citation48], being employed [Citation27,Citation32,Citation46,Citation48], higher economic status [Citation34] (except for Jayagobi PA et al. [Citation39]) and living in an urban area [Citation37,Citation43] are associated with better vaccine compliance. The second and third trimesters of pregnancy [Citation46,Citation51] and confidence in routine childhood vaccines [Citation49] are other determinants of vaccination adherence.
As reported by most authors, it must be taken into account that pregnancy is considered an emotional phase in women’s lives and how it can impact women’s mental health [Citation31]; in fact, Ceulemans M et al. [Citation32] highlighted that 52% of those who had already been pregnant in their sample reported that the pandemic had a (rather) large impact on their current pregnancy experience compared with previous pregnancies. This phenomenon, called maternal anxiety, must be considered in the management of immunization of pregnant and breastfeeding women; indeed, it can be both a determinant of vaccine compliance and refusal [Citation27,Citation28,Citation36,Citation41,Citation45,Citation49].
In this light, many experiences are quoted to achieve better immunization coverage. Most authors [Citation24,Citation27,Citation29,Citation30,Citation33–35,Citation45–48,Citation54–56,Citation63] focused on the role of healthcare providers; in fact, the degree of trust in healthcare professionals to provide reliable and trustworthy information related to COVID-19 vaccine safety seems to be a strong predictor of vaccine acceptance. As noted by Schaal NK et al. [Citation47] it is important that healthcare professionals, especially gynecologists, discuss the benefits and risks of the vaccine and reach a common decision based on individual circumstances. Along these lines, it is important that health providers are continuously informed about the latest scientific updates [Citation47]. Indeed, as reported by Siegel MR et al. [Citation48] while HCWs reported high personal compliance with COVID-19 vaccination and felt that the benefits of vaccination outweighed the risks in pregnancy, less than one-third felt very confident in counseling pregnant patients about the available evidence for mRNA vaccine safety in pregnancy. Chervenak FA et al. [Citation54] identified three previously undefined root causes underlying physicians’ hesitation to recommend COVID-19 vaccinations for pregnant women: clinical misapplications of therapeutic nihilism, shared decision-making, and ethical issues. Furthermore, expectant mothers who visit medical services and receive regular immunization counseling are more educated about their pregnancy an related risks and are more amenable to COVID-19 vaccination [Citation29,Citation43]. Finally, Sznajder KK et al. [Citation27] suggested that clinicians could offer COVID-19 vaccine along with influenza vaccine and remind women who feel overloaded that a COVID-19 vaccination could reduce their risk of severe COVID-19.
Much attention is paid to communicating evidence-based data of vaccine safety and efficacy to minimize vaccine hesitation in pregnant and breastfeeding women [Citation23,Citation24,Citation26,Citation31–35,Citation39–41,Citation45,Citation47,Citation51,Citation59,Citation63]; thus, public health strategies should prioritize providers and public education regarding adverse effects of COVID-19 in pregnancy and evolving safety data for vaccines in this group. Strategies to achieve this goal included: educational brochure [Citation39], community collaboration especially for ethnic minorities [Citation30], institutional communication from people who can provide leadership in society [Citation34], creation of websites and mobile apps that include correct and clear information about vaccination [Citation34,Citation63] as well as better use of mass media and social media and the internet to spread evidence-based facts on the vaccine and COVID-19 [Citation39]. Hirshberg JS et al. [Citation38] described their experience with vaccination uptake rates among high-risk obstetric patients before and after on-site availability of BNT162b2 messenger RNA vaccination in outpatient clinics as part of a pilot program to improve vaccine access among pregnant women; the authors concluded that increased access alone might not improve vaccination rates in obstetric patients even after consultation with experienced clinicians. Two studies [Citation52,Citation60] have proposed interventions based on an individual-level dialogue intervention using the components of a problem-based approach, the 5 C model of vaccine hesitancy, and motivational interviewing techniques. A key role should be played by the government, public health institutions, and scientific societies [Citation31,Citation53,Citation57,Citation59,Citation63] that should inform this subpopulation group about the true risks and benefits of vaccination; for any woman who is pregnant, nursing, or planning to conceive, contracting COVID-19 is certainly more dangerous than being vaccinated.
Finally, several studies [Citation40,Citation42,Citation53,Citation58,Citation61] have focused on excluding women from vaccine trials, leaving clinicians in the position of recommending vaccination to pregnant women without evidence of efficacy or safety, especially in the precocious phase of the vaccination campaign. Identifying the best way to include pregnant women in future vaccine studies rather than excluding them by default seems to be a priority for the scientific community and policy makers.
4. Conclusion
Our meta-analysis estimated vaccine hesitancy among pregnant and breastfeeding women worldwide to be 48% (95%CI = 43–53%); no differences were found between pregnant (49%) and lactating women (53%; p > 0.05) and between continents (p > 0.05); on the other hand, our analysis showed that vaccination hesitation varied by study period: assessed at 39% during 2020, before the start of the vaccination campaign, reached the highest values in the first semester of 2021 (58%), and then decreased in the second semester of 2021 (38%); this trend should be interpreted considering that in the first months of 2021 no data on vaccine safety and efficacy were available, and consequently healthcare providers could not rely on guidelines to recommend (or therefore) vaccination; further, reports of (rare) thromboembolic adverse events following vector adenoviral vaccines widely disseminated (and often distorted) by the mass media and social media [Citation69]) may have contributed to increase distrust and fear among patients. With increasing scientific evidence reported from phase IV studies and recommendations from scientific societies and international public health institutions, levels of unwillingness have returned to levels prior to the start of the vaccination campaign, although the percentage of hesitancy was still high and concerning.
Analysis of the evaluated determinants of vaccination hesitancy revealed that most of the evaluated determinants appeared not to influence the willingness of our population sub-group. The main determinants of improved vaccination compliance were white race, being employed, having previously received anti-influenza and/or dtaP vaccines, and being in the third trimester of pregnancy, as also confirmed by our systematic review. Social disparities as barriers to vaccination uptake (not specifically anti-COVID19) are determinants confirmed by other evidence in the literature; a 2022 systematic review reported that black ethnic minorities showed high vaccine hesitancy [Citation70]. Ayers KC et al. [Citation71] evaluated socioeconomic disparities and concluded that factors potentially contributing to vaccine adherence include barriers to vaccine access, inadequate information, and concerns about vaccine safety and efficacy. A 2019 systematic review reported that there was consistently a relationship between socioeconomic status and influenza immunization [Citation72]. These studies agreed that building trust, reducing physical barriers, and improving communication and transparency about vaccine development through healthcare workers, religious, and community leaders can improve access and facilitate vaccine adoption among ethnic minorities.
The third trimester seemed to be associated with a better readiness for vaccination, probably because women consider the fetus to be more mature and hence the vaccine may be less potentially dangerous. On the contrary, when considering the anti-influenza vaccine, a systematic review in 2021 found that one reason for not getting vaccinated was proximity to childbirth [Citation73], with some women fearing that getting vaccinated then may be dangerous or unnecessary because delivery is close [Citation73].
In each case, a major determinant of vaccination adherence was having received a previous vaccination, particularly the anti-influenza vaccine; this evidence had already been reported in the literature for anti-pertussis and anti-influenza vaccines [Citation73,74], as well as for other sub-group populations (i.e. HCWs) [Citation74–76].
The systematic review showed the main determinants of vaccine hesitation; lack of information about the vaccination, the opinion that the vaccine is not safe, and fear of adverse events for both mother and child are known determinants of vaccination refusal in the scientific literature [Citation77,Citation78]; in fact, these data confirmed the evidence already reported in the literature for other vaccinations in pregnancy [Citation73,74,Citation79]. On the other hand, the role of pharmaceutical companies in influencing vaccination policy decisions and the uncertainty associated with the rapid COVID-19 vaccine development process as determinants of hesitation are pathognomonic of COVID-19 vaccination compared with other vaccines; conversely, Prospero E et al. [Citation80] reported that the role of pharmaceutical companies is also a deterrent for anti-influenza vaccine.
History of disease or experience with it among family members and friends, fear of COVID-19 complications, and the safety and protection of mothers and fetuses/children seemed to increase the willingness to vaccinate. Higher education and scientific sources played a fundamental role in the attitudes of pregnant and breastfeeding women; trust in the scientific community has already been identified has a major determinant of vaccination compliance in the general population [Citation81] and thus, also plays a major role for this subgroup. Then again, the role of social media and the internet is discussed; although it is well known that fake news in mass and social media facilitates distrust of vaccines [Citation82], our systematic review did not clearly highlight this mechanism in pregnant and breastfeeding women [Citation34,Citation46]. Instead, extensive use of mass media, social media, and the internet to disseminate evidence-based facts about the vaccine and COVID-19 is advocated [Citation39].
The degree of reliance on healthcare professionals to provide reliable and trustworthy information related to COVID-19 vaccine safety, regular access to medical service by expectant mothers, and efficient immunization counseling seem to be strong predictors of vaccine acceptance. This evidence is also confirmed by considering the literature for anti-pertussis and anti-influenza vaccines [Citation73,74]. Indeed, as suggested by Sznajder KK et al. [Citation27] HCWs should offer COVID-19 vaccine along with influenza and pertussis vaccines to increase immunization rates. Finally, most authors agree on the need to promote vaccination through classical or modern sources of information, in order to combat fake news and ensure institutional communication based on scientific evidence.
The main limitation of this meta-analysis was the high heterogeneity between studies, as indicated by the I2 values; however, the use of a random-effects analysis minimized this bias. One major limitation is that the definition of ‘vaccine hesitancy’ is rather heterogeneous across studies, yet, it is very similar between them; therefore, this does not appear to be a critical issue. Another argument is that most surveys were administered online or on social media, and thus it is possible that HCWs responded to more than one questionnaire; this potential bias is unfortunately not detectable or correctable. However, a strength of our review and meta-analysis was the large sample size resulting from the collation of selected papers, which improved the statistical analysis and provided a better view of hesitancy to the anti-COVID-19 vaccine among pregnant and breastfeeding women. In addition, all studies were published as of 2021, so this view is up-to-date and reliable. Furthermore, many RR were calculated considering multiple determinants. Finally, the meta-analysis revealed a comparison of vaccine hesitancy before and during the vaccination campaign, not previously reported in the literature. Future studies should evaluate if the attitude tp vaccination change after the pregnancy and breastfeeding.
5. Expert opinion
Our study highlighted that half of pregnant and lactating women expressed hesitation to vaccinate and the main determinants of vaccination compliance were exposed. Many strategies have been proposed to deal with this criticism, and other approaches could be implemented considering those already suggested for other recommended vaccines in pregnancy [Citation73,74,Citation79], as well as for other fragile patients [Citation83]. In fact, available evidence in the literature has shown that fighting vaccine resistance is harsh and too slow as a process, considering the rapidity and unpredictability of a pandemic. Health education, and toward the anti-COVID19 vaccine, should be provided in order to improve the willingness of the community, especially for those with lower levels of education [Citation84].
Can mandatory vaccination be an option? This strategy has already been successful for other categories of the population [Citation85], but it seems difficult to propose this option considering the emotional fragility of the study population. Consequently, widespread action on multiple fronts is necessary to obtain good results in these patients.
Government and Public Health institutions should probably play the main role; in fact, clear and unambiguous communication is needed to express the risk/benefit ratio of the vaccine for both women and the fetus/child [Citation86]. Special measures are needed especially for that segment of the population of low economic status, which we have seen to be to be the most at risk of hesitation. Politicians should not be seduced by the vote pool of the no vax and anti-science community but should rely on scientific evidence and educate the population to data-based decisions.
Additionally, HCWs are among the most trusted sources of vaccine information and have direct influence on the vaccination decisions of their patients and social contacts [Citation87]. Hence, healthcare professionals must be empowered to play their role in the immunization campaign and pandemic management; it is also necessary to work on the hesitation of healthcare professionals, considering that a hesitant provider is less likely to recommend the vaccine to his or her patients [Citation74]. This last point is of crucial importance in the fight against the pandemic and the return to normalcy.
On the other hand, the role of information sources, particularly social media, must also be questioned. Many studies have reported the risk of vaccination campaign failure due to uncontrolled dissemination of erroneous information by the media [Citation69,Citation88]. Although the content of the media cannot be controlled, it must be taken into account that especially social platforms are the battlefield of no-vax groups that, even if small in number, are very organized and able to circulate fake news in a very short time [Citation69]. This is precisely why it is appropriate for public health institutions to ensure proper institutional and scientific communication, especially on social networks. Experiences reported in Italy, such as that of the website ‘Vaccinarsi,’ indicate that during the pandemic there was a strong increase in views, concluding that combining disciplines such as health education and digital communication through Information and Communication Technologies (ICT) represents the best strategy to support citizens [Citation89].
Finally, pregnant women have historically been excluded from most vaccine clinical trials because of ethical concerns and the potential for liability [Citation90–92]. So, there is a lack of evidence from phase III studies supporting the safety and efficacy of vaccines in this specific population. Data on vaccine safety in pregnant women are often drawn from the limited number of unintentional exposures during pregnancy. As assessed by Bianchi DW et al. [Citation93] additional protections that could be provided through rulemaking and development of regulations would help overcome these barriers to research on therapies during pregnancy and lactation, especially for those preventive or therapeutic interventions that are accelerated to address a public health emergency. There is a critical need to include pregnant women in vaccine trials, given the vulnerability of this population to developing severe illness with COVID-19 and other vaccine preventable diseases.
In conclusion, vaccination hesitancy toward the anti-COVID-19 vaccine among pregnant and breastfeeding women is an existing phenomenon. It must be considered that the changes in the practice of healthcare workers during the pandemic period [Citation94] and the impact of newer virus variants on health outcomes of pregnant [Citation95] put women’s health at even greater risk. Skjefte et al. [Citation49] showed how vaccination compliance is 50% higher in non-pregnant women compared with pregnant women. Achieving high vaccination coverage requires a multifactorial approach, which requires major social, scientific and health efforts. The success of the vaccination campaign in this population depends on the capillarity and consistency of the interventions implemented.
Author contributions
FPB and ST conceived the study. FPB and MCDG did the literature research. FPB did the metanalysis. SL and PS participated in the design of the metanalysis. NB supervisioned the metanalysis. FPB and ST codrafted the first version of the article.
Article highlights
Vaccine hesitancy can be a determining factor in the success (or otherwise) of the anti-COVID-19 immunization campaign
Vaccination hesitancy among pregnant and breastfeeding women is a topic already investigated in the literature
Insufficient vaccination coverage is reported, considering other vaccine-preventable diseases recommended for the category
Our study estimated the prevalence of vaccine hesitancy in pregnant and breastfeeding women, that was assessed around 48%.
No differences were found between pregnant (49%) and lactating women (53%; p>0.05).
The scenario of management strategies for hesitant individuals is very difficult
Our results highlight that vaccine hesitancy in pregnant and breastfeeding women is a genuine public health concern worldwide
Multifactorial strategy seems to be necessary to deal with low uptake
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download PDF (1.2 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2022.2100766
Additional information
Funding
References
- Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014 Jun 5;370(23):2211–2218.
- Sakala IG, Honda-Okubo Y, Fung J, et al. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;Dec;12(12):3065–3071.
- CDC. Guidelines for vaccinating pregnant women. Last updated: August 2016. [Accessed on 2022 Feb 10]. Available from: https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/guidelines.html
- Marchand G, Patil AS, Masoud AT, et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to June 3, 2021. AJOG Glob Rep. 2022 2;Feb(1):100049.
- Di Toro F, Gjoka M, Di Lorenzo G, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021 Jan;27(1):36–46.
- CDC. COVID-19 vaccines while pregnant or breastfeeding. Last updated January 28, 2022. accessed on 2022 Feb 11. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html.
- Fu W, Sivajohan B, McClymont E, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2022 Mar;156(3):406–417.
- Yan Z, Yang M, Lai CL. COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines (Basel). 2021 Sep 28;9(10):1097.
- Nir O, Schwartz A, Toussia-Cohen S, et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM. 2022 Jan;4(1): 100492.
- Perl SH, Uzan-Yulzari A, Klainer H, et al. Youngster I. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021 May 18;325(19):2013–2014.
- Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022 Feb;226(2):236.e1–236.e14.
- Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022 Jan 13. Epub ahead of print. Erratum in: Nat Med. 2022 Feb 4. DOI:10.1038/s41591-021-01666-2
- Má G-B, Gutiérrez-Calderón E, Pelcastre-Villafuerte BE, et al. Influenza vaccination hesitancy in five countries of South America. Confidence, complacency and convenience as determinants of immunization rates. PLoS One 2020 Dec 11 15(12):e0243833.
- de Munter AC, Ruijs WLM, Ruiter RAC, et al. Decision-making on maternal pertussis vaccination among women in a vaccine-hesitant religious group: stages and needs. PLoS One. 2020 Nov 12;15(11):e0242261.
- Martin S, Kilich E, Dada S, et al. “Vaccines for pregnant women … ?! Absurd” - Mapping maternal vaccination discourse and stance on social media over six months. Vaccine. 2020 Sep 29;38(42):6627–6637.
- Bianchi FP, Stefanizzi P, Lattanzio S, et al. Attitude for vaccination prophylaxis among pregnant women: a cross-sectional study. Hum Vaccin Immunother. 2022 Feb;18:1–5.
- Descamps A, Launay O, Bonnet C, et al. Seasonal influenza vaccine uptake and vaccine refusal among pregnant women in France: results from a national survey. Hum Vaccin Immunother. 2020 May 3;16(5):1093–1100.
- Doraivelu K, Boulet SL, Biswas HH, et al. Predictors of tetanus, diphtheria, acellular pertussis and influenza vaccination during pregnancy among full-term deliveries in a medically underserved population. Vaccine. 2019 Sep 24;37(41):6054–6059.
- Wales DP, Khan S, Suresh D, et al. Factors associated with Tdap vaccination receipt during pregnancy: a cross-sectional study. Public Health. 2020 Feb;179:38–44.
- Wilson RJ, Paterson P, Jarrett C, et al. Understanding factors influencing vaccination acceptance during pregnancy globally: a literature review. Vaccine. 2015 Nov 25;33(47):6420–6429.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- Hosokawa Y, Okawa S, Hori A, et al. The prevalence of COVID-19 vaccination and vaccine hesitancy in pregnant women: an internet-based cross-sectional study in Japan. J Epidemiol. 2022 Jan; 29.
- Huddleston HG, Jaswa EG, Lindquist KJ, et al. COVID-19 vaccination patterns and attitudes among American pregnant individuals. Am J Obstet Gynecol MFM. 2022 Jan;4(1): 100507.
- Kiefer MK, Mehl R, Costantine MM, et al. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: a cross-sectional study. BJOG. 2022 Feb; 1.
- Skirrow H, Barnett S, Bell S, et al. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022 Jan 14;22(1):33.
- Sznajder KK, Kjerulff KH, Wang M, et al. Covid-19 vaccine acceptance and associated factors among pregnant women in Pennsylvania 2020. Prev Med Rep. 2022 Apr;26:101713.
- Waring ME, Pagoto SL, Rudin LR, et al. Factors associated with mothers’ hesitancy to receive a COVID-19 vaccine. J Behav Med. 2022 Jan;4:1–6.
- Aisyah R, Fitriyani F, Pambudi D. determinant factors involved in pregnant women’s willingness to receive Covid-19 vaccine. Interest [Internet]. 6 Jan.2022;231–239. [cited 2022 Feb 16]. Available from: http://jurnalinterest.com/index.php/int/article/view/362
- Battarbee AN, Stockwell MS, Varner M, et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December 2020. Am J Perinatol. 2022 Jan;39(1):75–83.
- Carbone L, Mappa I, Sirico A, et al. Pregnant women’s perspectives on severe acute respiratory syndrome coronavirus 2 vaccine. Am J Obstet Gynecol MFM. 2021 Jul;3(4): 100352.
- Ceulemans M, Foulon V, and Panchaud A, et al. Vaccine willingness and impact of the COVID-19 pandemic on women’s perinatal experiences and practices-A multinational, cross-sectional study covering the first wave of the pandemic. Int J Environ Res Public Health. 2021 Mar 24;18(7):3367.
- Desai P, Kaur G, Dong F, et al. COVID-19 vaccine acceptance in pregnancy. Neonatol Today. 2021;16(7):11–15.
- Gencer H, Özkan S, Vardar O, et al. The effects of the COVID 19 pandemic on vaccine decisions in pregnant women. Women Birth. 2021 May;19(21):S1871–5192.
- Geoghegan S, Stephens LC, Feemster KA, et al. This choice does not just affect me.” Attitudes of pregnant women toward COVID-19 vaccines: a mixed-methods study. Hum Vaccin Immunother. 2021 Oct 3;17(10):3371–3376.
- Goncu Ayhan S, Oluklu D, Atalay A, et al. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet. 2021 Aug;154(2):291–296.
- Hailemariam S, Mekonnen B, Shifera N, et al. Predictors of pregnant women’s intention to vaccinate against coronavirus disease 2019: a facility-based cross-sectional study in southwest Ethiopia. SAGE Open Med. 2021 Aug 19;9:20503121211038454.
- Hirshberg JS, Huysman BC, Oakes MC, et al. Offering onsite COVID-19 vaccination to high-risk obstetrical patients: initial findings. Am J Obstet Gynecol MFM. 2021 Nov;3(6): 100478.
- Jayagobi PA, Ong C, Thai YK, et al. Perceptions and acceptance of COVID-19 vaccine among pregnant and lactating women in Singapore: a cross-sectional study. medRxiv. 2021;06:29.21259741.
- Levy AT, Singh S, Riley LE, et al. Acceptance of COVID-19 vaccination in pregnancy: a survey study. Am J Obstet Gynecol MFM. 2021 Sep;3(5): 100399.
- Mappa I, Luviso M, Distefano FA, et al. Women perception of SARS-CoV-2 vaccination during pregnancy and subsequent maternal anxiety: a prospective observational study. J Matern Fetal Neonatal Med. 2021 Apr;11:1–4.
- Mohan S, Reagu S, Lindow S, et al. COVID-19 vaccine hesitancy in perinatal women: a cross sectional survey. J Perinat Med. 2021 Apr 28;49(6):678–685.
- Mose A. Willingness to receive COVID-19 vaccine and its determinant factors among lactating mothers in Ethiopia: a cross-sectional study. Infect Drug Resist. 2021 Oct 14;14:4249–4259.
- Nguyen LH, Hoang MT, Nguyen LD, et al. Acceptance and willingness to pay for COVID-19 vaccines among pregnant women in Vietnam. Trop Med Int Health. 2021 Oct;26(10):1303–1313.
- Oluklu D, Goncu Ayhan S, Menekse Beser D, et al. Factors affecting the acceptability of COVID-19 vaccine in the postpartum period. Hum Vaccin Immunother. 2021 Nov 2;17(11):4043–4047.
- Riad A, Jouzová A, Üstün B, et al. COVID-19 vaccine acceptance of Pregnant and lactating women (PLW) in Czechia: an analytical cross-sectional study. Int J Environ Res Public Health. 2021 Dec 19;18(24):13373.
- Schaal NK, Zöllkau J, and Hepp P, et al. Pregnant and breastfeeding women’s attitudes and fears regarding the COVID-19 vaccination. Arch Gynecol Obstet. 2021 Oct;27:1–8.
- Siegel MR, Lumbreras-Marquez MI, James K, et al. Perceptions and attitudes towards COVID-19 vaccination amongst pregnant and postpartum individuals. medRxiv. 2021;12:17.21267997.
- Skjefte M, Ngirbabul M, and Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021 Feb;36(2):197–211.
- Sutton D, D’Alton M, Zhang Y, et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM. 2021 Sep;3(5): 100403.
- Tao L, Wang R, Han N, et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum Vaccin Immunother. 2021 Aug 3;17(8):2378–2388.
- Ransing R, Kukreti P, Raghuveer P, et al. A brief psycho-social intervention for COVID-19 vaccine hesitancy among perinatal women in low-and middle-income countries: need of the hour. Asian J Psychiatr. 2022 Jan;67:102929.
- Anderson E, Brigden A, Davies A, et al. Maternal vaccines during the Covid-19 pandemic: a qualitative interview study with UK pregnant women. Midwifery. 2021 Sep;100:103062.
- Chervenak FA, McCullough LB, Grünebaum A. Reversing physician hesitancy to recommend COVID-19 vaccination for pregnant patients. Am J Obstet Gynecol. 2021 Nov 8;S0002-9378(21):01210.
- Daskalakis G, Pergialiotis V, Antsaklis P, et al. Healthcare workers’ attitudes about vaccination of pregnant women and those wishing to become pregnant. J Perinat Med. 2021 Dec; 7.
- Deruelle P, Couffignal C, Sibiude J, et al. Prenatal care providers’ perceptions of the SARS-Cov-2 vaccine for themselves and for pregnant women.
- Hayakawa S, Komine-Aizawa S, Takada K, et al. Anti-SARS-CoV-2 vaccination strategy for pregnant women in Japan. J Obstet Gynaecol Res. 2021 Jun;47(6):1958–1964.
- Iacobucci G. Covid-19 and pregnancy: vaccine hesitancy and how to overcome it. BMJ. 2021 Nov;22(375):n2862.
- Moodley J, Khaliq OP, Mkhize PZ. Misrepresentation about vaccines that are scaring women. Afr J Prim Health Care Fam Med. 2021 Jun 9;13(1):e1–e2.
- Shook LL, Kishkovich TP, and Edlow AG. Countering COVID-19 Vaccine Hesitancy in Pregnancy: the “4 Cs.” Am J Perinatol. 2021 Oct;1055/a-1673-5546:19.
- Knight M, Morris RK, Furniss J, et al. Include pregnant women in research-particularly covid-19 research. BMJ. 2020 Aug;25(370):m3305.
- Perez MJ, Paul R, Raghuraman N, et al. Characterizing initial COVID-19 vaccine attitudes among pregnancy-capable healthcare workers. Am J Obstet Gynecol MFM. 2021 Dec;22 4(2):100557.
- Samannodi M. COVID-19 vaccine acceptability among women who are pregnant or planning for pregnancy in Saudi Arabia: a cross-sectional study. Patient Prefer Adherence. 2021 Nov 23;15:2609–2618.
- Stuckelberger S, Favre G, Ceulemans M, et al. SARS-CoV-2 vaccine willingness among pregnant and breastfeeding women during the first pandemic wave: a cross-sectional study in Switzerland. Viruses. 2021 Jun 22;13(7):1199.
- Bradfield Z, Wynter K, Hauck Y, et al. COVID-19 vaccination perceptions and intentions of maternity care consumers and providers in Australia. PLoS One. 2021 Nov 15;16(11):e0260049.
- Kansal N, Weaver K, Vasudevan, et al. Factors Increasing COVID-19 Vaccine Hesitancy Among Pregnant Women during a Global Pandemic. Am J Obstetrics & Ginecol. 2022 FEBRUARY 01;226(2):313–314.
- Mark E, Rick AM, Demirci J, et al. Vaccination beliefs and attitudes of lactating women during the SARS-CoV2 pandemic. Am J Obstetrics & Ginecol. 2022 Jan 01;226(1):S678.
- Saeb S, McCulloch J, Greene N, et al. The Childbirth Experience Survey (CBEX) and COVID-19: the ABCs of vaccine hesitancy in postpartum people. Am J Obstetrics & Ginecol. 2022 Jan 01;226(1):S227–S228.
- Bianchi FP, Tafuri S. A public health perspective on the responsibility of mass media for the outcome of the anti-COVID-19 vaccination campaign: the AstraZeneca case. Ann Ig. 2022 Feb; 3.
- Abba-Aji M, Stuckler D, Galea S, et al. Ethnic/racial minorities’ and migrants’ access to COVID-19 vaccines: a systematic review of barriers and facilitators. J Migr Health. 2022 Feb;18:100086.
- Ayers CK, Kondo KK, Williams BE, et al. Disparities in H1N1 vaccination rates: a systematic review and evidence synthesis to inform COVID-19 vaccination efforts. J Gen Intern Med. 2021 Jun;36(6):1734–1745.
- Lucyk K, Simmonds KA, Lorenzetti DL, et al. The association between influenza vaccination and socioeconomic status in high income countries varies by the measure used: a systematic review. BMC Med Res Methodol. 2019 Jul 17;19(1):153.
- Adeyanju GC, Engel E, Koch L, et al. Determinants of influenza vaccine hesitancy among pregnant women in Europe: a systematic review. Eur J Med Res. 2021 Sep 28;26(1):116.
- Biswas N, Mustapha T, Khubchandani J, Price JH. The Nature and. Extent of COVID-19 vaccination hesitancy in healthcare workers. J Community Health. 2021 Dec;46(6):1244–1251.
- Hall CM, Northam H, Webster A, et al. Determinants of seasonal influenza vaccination hesitancy among healthcare personnel: an integrative review. J Clin Nurs. 2021 Oct; 29.
- Bianchi FP, Vimercati L, Mansi F, et al. Compliance with immunization and a biological risk assessment of health care workers as part of an occupational health surveillance program: the experience of a university hospital in southern Italy. Am J Infect Control. 2020 Apr;48(4):368–374.
- Januszek SM, Faryniak-Zuzak A, Barnaś E, et al. The approach of pregnant women to vaccination based on a COVID-19 systematic review. Medicina (Kaunas). 2021;57(9):977. Published 2021 Sep 17.
- Truong J, Bakshi S, Wasim A, et al. What factors promote vaccine hesitancy or acceptance during pandemics? A systematic review and thematic analysis. Health Promot Int. 2022 Feb 17;37(1):daab105.
- Bisset KA, Paterson P. Strategies for increasing uptake of vaccination in pregnancy in high-income countries: a systematic review. Vaccine. 2018 May 11;36(20):2751–2759.
- Prospero E, Galmozzi S, Paris V, et al. Factors influencing refusing of flu vaccination among pregnant women in Italy: healthcare workers’ role. Influenza Other Respir Viruses. 2019 Mar;13(2):201–207.
- Sturgis P, Brunton-Smith I, Jackson J. Trust in science, social consensus and vaccine confidence. Nat Hum Behav. 2021 Nov;5(11):1528–1534.
- Muric G, Wu Y, Ferrara E. COVID-19 vaccine hesitancy on social media: building a public twitter data set of antivaccine content, vaccine misinformation, and conspiracies. JMIR Public Health Surveill. 2021 Nov 17;7(11):e30642.
- Bianchi FP, Stefanizzi P, Spinelli G, et al. Immunization coverage among asplenic patients and strategies to increase vaccination compliance: a systematic review and meta-analysis. Expert Rev Vaccines. 2021 Mar;20(3):297–308.
- Wake AD. The willingness to receive COVID-19 vaccine and its associated factors: “vaccination refusal could prolong the war of this pandemic” - A systematic review. Risk Manag Healthc Policy. 2021 Jun;21(14):2609–2623.
- Sindoni A, Baccolini V, Adamo G, et al. Effect of the mandatory vaccination law on measles and rubella incidence and vaccination coverage in Italy (2013-2019). Hum Vaccin Immunother. 2021 Aug;4:1–10.
- Chavan M, Qureshi H, Karnati S, et al. COVID-19 vaccination in pregnancy: the benefits outweigh the risks. J Obstet Gynaecol Can. 2021;43(7):814–816.
- Giambi C, Fabiani M, D’Ancona F, et al. Parental vaccine hesitancy in Italy - Results from a national survey. Vaccine. 2018 Feb 1;36(6):779–787.
- Signorelli C, Odone A, Conversano M, et al. Deaths after Fluad flu vaccine and the epidemic of panic in Italy. BMJ. 2015 Jan;14(350):h116.
- Arghittu A, Dettori M, Dempsey E, et al. Health communication in COVID-19 Era: experiences from the Italian vaccinarsì network websites. Int J Environ Res Public Health. 2021 May 25;18(11):5642.
- Denne SC. the pediatric policy council Including pregnant women in clinical research: time to overcome the barriers. Pediatr Res. 2019;86:554–555.
- Strömmer S, Lawrence W, Rose T, et al. Improving recruitment to clinical trials during pregnancy: a mixed methods investigation. Soc Sci Med. 2018 Mar;200:73–82.
- van der Graaf R, van der Zande ISE, den Ruijter HM, et al. Fair inclusion of pregnant women in clinical trials: an integrated scientific and ethical approach. Trials. 2018 Jan 29;19(1):78.
- Bianchi DW, Kaeser L, Cernich AN. Involving pregnant individuals in clinical research on COVID-19 vaccines. JAMA. 2021 Mar 16;325(11):1041–1042.
- Şahin D, Tanaçan A, Webster SN, et al. Pregnancy and COVID-19: prevention, vaccination, therapy, and beyond. Turk J Med Sci. 2021 Dec 17;51(SI–1):3312–3326.
- Sahin D, Tanacan A, Anuk AT, et al. Comparison of clinical features and perinatal outcomes between pre-variant and post-variant periods in pregnant women with SARS-CoV-2: analysis of 1935 cases. Arch Gynecol Obstet. 2022 Mar;7:1–10.