Abstract
The island of Trinidad has several endemic reptile species that in some cases are morphologically indistinguishable, or almost so, from their mainland counterparts. In particular, many snakes from the island have not been examined thoroughly with modern scientific methods and may therefore be misidentified. At least two species of sipo snakes (Chironius carinatus, C. septentrionalis) are reported to inhabit Trinidad, both being considered species already inhabiting the mainland, though their identities are based solely on morphology. Here, we evaluate the molecular distinctiveness of these Trinidadian snakes and assess their relationships to other members of the genus. We constructed a multi-locus data set (12S rRNA, 16S rRNA, cyt b, ND4, cmos, NT3, Rag-1, Rag-2) including novel sequences and those available on GenBank to perform Bayesian and maximum likelihood phylogenetic analyses. Our phylogenetic reconstruction elucidates the identity of both Trinidad species, which have been misidentified since their discovery, and we provide a literature review of their taxonomic history. More specifically, our results suggest that the Trinidad population of Chironius carinatus is an undescribed species, that we describe herein as Chironius nigelnoriegai sp. nov., and is the sister to C. flavopictus. Our results also demonstrate that the Trinidad population previously identified as C. septentrionalis is instead the mainland species C. cochranae, a member of a clade with C. foveatus, C. laurenti, and C. multiventris. Finally, we generated a time tree and inferred that our new species separated from C. flavopictus approximately 4 million years ago in the Pliocene, a time when the island of Trinidad detached from Northern Venezuela. This work contributes to a better understanding of species diversity of Trinidad and we hope that it assists in conservation efforts towards this important endemic region. These findings support the prospect of rapid speciation on Trinidad and implies that more extensive surveys of island squamates will reveal additional cryptic diversity. http://zoobank.org/urn:lsid:zoobank.org:pub:500DB91B-62CD-4051-9831-0BEEAC64C8E2
Introduction
Trinidad and its sister island Tobago have been connected to the mainland multiple times at low sea-level stands during the Pleistocene and researchers sometimes assume that because the islands were connected, the island species must be conspecific with mainland taxa (Kenny, Citation2000; Murphy et al., Citation2023; Scharff, Citation1922). The problem with this line of thinking is that it ignores geological and/or speciation events prior to the Pleistocene. In particular, Trinidad has a unique geography with a variety of distinct ecosystems resulting in a tendency of its mountainous landscape to promote speciation. More specifically Trinidad’s large size (∼4800 km2) and close proximity to the mainland (∼8 km) reduce the chance for the extirpation of colonizing fauna, while the three mountain or hill ranges separated by lowland basins allow for several distinct physiographic regions and in turn the prospect of both vicariance and ecological speciation (Boos, Citation2001; Murphy, Citation1997; Rivas et al., Citation2021). The island of Trinidad has endemic squamates that in some cases are morphologically indistinguishable, or almost so, from their mainland counterparts or sympatric species living on the island (e.g. Gonatodes ferrugineus Cope, 1864 (Sphaerodactylidae), Leptophis stimsoni Harding, 1995 (Colubridae), Ninia franciscoi Angarita-Sierra, 2014 (Dipsadinae), Plica caribeana Murphy & Jowers, 2013 (Tropiduridae), Polychrus auduboni (Hallowell, 1845) (Polychrotidae), (also on mainland and on Tobago) Tupinambis cryptus Murphy et al., Citation2018 (Teiidae)).
The Neotropical Whipsnakes or Sipo Snakes, genus Chironius Fitzinger, Citation1826, comprise one of the most speciose colubrid snake genera in Central and South America, with 23 currently recognized species (Entiauspe-Neto et al., Citation2020) distributed from the Caribbean coast of Honduras and Nicaragua southeast to northeastern Chile, Argentina, and the Río de la Plata of Uruguay (Hollis, Citation2006; Köhler, Citation2008). Most are widely distributed and occupy a variety of habitats ranging from savannas to cloud forests, and although some are generally ground dwelling others live in the canopy (Bailey, Citation1955; Dixon et al., Citation1993; Hollis, Citation2006). Species of Chironius are of interest partly because they have the lowest number (12) of dorsal scale rows at mid-body found in snakes, 11 species violate the general condition of male snakes having longer tails than female snakes, and they tend to be dietary specialists that feed primarily on amphibians, particularly anurans (Roberto & Souza, Citation2020). At least two species of Chironius inhabit Trinidad, with their identities to date based entirely on external appearances. The recognition of the presence of two species of Chironius on Trinidad can be traced back to Mole and Urich (1894a) when they recognized Herpetodryas carinatus and H. macrophthalmus as present. The one exception was a report of C. scurrulus (Dixon et al., Citation1993) from the island, which was based upon a specimen shown subsequently to have erroneous locality data (Murphy et al., Citation2018). Currently, all Chironius populations observed on the island have been identified as either C. carinatus or C. septentrionalis (formerly C. multiventris or C. m. septentronalis) (Murphy, Citation1997).
In this study, we construct the most taxonomically comprehensive molecular phylogeny of the genus Chironius and update our understanding of the evolutionary relationships within the clade. More specifically, we evaluate the evolutionary history and molecular distinctiveness of Sipo Snakes on Trinidad to better assess their relative endemism. Additionally, we assess the timing of diversification of this clade from its closest living relative by performing divergence time estimation. Our data include mitochondrial and nuclear genes for 20 species of Chironius available on GenBank, along with novel sequences that were generated from Trinidad snakes and one from Venezuela. From these findings we find justification to redescribe both taxa on Trinidad, one of which we describe as a new species.
Materials and methods
Morphological data
Ten preserved specimens of Chironius were examined in this study (Appendix). Specimen examination was conducted at the Field Museum of Natural History, the University of Wisconsin–Eau Claire, and the University of Wisconsin–Stevens Point Museum of Natural History. Standard scale counts and measurements were taken for the right side of the snake and follow Dixon et al. (Citation1993) and Entiauspe-Neto et al. (Citation2020). The dorsal scale arrangement is counted as the number of dorsal scale rows across the body one head length behind the head, at midbody, and one head length anterior to the vent. All counts and measurements were done under a dissection microscope and using digital calipers.
DNA sequencing and alignment
Genomic DNA from seven Chironius specimens was extracted using a Qiagen DNeasy extraction kit and protocol. Four mitochondrial (12S rRNA (12S), 16S rRNA (16S), cytochrome b (cyt b) and NADH dehydrogenase subunit 4 (ND4)) and four nuclear (cmos, NT3, Rag-1, Rag-2) gene fragments were independently amplified. Published sequence data for these gene fragments for most Chironius species and some closely related outgroup taxa were acquired from GenBank (Supplemental Table S1) and combined in data sets with our novel sequences (). Chironius is considered monophyletic (Hamdan et al., Citation2017; Hollis, Citation2006; Klaczko et al., Citation2014) and we therefore did not test this hypothesis. Each gene fragment was aligned using the program MUSCLE (Edgar, Citation2004) with default settings and then edited and concatenated manually using Se-Al v.2.0a11 (Rambaut, Citation2002).
Table 1. GenBank numbers from both Trinidadian Chironius taxa. Bolded sequence numbers were generated in this study.
Phylogenetic analyses
We performed maximum likelihood (ML) phylogenetic analyses in IQ-TREE v2.2.2.6 (Minh et al., Citation2020). Multiple analyses on different data sets were performed to assess congruence. We first performed an unpartitioned analysis of the concatenated eight gene (mtDNA + nDNA) data set. We then performed a partitioned analysis on the same matrix, with the best-fitting partition scheme and substitution models identified using BIC in ModelFinder (MFP + MERGE; Kalyaanamoorthy et al., Citation2017; Lanfear et al., Citation2012). Next, we performed ML analyses on only the concatenated mtDNA data. Again, both unpartitioned and partitioned models were implemented to assess congruence. For all analyses, support was determined using both the ultrafast bootstrap (Hoang et al., Citation2018) and SH-aLRT tests (Guindon et al., Citation2010) with 1000 replicates each. Bayesian inference was implemented using MrBayes v.3.0b4 with default Markov chain Monte Carlo (MCMC) settings, for a total of 6 × 106 generations per run, sampling trees and parameters every 100 generations and the first 1.5 × 106 generations from each run discarded as burn-in. The data set was concatenated including all mtDNA and nuclear fragments and partitioned by fragment and codon position. Appropriate models of nucleotide substitution for Bayesian analyses were identified using the program MrModeltest v2.2 (Nylander, Citation2004), run in PAUP* v4.0b10 (Swofford, Citation2002). The best-fit models were selected using the Akaike information criterion (AIC). All trees were rooted using the outgroup taxa Dendrophidion brunneum, Drymoluber dichrous, Oxybelis koehleri, and Spilotes sulphureus per phylogenetic estimates by Jadin et al. (Citation2014, Citation2019).
Divergence time estimation
We used BEAST v2.7.5 (Bouckaert et al., Citation2019) to estimate divergence times within Chironius. We began with the concatenated mtDNA + nDNA matrix and subsampled one individual per lineage. Representative individuals were selected based primarily on the availability of sequence data. This resulted in a matrix of 38 taxa and 5229 bp. We partitioned analyses by independent locus (i.e., concatenated mtDNA, cmos, RAG1, RAG2, NT3) to minimize overparameterization. Tree models were linked and site models were unlinked. We specified two separate clock models, one for mtDNA and one for nDNA. We initially attempted separate clock models for each nuclear locus, but this resulted in poor mixing of the MCMC. To account for substitution model uncertainty we utilized Bayesian model selection (bModelTest; Bouckaert & Drummond, Citation2017). Because the data consisted of multiple species with a single sample per lineage, we specified an optimized relaxed clock model (Douglas et al., Citation2021; Zhang & Drummond, Citation2020) for both the mtDNA and nDNA. A Yule tree prior was used and the remaining priors were left as defaults. To temporally calibrate the analysis we followed the approach of Torres-Carvajal et al. (Citation2019), who based their secondary calibration scheme on the results of Hamdan et al. (Citation2017) and Zheng and Wiens (Citation2016). Specifically, we calibrated the crown-group age of Chironius using a normal prior (mean = 20 million years ago (mya), sigma = 1 mya). Two independent runs were executed for 20 million generations each, sampling every 2000 generations (10,000 posterior samples per run). Mixing and effective sample sizes (ESS; target >200) were monitored in Tracer v1.7.2 (Rambaut et al., Citation2018). Upon adequate sampling, LogCombiner was used to combine tree files following a burn-in of 10%. TreeAnnotator was then used to construct a maximum clade credibility (MCC) tree with median node heights.
Results
Phylogenetic analysis
The concatenated eight gene (mtDNA + nDNA) data set consisted of 133 individuals and 5229 sites. The number of constant sites was 4143 and the number of parsimony informative sites 776. The best-fit substitution model for the unpartitioned analysis according to BIC was TIM2 + F + I + R3. ModelFinder indicated that the best-fit scheme comprised four partitions (Supplemental Table S2). This same data set was utilized for the Bayesian estimate but was partitioned by gene fragment and by codon position and the best model for each partition was estimated using MrModeltest (Supplemental Table S3).
The concatenated mtDNA data consisted of 133 sequences and 2598 sites. The number of constant sites was 1635 and the number of parsimony informative sites 715. The best-fit substitution model for the unpartitioned analysis according to BIC was TIM2 + F + I + R3. ModelFinder indicated that the best-fit scheme comprised three partitions (Supplemental Table S4).
All of our phylogenetic hypotheses were highly congruent showing consistent relationships (described thoroughly in the discussion) regardless of methodology or data set. Our two most important findings however were: (1) the Trinidadian population previously identified as C. septentrionalis instead belongs to the mainland South American taxon C. cochranae, that was sister to C. multiventris and appears to be a paraphyletic species complex with respect to C. foveatus and C. laurenti; and (2) the taxon previously identified as C. carinatus from Trinidad is a distinct species sister to C. flavopictus, which together are sister to a clade of C. quadricarinatus, C. maculoventris and C. exoletus ().
Fig. 1. Phylogenetic relationships of Chironius taxa estimated from a Bayesian 50% majority-rule consensus phylogram using a multilocus dataset (i.e., 12S, 16S, cyt b, ND4, cmos, NT3, Rag-1, Rag-2; total of 5229 bp) with posterior probabilities (≥95) represented at the node (red circles) and additional support values (SH-aLRT > 80%/UFboot <95%) from maximum likelihood (ML) analyses of the partitioned dataset obtained from IQ-TREE. Inserted photographs are top right Chironius nigelnoriegai sp. nov. from Trinidad’s Northern Basin; bottom left is a C. cochranae from the Arima Valley, Trinidad. Photographs by Saifudeen Muhammad and JCM, respectively.
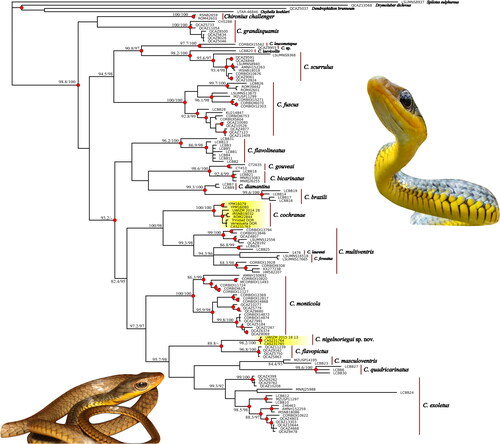
Fig. 2. Divergence time estimates from representative Chironius samples. Inference was based on the combined mtDNA and nDNA data (5229 bp). Red dots indicate mean divergence data. Plio. = Pliocene; Plei. = Pleistocene. Insert is Chironius cochranae from southern Trinidad north of Moruga, photograph by Rainer N. Deo.
Divergence times
ESS values for all parameters for each BEAST run were >100, with nearly all >200. ESS values for the combined runs were all >200 indicating adequate sampling of the posterior. Assuming a normal distribution with a mean divergence time of 20 mya for crown Chironius resulted in a mean mtDNA substitution rate of 0.0064529 substitutions per site per million years (95% highest posterior density (HPD) = 0.004940 − 0.0080905). The estimated mean substitution rate for the nuclear loci was 0.0003091 substitutions per site per million years (95% HPD = 0.0002195, 0.00040956). For both data sets the 95% HPD for the coefficient of variation parameter did not include zero, indicating that relaxed clock models were appropriate. Our analysis recovered an Early Miocene origin for Chironius, at 19.9 mya (95% HPD: 17.8–21.8 mya, ). Within that time period, the divergence of C. cochranae from its sister clade of Southern American C. multiventris, C. laurenti and C. foveatus was inferred to be 9.5 mya (95% HPD: 6.6–12.4 mya) while Chironius sp. nov. from Trinidad and C. flavopictus from Ecuador were inferred to have separated in the Miocene, 4.2 mya (95% HPD: 1.7–7.3 mya).
Taxonomic implications
Three species of Coluber (i.e., C. carinatus, C. exoletus and C. fuscus) described by Linnaeus (Citation1758) are still considered valid and are now placed in the genus Chironius. A fourth species, C. saturninus is in the synonymy of C. fuscus. Fitzinger (Citation1826) designated Coluber carinatus as the type species for his genus Chironius. Ten species of Chironius had been named by 1827 by Linnaeus, Wied, Wagler and Boïé, assigned to the genera Coluber (Linnaeus, Citation1758 and Wied-Neuwied, Citation1820), Natrix (Wagler, Citation1824), Chironius (Fitzinger, Citation1826) and Erpetodryas (Boie, Citation1827). By 1830, Wagler was referring to Erpedoryas as Herpetodryas (an emendation) The new spelling was widely used by Schlegel (1837), Lichtenstein (1856), Jan (Citation1863), Jan and Sordelli (Citation1869), and by Cope in multiple papers between 1860 and 1893. Boulenger (Citation1894) created more confusion with Herpetodryas (= Chironius) by placing several names in the synonymy. However, other late 19th and early 20th century European and America herpetologists perpetuated the use of Herpetodryas. No one had assigned a type species for the genus Erpetodryas or Herpetodryas until Montingelli et al. (Citation2011) assigned Herpetodryas reticulata Peters, 1863, as revalidated and assigned as a junior synonymy of the genus Mastigodryas.
In the 1890s F. W. Urich and R. R. Mole collected Trinidad reptiles, particularly snakes. At that time, any literature on snakes was unavailable on Trinidad. Therefore, they sent specimens to O. Boettger at the Senckenberg Museum in Frankfurt and G. A. Boulenger at the British Museum of Natural History in London. In their 1894 checklist Mole and Urich included the new descriptions of a frog and gecko from Trinidad written by Boettger. And, he provided names to apply to two species of Chironius previously described. Herpetodryas carinatus, Linnaeus, Citation1758, which had 12 mid-dorsal scales rows, 165 ventrals, a divided anal plate and 93 subcaudals, and Herpetodryas macrophthalmus Jan, Citation1863, which had 12 mid-dorsal scale rows, 173 ventrals, a divided anal plate and 171 subcaudals. Mole and Urich (1894b) listed the species as Herpetodryas macrophthalma Jan and considered it to be a variety of Herpetodryas carinatus. Wallach et al. (Citation2014) considered Jan’s Herpetodryas macrophthalma to be a nomen nudum as Jan (Citation1863: 80) stated that the species occurs in Brazil but provides no other details. Mole’s (Citation1924) detailed survey of Trinidad snakes mentioned one species of Chironius, H. carinatus. In a paper on the ecology of the Arima Valley, Beebe (Citation1952) listed only Chironius carinatus as present in Trinidad’s Arima Valley. Wehekind (Citation1955), Emsley (Citation1977) and Murphy (Citation1997) also listed only C. carinatus as present on the island.
The confusion over the identity of Trinidad Chironius becomes apparent and unsurprising when you consider how similar the snakes involved are. Examining specimens and published accounts does not easily resolve the confusion. For example, data presented in Dixon et al. (Citation1993) accounts for C. m. septentrionalis include data from Trinidad (and probably Venezuela), C. cochranae, and their account for Carinatus carinatus carinatus includes data from Trinidad and British Guiana. Dixon et al. (Citation1993:177–8) stated that C. m. septentrionalis specimens from Trinidad and extreme northwestern Venezuela were more like C. m. cochranae because they inhabited rainforests instead of cloud forests; consistently had fewer than 175 ventrals versus more than 177 ventrals in C. m. cochranae; the tail had yellow subcaudals and a brown dorsum extending to the tips of the subcaudals, producing a straight ventrolateral line of contrasting colours usually present in C. m. cochranae; they have a high incidence (76.9%) of one apical pit on dorsal scales just behind the head; a high incidence (64.2%) of three postocular scales and a 35.8% incidence of two postoculars (northern Venezuelan specimens have a 100% incidence of two postoculars). In this study, our phylogenetic analyses () confirm their identities as C. cochranae and a novel taxon and we hope to clarify this confusion by describing both below.
Chironius cochranae Hoge and Romano Citation1969
Herpetodryas macrophthalmus – Mole and Urich, Citation1894b: 518.
Chironius carinatus–Beebe, Citation1946: 21.
Chironius multiventris cochranae Hoge and Romano, Citation1969: 93. Type locality: Agua Preta, Estado Pará, Brazil. Abuys, Citation1982: 243. Holotype USNM 158103 (not 1581103 as reported in original description) from Agua Prete Utinga (near Belem) State Para, Brazil. The holotype is a male collected by E. Dente on 19 July 1965 ().
Fig. 3. Holotype of Chironius carinatus Linne 1758, NRM 33. Photograph taken from the Museum Adolphi Friderici collection of the Swedish Museum of Natural History.

Chironius multiventris septentrionalis–Boos, Citation2001: 120.
Chironius cochranae–Hollis, Citation2006: 445.
Chironius cochranae was described from a holotype from northeastern Brazil, near Belém and paratypes from Guyana and Suriname. However, one of the paratypes (AMNH 6801 from Guyana) was later found to be C. exoletus by Dixon et al. (Citation1993). Abuys (Citation1982) recognized C. cochranae as a subspecies of C. multiventris. This was followed by Dixon et al. (Citation1993), who recognized four subspecies using morphology within C. multiventris: C. m. cochranae, C. m. foveatus, C. m. multiventris and C. m. septentrionalis. Dixon et al. (Citation1993) assigned specimens from northeastern Brazil, Guyana and Suriname to C. m. cochranae. A phylogenetic analysis based upon Chironius morphological data provided by Dixon et al. (Citation1993) was used as the basis for resurrecting C. cochranae (Hollis, Citation2006). Klaczko et al. (Citation2014) synonymized C. cochranae with C. multiventris based on morphology, but the holotype of C. cochranae was not included in their study, nor were specimens from the type locality, nor any specimen from Guyana or Suriname. Torres-Carvajal et al. (Citation2019) provided a phylogeny of Chironius using molecular data and their results strongly supported Guyana and Suriname specimens of C. multiventris as distinct species. Our tree strongly supports C. cochranae as a valid species present on Trinidad (). Therefore, we remove C. cochranae from the synonymy of C. multiventris and return it to species status.
Diagnosis
Chironius cochranae can be distinguished from its congeners by the following characteristics: (1) dorsal scale row arrangement is 12-12-10 in females, 12-12-8 in males (); (2) preocular single; (3) postoculars, two; (4) loreal present, single, rectangular; (5) temporals 1 + 2; (6) supralabials 9, with fifth and six or four-six bordering orbit; (7) 10 lower labials, with first six in contact with chin shields; (8) ventrals 163–173 Trinidad (mainland 178–196) both sexes (9) paired subcaudals 156–172 both sexes, Trinidad (mainland 180–199) both sexes; (10) juveniles brown with subtle transverse bands and a pale vertebral stripe; (11) adults dark blue-grey to black, first dorsal scale row olive-green with a dull yellow smudge; (12) vertebral stripe present between the keels on the paravertebral scales; (13) apical pits large and prominent on dorsal scales behind the head, and on the paravertebral scales and adjacent scales; (14) the first row of dorsal scales are quadrangular. Pale crossbands may be present on the otherwise brown dorsum of the anterior body in juveniles ().
Comparisons
Chironius cochranae has 12 scale rows at midbody, distinguishing it from C. challenger, C. leucometapus, C. fuscus and C. scurrulus; all of which have 10 rows of scales at midbody. Chironius cochranae has a divided anal plate that distinguishes it from C. diamentia, C. exoletus, C. leucometapus, C. laevicollis, C. monticola and C. multiventris all of which have a single anal plate. Chironius cochranae has apical pits on some scales, which separates it from C. gouveai, and C. quadracarinatus that have no apical pits. Chironius cochranae has a vertebral stripe, which separates it from C. flavopictus, C. grandisquamis, C. maculoventris and C. vincenti which have no vertebral stripe. Chironius cochranae has three upper labials contacting the loreal which distinguishes it from C. bicarinatus, C. brazili, C. carinatus, C. flavolineatus, C. foveatus, and C. spixii and C. nigelnoriegai sp. nov. which have two upper labials contacting the loreal. The ventrals have no black edges (they do in C. nigelnoriegai sp. nov.), but the subcaudals have black on the midline suture.
Habitat
A species inhabiting tropical evergreen rainforest on Trinidad (unknown from Tobago), eastern Venezuela, Guiana, Suriname, French Guiana and northeast Brazil.
Etymology
Named after Doris Cochran, USNM Curator.
Natural history
Beebe (Citation1946) considered this species to be Chironius carinatus at his Kartabo, British Guyana study site (noting it was not present at the Caripito, Venezuelan study site). He considered this an arboreal snake but reported that about half his specimens were collected on the ground. Beebe (Citation1946) also reported a 2.85 m specimen, making it one of the longest colubrid snakes. Hoogmoed and Avila-Pires (Citation1991) reported this species as C. multiventris from secondary forests in French Guiana (Hoogmoed & Avila-Pires, Citation1991). Diet includes Boana boans (formerly Hyla maximum), Leptodactylus sp. and nestling antbirds (Beebe, Citation1946; Murphy et al., Citation2018).
Chironius nigelnoriegai sp. nov.
Coluber carinatus Linnaeus Citation1758: 223.
Coluber (Chironius) Donndorff Citation1798: 209.
Natrix carinatus–Merrem Citation1820: 120.
Chironius carinatus–Fitzinger 1826: 31, 60; Ruthven Citation1922: 65; Amaral Citation1925: 4; Beebe Citation1946: 21; Duellman Citation1978: 231; Roze Citation1966: 93; Gorzula & Señaris Citation1999: 162; Savage Citation2002: 648; Wallach et al. Citation2014: 159; Nogueira et al. Citation2019.
Herpetodryas carinatus–Wagler Citation1830: 180; Boulenger Citation1886: 433; Boulenger Citation1894: 73; Barbour & Cole Citation1906: 152; Barbour Citation1915: 77.
Herpetodryas fuscus Reinhardt and Lütken Citation1862: 10.
Herpetodryas carinatus–Wagler Citation1830: 180; Mole and Urich Citation1894a: 85; Mole and Urich Citation1894b: 518; Mole Citation1924: 246.
Herpetodryas carinatus var. carinatissima Jan and Sordelli Citation1869: 31, plate 2 (nomen oblitum).
Herpetodryas carinatus var. decalepis Jan and Sordelli Citation1869: 31, plate 2 (nomen dubium).
Herpetodryas carinatus var. glabra Jan and Sordelli Citation1869: 31, plate 2 (nomen dubium).
Herpetodryas carinatus var. macrophthalma Jan and Sordelli Citation1869: 31, plate 2 (nomen dubium) ().
Fig. 6. Illustration of Herpetodryas carinatus reproduced from Jan and Sordelli (Citation1869).
Erpetodryas sexcarinatus—Fowler Citation1913: 171.
Chironius carinatus Wehekind L. Citation1960:73; Emsley, Citation1977: 239; Murphy Citation1997: 168; Murphy et al. Citation2018.
Chironius carinatus carinatus–Dixon et al. Citation1993: 73; Boos Citation2001: 119.
Type specimens
Holotype.–CAS 231765 (field number PGF 364), collected on 18 July 2004 by R. Lawson, P.G. Frank, and P.J. Frank, an adult female from Nariva Road, Manzanilla Beach (georeferenced, 10°29’25.6”N, 61°03’16.8”W), Trinidad (). The same information is also provided for the designated paratype CAS 231764 (PGF 363).
Common name
Proposed standard English name: Trinidadian Sipo.
ZooBank. urn:lsid:zoobank.org:act:885B39C1-6D7A-4E81-8B32-6DF530648378.
Diagnosis
A dark olive green to black Chironius with heavily keeled paravertebral scales that form a double ridge (less noticeable in females) (). The edges of the scales between the ridges alternate yellow or cream spots that appear as a stripe from a distance. The vertebral stripe fades anterior to the cloaca; the upper eight labials are primarily yellow. The first row of dorsal scales are bright yellow to yellow-green; this colouration extends onto rows two and three in some specimens; the first row of scales on the tail have a bright yellow spot on each scale; the posterior edge of each ventral is trimmed with dark pigment; ventrals 156–167 (Dixon et al. Citation1993 reported 146–167 in C. c. carinatus), subcaudals 127–135 (Dixon et al. Citation1993 reported 108–145 which is strikingly fewer than C. cochranae, ); upper labials 4 + 5 or 5 + 6 border the orbit; usually two postoculars; six lower labials contact each chin shield (). The dorsal scale row arrangement is 12-12-8 in males and 12-12-10 in females. Anterior dorsal scale rows 2–5 have a single apical pit. The dorsal scales in this species are ovate and about as tall as they are long (in C. cochranae they are longer than they are tall).
Fig. 9. Head comparison. A–C Chironius cochranae CAS 231763. D–F Chironius nigelnoriegai CAS 231764. Photographs by JCM.
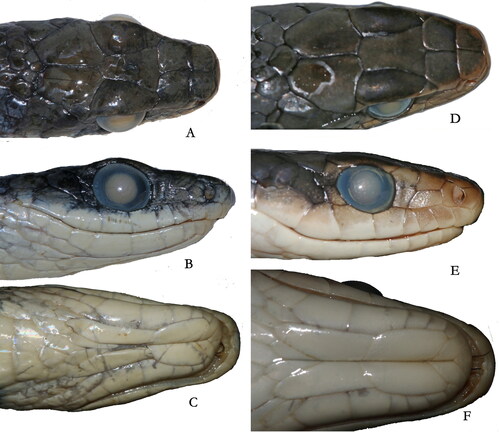
Table 2. Morphological comparison of Chironius nigelnoriegai sp. nov. and C. cochranae. Values for C. cochranae derived from Dixon et al. (Citation1993).
Comparisons
Chironius nigelnoriegai has 12 scale rows at mid body that will distinguish it from the following species with 10 scale rows at midbody: C. challenger, C. fuscus, C. leucometapus and C. scurrulus. Chironius nigelnoriegai has a divided anal plate that will distinguish it from C. diamentia, C. exoletus, C. laevicollis, C. leucometapus, C. monticola and C. multiventris all of which have a single anal plate. Chironius nigelnoriegai has apical pits on some scales, which separates it from C. gouveai and C. quadracarinatus that have no apical pits. Chironius nigelnoriegai has a vertebral stripe, which separates it from C. flavopictus, C. grandisquamis, C. maculoventris and C. vincenti, which have no vertebral stripe. Chironius nigelnoriegai has two upper labials contacting the loreal which distinguishes it from C. bicarinatus, C. brazili, C carinatus, C. flavolineatus, C. foveatus and C. spixii. Chironius nigelnoriegai has two upper labials contacting the loreal which will separate it from the following species which have three upper labials at the loreal: C. brazili, C. carinatus, C. cochranae, C. exoletus, C. gouveai, C. grandisquamis, C. laurenti, C. maculoventris, C. multiventris, C. quadracarinatus, C. septentrionalis and C. spixii.
Description of holotype
Adult female (); total length 1721 mm; SVL 1175 mm; tail length 546 mm (31.73% of total length); head length 42.03 mm; head width 24.18 mm; interorbital distance 16.89 mm; head notably wider than neck, narrow anteriorly, slightly triangular in dorsal view; snout round in dorsal and lateral views; rostral broader than long, 7.25 mm wide, 5.20 mm tall, visible from above; internasals square shaped, 5.09 mm long, 4.71 mm wide, smaller than prefrontals, in contact with nasals; prefrontals 4.91 mm long, 6.21 mm wide, extend down to loreal, separated from eye by supraocular and preocular; supraocular 10.18 mm long, 5.25 mm wide; frontal bell-shaped, 9.71 mm long, 8.20 mm wide; nasal divided; nostril located between prenasal and postnasal; loreal 4.59 mm long, 2.16 mm high; eye large, diameter 7.41 mm long; round pupil; parietal 11.00 mm long, 8.45 mm wide; two postoculars; nine supralabial scales, with fifth and sixth in contact with orbit; eight largest, first contacting nasal, second contacting nasal and loreal, third and fourth also contacting loreal while fourth and fifth contact the preocular, sixth and seventh in contact with postocular, seventh to ninth in contact with temporals; 10 infralabials, with first to sixth (seventh on left) in contact with chin shield; two gular scale rows; two preventral scale rows; 12-12-8 dorsal scale row arrangement with the top two dorsal scale rows being strongly keeled and no keel on remainder; anal plate divided; 161 entire ventral scales; 127 divided subcaudal scales with pointed terminal scute.
Etymology
The specific epithet, nigelnoriegai is a patronym honouring Dr Nigel Noriega, an integrative biologist who now operates Sustainable Innovation Initiatives, an organization focused on enabling ecologically regenerative economies in Trinidad and the southern Caribbean.
Natural history
This forest and forest-edge species spends time in the canopy, understorey and leaf litter. It is active during the day and sleeps off the ground. It will use riparian habitats but is not restricted to them. Wehekind (Citation1955: 11) wrote that it feeds principally on frogs, but suggested it will take mice and birds. However, before that Mole (Citation1924: 246) found that captives would eat only frogs. All currently recognized species of Chironius are known to eat anurans while four species are known to eat birds, seven are known to feed on lizards and two are known to feed on rodents (Roberto & Souza, Citation2020). The literature indicates that typically frogs compose the bulk of the diet of these snakes (Beebe, Citation1946; Brongersma, Citation1956; Test et al., Citation1966). Roberto and Souza (Citation2020) suggest that Chironius may specialize on specific kinds of frogs, unfortunately, more data are needed to test that. On Trinidad, clutch sizes of four and five eggs were reported by Mole and Urich (Citation1894b), Mole (Citation1924) and Test et al. (Citation1966).
Discussion
Our phylogenetic reconstruction is congruent with most relationships published in other studies, though the prior works do not include all of the taxa that we sampled. For instance, C. grandisquamis is typically found outside a clade comprising most other species of Chironius (Hamdan et al., Citation2017; Klaczko et al., Citation2014) and we recover this species in a sister relationship with C. challenger. We further recovered a clade of C. laevicollis and C. scurrulus that is also recovered in Hamdan et al. (Citation2017), Hollis (Citation2006) and Klaczko et al. (Citation2014), though our clade includes C. leucometapus that together are sister to C. fuscus. Thirdly, our sister relationships between C. brazili and C. diamantina and C. bicarinatus and C. gouveai which together formed a clade with C. flavolineatus that were sister to the remaining Chironius taxa concur with Entiauspe-Neto et al. (Citation2020) and Hamdan et al. (Citation2017), though C. gouveai was not yet described in 2017. Although C. brazili, C. diamantina and C. gouveai were not sampled by Hollis (Citation2006) or Klaczko et al. (Citation2014), C. bicarinatus and C. flavolineatus were part of a basal split from the remaining Chironius taxa in their analyses. Next we recovered the C. multiventris complex that includes C. foveatus and C. laurenti (Hamdan et al., Citation2017; Hollis, Citation2006; Klaczko et al., Citation2014). This complex was sister to the South American taxon C. cochranae to which our Trinidadian “C. septentrionalis” belongs. Finally, we recover C. nigelnoriegai as sister to C. flavopictus. This clade is sister to a clade of C. exoletus, C. quadricarinatus and C. carinatus/C. maculoventris, while all of these species are sister to C. monticola. These species and relationships are often but not exactly associated with each other in Hamdan et al. (Citation2017), Hollis (Citation2006) and Klaczko et al. (Citation2014).
This close relationship among the taxa are in agreement with Dixon et al. (Citation1993) and Hollis (Citation2006) but this placement is at odds with the description of C. vincenti by Klaczko et al. (Citation2014) that recovered C. vincenti as a sister species to a clade formed by C. bicarinatus and C. flavolineatus.
In recent years, genetic sequencing of many snakes in Trinidad and Tobago has resulted in the identification of cryptic lineages in the region with the subsequent description of new species. The high snake diversity of Trinidad is partly a reflection of Trinidad having been connected to the mainland until its separation approximately 4 mya (Murphy et al., Citation2023). Since then multiple colonization events have occurred from the proximal Paria Peninsula in Northern Venezuela, as well as from other mainland regions such as the Guianas (Murphy et al., Citation2023; Rivas et al., Citation2021). Snake colonization events have been intrinsically linked to the changing topographic conditions in the region during multiple glacial periods when the shallow marine depths resulted in the connection of Northern Venezuela and Trinidad (Jowers et al., Citation2019, Citation2020, Citation2021; Murphy et al., Citation2016, Citation2018, Citation2019; Rivas et al., Citation2021).
Our time tree recovers an Early Miocene origin for Chironius, at approximately 19.9 million years ago (95% HPD: 17.8–21.8 mya; ). The two Chironius on Trinidad are distantly related on the phylogenetic tree and likely differ in their ancestral origins and colonization routes. More specifically, the sister-group relationship between C. nigelnoriegai sp. nov. from Trinidad and C. flavopictus from Ecuador may have resulted in a colonization event from the west, as has been observed in other Trinidad and Tobago snakes with western origins (Murphy et al., Citation2023). These two species appear to have diverged in the Miocene (4.2 mya 95% HPD: 1.7–7.3 mya), an estimate congruent with Trinidad’s detachment from the mainland (Murphy et al., Citation2023) and suggesting a vicariant speciation event. Chironius cochranae on the other hand is composed of populations from Suriname (YPM16079 and YPM16080), Guyana (IRSNB18032, ROM 22844), Trinidad and Venezuela (Paria Peninsula) and is sister to a clade of South American C. foveatus, C. laurenti and C. multiventris with a divergence between clades dating to 9.5 mya (95% HPD: 6.6–12.4 mya). The clade composed by the two Trinidad and Paria Peninsula individuals of C. cochranae reflect recent gene flow in the region () suggesting very recent colonization. This pattern of colonization from north-western Venezuela has been discovered in several Trinidad species, for example Atractus trilineatus (Murphy et al., Citation2019), Oxybelis rutherfordi (Jadin et al., Citation2020) and Tantilla melanocephala (Jowers et al., Citation2020). Similarly, this is not the first time that more than one Trinidadian species belonging to the same genus have colonized at different times from the north-west or the south-east of the mainland. Examples are the New World Coral Snakes Micrurus (Jowers et al., Citation2019) and the microhylid frogs Elachistocleis (Jowers et al., Citation2021) with very similar biogeographic patterns as shown here. Finding a distinct island species of Chironius is not unprecedented because C. vincenti (Boulenger, Citation1891) is endemic to the island of St. Vincent (Henderson & Powell, Citation1996). Furthermore, the recent number of additions to the Trinidad fauna and name changes within the island’s herpetofauna suggests that further unrecognized endemic species remain to be described (Murphy et al., Citation2018; Murphy & Downie, Citation2012).
Associate Editor: Dr David Gower
Supplemental Material
Download MS Word (46.4 KB)Acknowledgements
We thank E.J. Ely and L.A. Scheinberg (CAS) and J. Rosado, T. Takahashi and J. Hanken (MCZ) for allowing us to examine specimens under their care. We thank Gilson A. Rivas for a tissue sample from near Cerro Humo in 2016 from the Paria Peninsula in Venezuela (permit number 0877) and Mike Rutherford and Renoir Auguste for logistical support in Trinidad. The UWSP College of Letters and Science and Olson Museum of Natural History provided funding to RCJ for a specimen imaging station used for photographing type material. We thank R.N. Deo, A. Fifi and S. Muhammad for providing us with additional photographs in life.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental material
Supplemental material for this article can be accessed here: https://dx.doi.org/10.1080/14772000.2024.2338064.
References
- Abuys, A. (1982). De slangen van Suriname, deel III: De familie Colubridae (algemene gegevens en het genus Chironius)/The snakes of Surinam, part III: The family Colubridae (General data and the genus Chironius). Litteratura Serpentium, 2, 226–245.
- Amaral, A. d. (1925). South American snakes in the collection of the United States National Museum. Proceedings of the United States National Museum, 67, 1–30. https://doi.org/10.5479/si.00963801.2596
- Bailey, J. R. (1955). The snakes of the genus Chironius in southeastern South America. Occasional Papers of the Museum of Zoology, University of Michigan, 571, 1–21.
- Barbour, T. (1915). Recent notes regarding West Indian reptiles and amphibians. Proceedings of the Biological Society of Washington, 1915, 71–77.
- Barbour, T., & Cole, L. J. (1906). Vertebrata from Yucatan, Reptilia, Amphibia, and Pisces. Bulletin of the Museum of Comparative Zoology, 50, 101–159.
- Beebe, W. (1946). Field notes on the snakes of Kartabo, British Guiana, and Caripito, Venezuela. Zoologica: Scientific Contributions of the New York Zoological Society, 31, 11–52. https://doi.org/10.5962/p.203521
- Beebe, W. (1952). Introduction to the ecology of the Arima Valley. Zoologica: Scientific Contributions of the New York Zoological Society, 37, 157–183. https://doi.org/10.5962/p.203464
- Boie, F. (1827). Bemerkungen über Merrem’s Versuch eines Systems der Amphibien, 1. Lieferung: Ophidier. Isis von Oken, 20, 508–566.
- Boos, H. E. (2001). The snakes of Trinidad and Tobago. Texas A&M University Press.
- Bouckaert, R., & Drummond, A. J. (2017). bModelTest: Bayesian phylogenetic site model averaging and model comparison. BioMedCentral Ecology and Evolution, 17, 42.
- Bouckaert, R., Vaughan, T. G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., Heled, J., Jones, G., Kühnert, D., De Maio, N., Matschiner, M., Mendes, F. K., Müller, N. F., Ogilvie, H. A., Du Plessis, L., Popinga, A., Rambaut, A., Rasmussen, D., Siveroni, I., … Drummond, A. J. (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. Public Library of Science Computational Biology, 15, e1006650. https://doi.org/10.1371/journal.pcbi.1006650
- Boulenger, G. A. (1886). A synopsis of the reptiles and batrachians of the province Rio Grande do Sul, Brazil. Annals and Magazine of Natural History, 18(5), 423–445. https://doi.org/10.1080/00222938609459995
- Boulenger, G. A. (1891). On reptiles, batrachians and fishes from the Lesser West Indies. Proceedings Zoological Society of London, 1891, 351–357.
- Boulenger, G. A. (1894). Catalogue of snakes in the British Museum (Natural History) (Vol. 2, pp. 382). Trustees of the British Museum.
- Brongersma, L. D. (1956). On some reptiles and amphibians from Trinidad and Tobago, B.W.I. I & II. Proceedings Koninklijke Nederland Akademie van Wetenschappen Series C, 59, 165–188.
- Dixon, J., Wiest, J. A., & Cei, J. M. (1993). Revision of neotropical snake genus Chironius Fitzinger (Serpentes, Colubridae). Museo Regionale di Scienze Naturali.
- Donndorff, J. A. (1798). Zoologische [Amphibiologische und Ichthyologische] Beyträge zur XIII. Ausgabe des Linneischen Natursystems. Dritter Band:Amphibien und Fische. Amphibien und Fische. Weidmannschen Buchhandlung, Leipzig.
- Douglas, J., Zhang, R., & Bouckaert, R. (2021). Adaptive dating and fast proposals: Revisiting the phylogenetic relaxed clock model. Public Library of Science Computational Biology, 17, e1008322. https://doi.org/10.1371/journal.pcbi.1008322
- Duellman, W. E. (1978). The biology of an equatorial herpetofauna in Amazonian Ecuador. Miscellaneous Publication of the University of Kansas Museum of Natural History, 65, 1–352.
- Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. https://doi.org/10.1093/nar/gkh340
- Emsley, M. G. (1977). Snakes, and Trinidad and Tobago. Bulletin of the Maryland Herpetological Society, 13, 201–304.
- Entiauspe-Neto, O. M., Lyra, M. L., Koch, C., Quintela, F. M., Abegg, A. D., & Loebmann, D. (2020). Taxonomic revision of Chironius bicarinatus (Wied 1820) (Serpentes: Colubridae), with description of a new species. Herpetological Monographs, 34, 98–115. https://doi.org/10.1655/HERPMONOGRAPHS-D-19-00013.1
- Fitzinger, L. J. F. J. (1826). Neue Classification der Reptilien nach ihren Natürlichen Verwandtschaften nebst einer Verwandtschafts-Tafel und einem Verzeichnisse der Reptilien-Sammlung des K. K. Zoologisch Museum’s zu Wien. J. G. Heubner.
- Flower, H. W. (1913). Amphibians and reptiles from Ecuador, Venezuela and the Yucatan. Proceedings of the Academy of Natural Sciences of Philadelphia, 1913, 153–176.
- Gorzula, S., & Señaris, J. C. (1999). Contribution to the herpetofauna of the Venezuelan Guayana. I. A data base. Scientia Guaianae, 8, 1–269.
- Guindon, S., Dufayard, J.-F., Lefort, V., Anisimova, M., Hordijk, W., & Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321. https://doi.org/10.1093/sysbio/syq010
- Hamdan, B., Pereira, A. G., Loss-Oliveira, L., Rödder, D., & Schrago, C. G. (2017). Evolutionary analysis of Chironius snakes unveils cryptic diversity and provides clues to diversification in the Neotropics. Molecular Phylogenetics and Evolution, 116, 108–119. https://doi.org/10.1016/j.ympev.2017.08.004
- Henderson, R. W., & Powell, R. (1996). Chironius vincenti. Society for the Study of Amphibians and Reptiles. Catalogue of American Amphibians and Reptiles, 635, 1–2
- Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q., & Vinh, L. S. (2018). UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution, 35, 518–522. https://doi.org/10.1093/molbev/msx281
- Hoge, A. R., & Romano, S. A. (1969). A new species of Chironius (Serpentes–Colubridae). Memórias do Instituto Butantan, 34, 93–96.
- Hollis, L. (2006). Phylogenetics of the genus Chironius Fitzinger, 1826 (Serpentes, Colubridae) based on morphology. Herpetologica, 62, 435–453. https://doi.org/10.1655/0018-0831(2006)62[435:POTGCF]2.0.CO;2
- Hoogmoed, M. S., & Avila-Pires, T. C. S. (1991). Annotated checklist of the herpetofauna of Petit Saut, Sinnamary river, French Guiana. Zoologische Mededelingen, 65, 53–88.
- Jadin, R. C., Burbrink, F. T., Rivas, G. A., Vitt, L. J., Barrio-Amorós, C. L., & Guralnick, R. P. (2014). Finding arboreal snakes in an evolutionary tree: Phylogenetic placement and systematic revision of the Neotropical birdsnakes. Journal of Zoological Systematics and Evolutionary Research, 52, 257–264. https://doi.org/10.1111/jzs.12055
- Jadin, R. C., Blair, C., Jowers, M. J., Carmona, A., & Murphy, J. C. (2019). Hiding in the lianas of the tree of life: Molecular phylogenetics and species delimitation reveal considerable cryptic diversity of New World Vine Snakes. Molecular Phylogenetics and Evolution, 134, 61–65. https://doi.org/10.1016/j.ympev.2019.01.022
- Jadin, R. C., Blair, C., Orlofske, S. A., Jowers, M. J., Rivas, G. A., Vitt, L. J., Ray, J. M., Smith, E. N., & Murphy, J. C. (2020). Not withering on the evolutionary vine: Systematic revision of the Brown Vine Snake (Reptilia: Squamata: Oxybelis) from its northern distribution. Organisms Diversity & Evolution, 20, 723–746. https://doi.org/10.1007/s13127-020-00461-0
- Jan, G. (1863). Elenco olonizatio degli ofidi descritti e disegnati per l’lconografis generale. A. Lombardi, Milano vii + 143 pp.
- Jan, G., & Sordelli, F. (1869). Iconographie generale de ophidiens. Tome Second, Livr, 31–33.
- Jowers, M. J., Garcia Mudarra, J. L., Charles, S. P., & Murphy, J. C. (2019). Phylogeography of West Indies Coral snakes (Micrurus): Island colonization and banding patterns. Zoologica Scripta, 48, 263–276. https://doi.org/10.1111/zsc.12346
- Jowers, M. J., Schargel, W. E., Muñoz‐Mérida, A., Sánchez‐Ramírez, S., Weber, J. C., Faria, J. F., Harris, D. J., & Murphy, J. C. (2021). The enigmatic biogeography of Tobagós marooned relics: The case study of a fossorial snake (Squamata, Diapsididae). Journal of Zoological Systematics and Evolutionary Research, 59, 1382–1389. https://doi.org/10.1111/jzs.12509
- Jowers, M. J., Rivas, G. A., Jadin, R. C., Braswell, A. L., Auguste, R. J., Borzée, Z., & Murphy, J. C. (2020). Unearthing the species diversity of a cryptozoic snake, Tantilla melanocephala, in its northern distribution with emphasis to the colonization of the Lesser Antilles. Amphibian and Reptile Conservation, 14, 206–217.
- Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589. https://doi.org/10.1038/nmeth.4285
- Kenny, J. (2000). View from the ridge, exploring the natural history of Trinidad and Tobago. Prospect Press, Port-of-Spain.
- Klaczko, J., Montingelli, G. G., & Zaher, H. (2014). A combined morphological and molecular phylogeny of the genus Chironius Fitzinger, 1826 (Serpentes: Colubridae). Zoological Journal of the Linnean Society, 171, 656–667. https://doi.org/10.1111/zoj.12147
- Köhler, G. (2008). Reptiles of Central America (2nd ed.). Herpeton Verlag.
- Lanfear, R., Calcott, B., Ho, S. Y. W., & Guindon, S. (2012). PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29, 1695–1701. https://doi.org/10.1093/molbev/mss020
- Linnaeus, C. (1758). Systemae naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, Reformata. Laurentii Salvii, Holmiae.
- Merrem, B. (1820). Versuch eines Systems der Amphibien. Johann Christian Krieger.
- Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., von Haeseler, A., & Lanfear, R. (2020). IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37, 1530–1534. https://doi.org/10.1093/molbev/msaa015
- Mole, R. R. (1924). The Trinidad snakes. Proceedings of the Zoological Society of London, 94, 235–278. https://doi.org/10.1111/j.1096-3642.1924.tb01500.x
- Mole, R. R., & Urich, F. W. (1894a). A preliminary list of the reptiles and batrachians of the island of Trinidad. Journal of the Trinidad Field Naturalist’s Club, 2, 77–90.
- Mole, R. R., Urich, F. W. (1894b). Biological notes upon some of the ophidian of Trinidad, B.W.I. with a preliminary list of species recorded from the island. Proceedings of the Biological Society of London, 1894, 499–518.
- Montingelli, G. G., Valencia, J. H., Benavides, M. A., & Zaher, H. (2011). Revalidation of Herpetodryas reticulata (Peters, 1863) (Serpentes: Colubridae) from Ecuador. South American Journal of Herpetology, 6, 189–197. https://doi.org/10.2994/057.006.0304
- Murphy, J. C. (1997). Amphibians and reptiles of Trinidad and Tobago (p. 245). Krieger. Malabar, Florida.
- Murphy, J. C., & Downie, J. R. (2012). The changing Trinidad and Tobago herpetofauna. Living World, Journal of the Trinidad And Tobago Field Naturalists’ Club, 2012, 87–95.
- Murphy, J. C., Rutherford, M. G., & Jowers, M. J. (2016). The threadsnake tangle: Lack of genetic divergence in Epictia tenella (Squamata, Leptotyphlopidae): Evidence for introductions or recent rafting to the West Indies. Studies on Neotropical Fauna and Environment, 51, 197–205. https://doi.org/10.1080/01650521.2016.1234358
- Murphy, J. C., Downie, J. R., Smith, J. M., Livingstone, S. R., Mohammed, R. S., Lehtinen, R. M., Eyre, M., Sewlal, J.-A., Noriega, N., Casper, G. S., Anton, T., Rutherford, M. G., Braswell, A. L., & Jowers, M. J. (2018). A field guide to the amphibians and reptiles of Trinidad & Tobago. Trinidad & Tobago Field Naturalists Club.
- Murphy, J. C., Salvi, D., Braswell, A. L., & Jowers, M. J. (2019). Phylogenetic position and biogeography of the three-lined snake Atractus trilineatus (Squamata, Dipsadidae) in the Eastern Caribbean. Herpetologica, 75, 247–253. https://doi.org/10.1655/D-18-00043
- Murphy, J. C., Weber, J., Jowers, M. J., & Jadin, R. C. (2023). Two islands, two origins: The snakes of Trinidad and Tobago. In H. B. Lillywhite & M. Martins (Eds.), Islands and Snakes Vol. II: Diversity and conservation (pp. 81–99). Oxford University Press.
- Nogueira, C. C., Argôlo, A. J. S., Arzamendia, V., Azevedo, J. A., Barbo, F. E., Bérnils, R. S., Bolochio, B. E., Borges-Martins, M., Brasil-Godinho, M., Braz, H., Buononato, M. A., Cisneros-Heredia, D. F., Colli, G. R., Costa, H. C., Franco, F. L., Giraudo, A., Gonzalez, R. C., Guedes, T., Hoogmoed, M. S., … Martins, M. (2019). Atlas of Brazilian snakes: Verified point-locality maps to mitigate the Wallacean shortfall in a megadiverse snake fauna. South American Journal of Herpetology, 14, 1–274. https://doi.org/10.2994/SAJH-D-19-00120.1
- Nylander, J. A. A. (2004). MrModeltest v2.2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden.
- Rambaut, A. (2002). Se-Al v2.0a11. University of Oxford.
- Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032
- Reinhardt, J., & Lütken, C. (1862). Bidrag til Kundskab om Brasiliens Padder og Krybdyr. Videnskabelige Meddelelser Forening I Kjobenhavn, 3, 143–242.
- Rivas, G. A., Lasso-Alcalá, O. M., Rodríguez-Olarte, D., Freitas, M., Murphy, J. C., Pizzigalli, C., Weber, J. C., Verteuil, L., & Jowers, M. J. (2021). Biogeographical patterns of amphibians and reptiles in the northernmost coastal montane complex of South America. Public Library of Science One, 16, e0246829. https://doi.org/10.1371/journal.pone.0246829
- Roberto, I. J., & Souza, A. R. (2020). Review of prey items recorded for snakes of the genus Chironius (Squamata, Colubridae), including the first record of Osteocephalus as prey. Herpetology Notes, 13, 1–5.
- Roze, J. A. (1966). La Taxonomia y Zoogeograjia de los Ojidios en Venezuela (p. 362). Ediciones de la Biblioteca.
- Ruthven, A. G. (1922). The amphibians and reptiles of the Sierra Nevada de Santa Marta, Colombia. Miscellaneous Publications. Museum of Zoology, University of Michigan, 8, 1–69.
- Savage, J. M. (2002). The Amphibians and Reptiles of Costa Rica: A herpetofauna between two continents, between two seas. University of Chicago Press.
- Scharff, R. F. (1922). On the origin of the West Indian Fauna. Bijdragen Tot de Dierkunde, 22, 65–72. https://doi.org/10.1163/26660644-02201011
- Swofford, D. L. (2002). PAUP*: Phylogenetic analyses using parsimony (*and other methods), version 4.0b10. Sinauer Associates.
- Test, F. H., Sexton, O. J., & Heatwole, H. (1966). Reptiles of Rancho Grande and vicinity, Estado Aragua, Venezuela. Miscellaneous Publication Museum of Zoology University Michigan, 128, 1–63.
- Torres-Carvajal, O., Echevarría, L. Y., Lobos, S. E., Venegas, P. J., & Kok, P. J. (2019). Phylogeny, diversity, and biogeography of Neotropical sipo snakes (Serpentes: Colubrinae: Chironius). Molecular Phylogenetics and Evolution, 130, 315–329. https://doi.org/10.1016/j.ympev.2018.10.022
- Wagler, J. (1824). Serpentum Brasiliensium species novae, ou histoire naturelle des espèces nouvelles de serpens. In Jean de Spix, Animalia nova sive species novae. Natrix bahiensis: 27 (pp. vii + 75). Monaco, Typis Franc. Seraph. Hübschmanni.
- Wagler, J. G. (1830). Natürliches System der Amphibien, mit vorangehender Classification der Saugthiere und Vogel. Ein Beitrag zur vergleichenden Zoologie. J.G. Cotta schen Buchhandlung. Munchen, Stuttgart und Tubingen.
- Wallach, V., Williams, K. L., & Boundy, J. (2014). Snakes of the world: A catalogue of living and extinct species. CRC Press.
- Wehekind, L. (1955). Notes on the food of Trinidad snake. British Journal of Herpetology, 2, 9–13.
- Wehekind, L. (1960). Trinidad snakes. Journal of the British Guiana Museum of Zoology, 27, 71–76.
- Wied-Neuwied, M. P. z. (1820). Reise nach Brasilien in den Jahren 1815 bis 1817 (vol. 1). Heinrich Ludwig Bronner.
- Zhang, R., & Drummond, A. (2020). Improving the performance of Bayesian phylogenetic inference under relaxed clock models. BioMedCentral Evolutionary Biology, 20, 54. https://doi.org/10.1186/s12862-020-01609-4
- Zheng, Y., & Wiens, J. J. (2016). Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Molecular Phylogenetics and Evolution, 94, 537–547. https://doi.org/10.1016/j.ympev.2015.10.009
Appendix.
Specimens examined
Chironius cochranae: TRINIDAD & TOBAGO: Trinidad: Nariva Road, Manzanilla Beach (CAS 231763, 10°29’25.6”N, 61°03’16.8”W).
Chironius flavopictus: COLOMBIA: Choco: Quebrada Taparral, about 20 km N. of Palestina, on Rio San Juan (CAS 119918, 4.3135638889°N, 77.0340472222°W); ECUADOR: Pastaza: Pastaza River, Canelos to Marañón River (MCZ R-36957 & 36958, 2.5721330°S, 76.744895°W); Ecuador: Zamora: vicinity Rio Zamora (CAS 94094, 4.069167°S, 78.956665°W); PANAMA: Colon: Ciricito (CAS 71424, 9.0027083°N, 80.074578°W); PERU: Junín: Chanchamayo: Hacienda Naranjal (MCZ R-45924, 11.134803°S, 75.405326°W); Junín: (MCZ 42441, 11.086747°S, 75.135437°W).
Chironius nigelnoriegai sp. nov.: TRINIDAD & TOBAGO: Trinidad: Nariva Road, Manzanilla Beach (CAS 231765 [holotype] & CAS 231764 [paratype], 10°29’25.6”N, 61°03’16.8”W).




