ABSTRACT
Introduction
The evolution of endovascular surgery over the past 30 years has made it possible to treat increasingly complex vascular pathologies with an endovascular method. Although this generally speeds up the patient’s recovery, the risks of health problems caused by long-term exposure to radioactive radiation increase. This warrants the demand for radiation-reducing tools to reduce radiation exposure during these procedures.
Areas covered
For this systematic review Pubmed, Embase and Cochrane library databases were searched on 28 December 2021 to provide an overview of tools that are currently used or have the potential to contribute to reducing radiation exposure during endovascular aortic procedures. In addition, an overview is presented of radiation characteristics of clinical studies comparing a (potential) radiation-reducing device with conventional fluoroscopy use.
Expert opinion
Radiation-reducing instruments such as fiber optic shape sensing or electromagnetic tracking devices offer the possibility to further reduce or even eliminate the use of radiation during endovascular procedures. In an era of increasing endovascular interventional complexity and awareness of the health risks of long-term radiation exposure, the use of these technologies could have a major impact on an ongoing challenge to move toward radiation-free endovascular surgery.
1. Introduction
Since the first endovascular aortic aneurysm repairs described by Volodos et al [Citation1]. and Parodi et al [Citation2]. in 1991, there has been a major shift from open to endovascular aortic treatment. These newly developed endovascular methods offer new treatment options for the management of vascular pathologies that are often less invasive than conventional open surgery and lead, among other things, to a reduced risk of complications and a shorter hospital stay [Citation3–6]. On the other hand, there are also limitations associated with their application. When using fluoroscopy, three-dimensional (3D) structures are projected onto monitors only in two-dimensional (2D) grayscale images. Spatial perception of tortuosity and depth is thereby eliminated, making navigation more difficult and increasing the potential for procedural errors, prolonged procedural time, and increased radiation exposure. Exposure to fluoroscopy imposes potential long-term DNA damage and health effects, which limits the risks of perioperative radiation exposure, and therefore plays a major role during these procedures [Citation7,Citation8]. In order to achieve the lowest possible radiation exposure at which the endovascular procedure can be performed safely, current radiation guidelines are based on the ‘as low as reasonably achievable’ (ALARA) principle. However, the increasing complexity of endovascular treatments of vascular pathologies prolongs the procedure time, which despite ALARA guidelines still results in increased radiation exposure for both the patient and the treatment team [Citation9–11].
Over the past decade, there have been many new developments that help mitigate the risks of perioperative radiation exposure during endovascular procedures. Studies show that the introduction of hybrid operating rooms with a fixed C-arm and advanced applications such as image fusion (IF) of preoperatively acquired Magnetic Resonance Angiography (MRA) or Computed Tomography Angiography (CTA) images contribute to the reduction of radiation exposure and contrast agent [Citation12–14]. However, its application is limited to projecting a 3D outline of the anatomical structures onto real-time fluoroscopy images with only 2D information about the positioning and orientation of the endovascular devices used. As a result, with increasing complexity of the endovascular procedure, radiation exposure will also increase. Developed endovascular navigation systems using, for example, electromagnetic (EM) tracking or fiber optic shape sensing (FOSS) methods may provide the solution to overcome this problem [Citation15–17]. These techniques improve the spatial perception of the devices within anatomical imaging and lead to a potential reduction of radiation exposure during the navigation phase of endovascular procedures. However, most of these techniques are still under development and the added value in reducing radiation exposure has not yet been proven. Therefore, the aim of this review is to provide an overview of current and potential radiation reducing techniques used during aortic endovascular procedures.
2. Method
2.1. Data search and collection
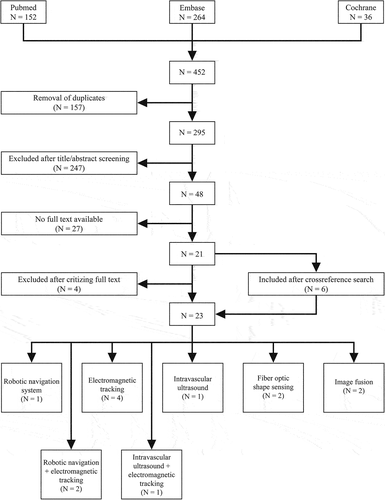
This literature review was performed according to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analysis’ (PRISMA) statement [Citation18]. Pubmed, Embase, and Cochrane library databases were searched on 28 December 2021, using the following MeSH terms: ‘Aorta,’ ‘Endovascular procedures,’ ‘Radiation,’ ‘Radiation monitoring,’ ‘Radiation dosage,’ ‘Fluoroscopy.’ Only articles with publication date from 2011 until moment of search. The applied search strategy yielded 452 potentially interesting articles; 152 in Pubmed, 264 in Embase, and 36 in Cohrance library, of which 295 remained after duplicate removal. An overview of the complete electronic search strategy is presented in supplementary material A.
Two independent qualified observers (J.K. and L.J.V.) performed title and abstract screening of the remaining 295 potentially relevant articles. All studies describing interventional tools that can be used radiation-free, simplify navigation tasks or reduce navigation time and are used in aortic endovascular interventions were included in this review. No distinction was made between the aortic pathology for which the treatment was performed. Articles focusing on pre- or post-operative radiation exposure reduction protocols, as well as C-arm hardware or integrated dose reduction software, such as AlluraClarity, were excluded. In addition, articles were excluded that are not written in the English language or where the full text was not available. Applying these criteria in combination with cross-references ultimately leads to the discussion of 23 remaining articles that focus on the current or potential reduction of radiation exposure in endovascular aortic procedures. An overview of data collection process is presented in .
2.2. Data extraction
Selected studies were grouped based on the examined tool that contributes to reduction of radiation exposure. The following main outcome characteristics were extracted from the selected studies: first author, year of publication and type of study, type of intervention or used model, conclusion, and limitations. Subsequently, if reported, the following characteristics were extracted from all included clinical studies in which (potential) radiation reducing tool is compared with a conventional control group: used tool, number of patients included, type of intervention, procedure time (minutes), cumulative radiation dose expressed in air kerma (AK, mGy) or dose area product (DAP, Gycm2), fluoroscopy time (minutes), and contrast volume (mL). In addition, for studies describing tools used during endovascular cannulation of target vessels, the following characteristics were extracted: used tool, study type, model/intervention, cannulation tasks, cannulation attempts, vessel wall hits, cannulation time (minutes), and fluoroscopy time (minutes).
3. Results
In this review 23 articles were included describing the robotic navigation system (RNS), EM-tracking, Intravascular Ultrasound (IVUS), FOSS and IF. An overview of all included articles is presented in .
Table 1. Overview of articles included in the review.
3.1. Overview of included techniques
3.1.1. Electromagnetic tracking
Four articles [Citation19–22] described the standalone use of EM tracking and reported the ability of EM-tracked devices to support guidance during endovascular interventions. Three of the articles describing EM-tracking reported results of phantom studies [Citation19,Citation20,Citation22] and one a prospective clinical study [Citation21]. Three of these studies use the Aurora tracking system [Citation20–22] and one the StealthStation Treon plus system [Citation19].
EM-tracking systems consist of a low magnetic field generator and EM position coils integrated within the tip of the used catheter or guidewire. Information about the EM field within the EM coils at the tip of the devices is analyzed in a control box that converts this information into a 3D position of the coil. In combination with navigation software, the system can visualize the 3D position and orientation of the devices relative to the anatomy, segmented from a preoperative CTA. This allows this technology to be used in addition to or even without the use of fluoroscopy when performing navigation or cannulation tasks. However, visual cues that indicate the full configuration and tension of the instrument body are limited by the number of sensor coils placed in the EM tracking devices, as the system can only track the points where the sensor coils are located. Three studies reported the specifications of the five degrees of freedom EM sensor coils (0.5x8mm [Citation20,Citation22] and 0.5x11mm [Citation21]) placed into catheters and two studies of the five degrees of freedom EM sensor coils (0.3x12mm [Citation20] and 0.5x8mm [Citation22]) placed into guidewires. In addition, commercially available interventional devices hardly support the use of EM tracking, often requiring additional and custom-built devices during these procedures. For example, in the registered studies, the control cables of steerable catheters [Citation20] or the balloon of percutaneous transluminal angioplasty (PTA) balloon catheters [Citation21,Citation22] have been removed to use the extra lumen in these catheters to insert the EM sensor coils, making these devices suitable for EM tracking. Another limitation is that nearby electronic devices such as the C-arm, for example, can cause interference in the EM field, reducing the tracking quality of the system. To minimize the impact of this, it is recommended to position the C-arm away from the navigation field when using EM tracking. Moreover, the system setup time and learning curve during use also cause the procedure time to be extended at the moment.
3.1.2. Image fusion
IF as radiation exposure reducing technology is discussed in 12 of the articles in this review. Eight of the included articles on IF were retrospective clinical studies [Citation13,Citation14,Citation23–28], while four studies described prospective clinical studies [Citation29–32]. Of these studies, three described the VesselNavigator application [Citation13,Citation23,Citation27], one the CYDAR application [Citation29], three the Syngo application [Citation24,Citation25,Citation31], one the Advantage Workstation application [Citation30], two the Ziostation 2 Plus ZWS-2000 application [Citation26,Citation28], one the Leonardo application [Citation14] and one the Xtra vision 8.3 application [Citation32]. The IF studies included in this review reported three different methods by which preoperatively acquired CTA images can be registered to the live situation and then projected onto fluoroscopy images by IF. Four studies described a two-dimensional (2D) registration method (2D/3D) in which two bi-planar fluoroscopy images are acquired to register the preoperative CTA images [Citation13,Citation23,Citation26,Citation30]. This registration method requires the user to manually position the segmentation of the preoperatively acquired CTA in the correct position on these two fluoroscopy images. Six studies reported the use of a 3D registration method (3D/3D) in which a preoperatively acquired CBCT is used to register the segmentation of the preoperatively acquired CTA [Citation14,Citation24,Citation25,Citation28,Citation31,Citation32]. This method allows the user to select corresponding locations in the preoperative and perioperative scan based on anatomical landmarks such as atherosclerotic plaques. The software then automatically positions the preoperative CTA over the live fluoroscopy images. One selected study reported the use of both 2D/3D and 3D/3D IF registration methods [Citation27]. Finally, one study reported the use of an automatic registration method for IF [Citation29]. This technique compares the anatomy, mainly vertebrae, visible on live fluoroscopy images with the anatomy of the preoperative CTA and then automatically produces a 3D overlay. In contrast to the 2D/3D and 3D/3D registration methods, this technique is able to automatically update the roadmap during the endovascular procedure by adapting the roadmap to the new position visible anatomy and mostly the position of the visible vertebrae.
IF is a technology that improves anatomical understanding during endovascular procedures without the need to perform digital subtraction angiography (DSA). It is a relatively easy-to-use method that significantly reduces radiation exposure and contrast volume, but does not eliminate the need to use fluoroscopy or reduce overall procedure time. Moreover, only the CYDAR image fusion technology is able to automatically adapt the anatomical roadmap according to the real-time situation. All other described image fusion methods are based on a rigid registration method of the preoperatively acquired CTA with real-time fluoroscopy images. As a result, the roadmap created with these methods must therefore be adjusted manually, whether or not on the basis of a DSA, to changes in the real-time situation that arise due to, for example, the introduction of a rigid guidewire.
3.1.3. Intravascular ultrasound
One of the included articles [Citation33] for this review reported the use of IVUS during endovascular aortic repair (EVAR) procedures in a retrospective clinical study. The study [Citation34] discussing a combination of IVUS with EM tracking is presented in the section ‘combined tools.’ IVUS creates intraluminal images of the blood vessel using high-frequency sound waves. This technique allows users to determine, among other things, characteristics of the aneurysm neck, such as diameter, and compare them with the values found in CTA obtained preoperatively. In addition, IVUS can also determine the location of branches without using radiation and thus has the potential to reduce radiation exposure.
Pecoraro et al [Citation33]. reported results of a retrospective study on the feasibility of using IVUS and its ability to significantly reduce the amount of radiation exposure and contrast volume during EVAR procedures. In addition, IVUS reduced the rate of endoleaks, did not lead to an increase in operative time, and no adverse events associated with IVUS use were reported.
3.1.4. Robotic navigation system
The use of a RNS was reported by one included study [Citation35] and two studies [Citation16,Citation36] discussing a combination of RNS with EM tracking are presented in the section ‘combined tools.’ This study describes the Sensei Robotic Catheter System that consists of a hand operated joystick and an instinctive motion controller with a steerable catheter. Since this joystick for operating this device can be placed at a distance from the radiation source, the use of this technology reduces radiation exposure for the operator.
The results presented by Riga et al [Citation35] of an in vitro study of cannulation tasks in a type I and a type III aortic arch phantom show that a robotic navigation system can contribute to reducing radiation exposure and operation duration. The increased accuracy of catheter positioning makes this technique a suitable adjunct for navigation during complex endovascular procedures. However, a limitation to the use of this technology is that larger sheaths must be used for vascular access and that careless advancing of the devices increases the risk of dissections.
3.1.5. Fiber optic shape sensing
Two included studies describe the use of multicore fiber integrated shape sensing devices for navigation during endovascular procedures [Citation17,Citation37]. These devices are connected to the system that sends laser light through the optical fibers. Shape changes in the integrated optical fiber caused by the twisting and bending of the devices influences the returning light. By analyzing these changes in the returning light spectrum, the system is able to create three-dimensional reconstructions along the full length of the integrated optical fiber, making it possible to visualize the devices in real time without the need for radiation.
One study reported promising results of a shape detection multicore fiber used in a 3D printed aortic model [Citation37]. Tests in the endovascular model show high accuracy. However, these experiments only describe the use of optical fibers that have not yet been integrated into endovascular devices. In addition, the measurements were performed under ideal conditions, which may reduce the accuracy under more complex conditions. Van Herwaarden et al [Citation17], on the other hand, described the first in human use of optical shape-sensitive devices with a prospective study design. In this study, they describe the use of Fiber Optic RealShape (FORS) technology to perform navigation tasks during endovascular procedures and demonstrate the safety and feasibility of its use. Improving the understanding of the spatial course of the devices and the unrestricted viewing angles in particular is considered to be of added value. In addition, it is shown that cannulation tasks can be performed with minimal radiation exposure. However, although it has been reported that cannulation tasks can be performed with minimal use of radiation, this study does not demonstrate the reduction in radiation exposure.
3.1.6. Combined tools
Two studies [Citation16,Citation36] reported the combination of a Magellan robotic navigation system with Aurora EM-tracking technology. Schwein et al [Citation16,Citation36]. reported the value of remotical 3D catheter control in combination with improved 3D device orientation using EM-tracking technology in two subsequent studies. Outcomes show a reduction in fluoroscopy and cannulation time.
One study reported the combination of IVUS and Aurora EM-tracking technology. Shi et al [Citation34]. proposed a new real-time intravascular reconstruction and navigation for endovascular aortic stent grafting. In this method, anatomical information is obtained with IVUS and linked to the catheter position obtained via EM tracking. Results show significant potential for clinical applications, efficiency improvement of precision alignment and placement of grafts by real-time reconstruction and navigation.
3.2. Radiation reduction in clinical studies
In this review, 14 articles were selected in which outcomes of radiation-related measured parameters of a (potentially) radiation-reducing tool were compared with outcomes in a control group. Twelve articles examined the influence of IF [Citation13,Citation14,Citation23–32], one the influence of EM tracking [Citation21] and one the influence of IVUS [Citation33] on radiation exposure reduction. presents an overview of extracted radiation-related measured parameters, if available, of all included clinical studies.
Table 2. Overview of clinical studies in which a (potential) radiation reducing tool is compared with a conventional control group.
One study [Citation21] presented results of a prospective clinical study on the influence of the use of EM tracking on the reduction of radiation exposure in EVAR procedures. This study showed that compared to the use of conventional fluoroscopy, the use of EM tracking significantly increased radiation dose, prolonged procedure time and comparable contrast volume utilization. No results were presented in this study for fluoroscopy time. While the results actually show increased radiation exposure, it should be noted that the intraoperative CBCT performed and the setup and testing of the EM tracking equipment also influence these parameters and thus may not affect the true contribution of EM tracking to the reduction of the radiation dose. Due to the available 3D information about the position of the EM-tracked device, the authors certainly see this technology of added value in cannulation tasks during complex procedures. It is therefore expected that with increasing experience with this navigation technology, radiation exposure, and procedure time will eventually decrease.
In two of the twelve clinical studies examining the influence of IF on radiation reduction, the use of IF resulted in a significant reduction in procedure time, radiation dose, fluoroscopy time and contrast volume. Stangenberg et al [Citation27]. reported a significant reduction in the above mentioned parameters during EVAR procedures and McNally et al [Citation14]. during fenestrated endovascular aortic repair (FEVAR) procedures when using IF. In addition, eight studies [Citation23–25,Citation28–32] showed a trend toward a reduction in radiation exposure with IF. In fact, one of these studies [Citation31] showed a significant reduction in radiation exposure during FEVAR procedures, while in two studies [Citation24,Citation30], this reduction was significant only in the subgroups of EVAR, FEVAR, and branched endovascular aortic repair (BrEVAR) procedures. One study [Citation28] showed a significant reduction in fluoroscopy time during complex EVAR procedures and another study [Citation13] only in a subset of TEVAR procedures Furthermore, two studies [Citation24,Citation32] reported a trend toward reduction in fluoroscopy time when using IF during standard or complex EVAR procedures and one study [Citation29] during treatment of aortoiliac occlusive disease. The procedure time was significantly shorter in two studies [Citation31,Citation32] reporting the use of IF during FEVAR procedures, while in one two studies [Citation13,Citation26] subgroup results with TEVAR and one study [Citation24] with subgroup results with complex EVAR procedures yielded the same result. In addition, three other studies [Citation25,Citation28,Citation30] and the remaining subgroups of two studies [Citation13,Citation24] show a trend toward shortening of procedure time when using IF, while only one study [Citation23] reported no results. Eight studies [Citation13,Citation23–26,Citation28,Citation30,Citation32] showed a significant decrease in contrast volume. In contrast, one study [Citation29] reported a non-significant increase in contrast volume and one study [Citation31] reported no outcomes for contrast volume.
Finally, one study [Citation33] presented the results of a prospective clinical study comparing the influence of IVUS on total radiation exposure during EVAR procedures with conventional fluoroscopy. In this study, radiation dose, fluoroscopy time, and contrast volume are significantly decreased, while procedure time remains almost the same.
3.3. Cannulation task characteristics
Cannulation task characteristics were reported in seven studies included in this review, of which five studies [Citation16,Citation19,Citation20,Citation35,Citation36] reported an in vitro design and two studies [Citation17,Citation21] a prospective in vivo design. An overview of all articles with, if available, cannulation task characteristics can be found in . Three studies reported characteristics of cannulation tasks while using an EM tracking device; two studies [Citation19,Citation20] presented the results of phantom studies, while one study [Citation21] presented the outcome of a prospective clinical trial. Two studies [Citation19,Citation20] reported results of fluoroscopy time and showed a reduction in the use of EM-tracking devices. Two studies also presented cannulation time results, with one study [Citation19] showing a reduction in cannulation time while using EM tracking equipment and the other study [Citation21] reporting an increase. Furthermore, one study [Citation19] reported only on vessel wall hits, one study [Citation21] only the number of cannulation attempts and one study [Citation20] reported both. In all these cases, the use of EM tracking equipment resulted in a reduction compared to the use of conventional fluoroscopy.
Table 3. Overview of studies reporting cannulation task characteristics.
One study [Citation35] reported the use of RNS while performing four cannulation tasks in a type 1 and four in a type 3 aortic arch phantom. This study reported a reduction in cannulation time in five of the eight cannulation tasks, a reduction in the number of vessel wall hits in four of the eight tasks, and a reduction in the number of cannulation attempts in all cases. However, no elapsed fluoroscopy time information is presented.
Finally, there are three studies that only described the cannulation and fluoroscopy time; two studies [Citation16,Citation36] described the influence of the use of combination of EM and RNS in an aortic aneurysm phantom and one study [Citation17] the use of FOSS in a prospective clinical trial. Both studies describing a combination of EM and RNS showed a reduction in both cannulation time and fluoroscopy time. In contrast, the study describing the use of FOSS only reported results while using this technology. As a result, there are no results of comparable tasks performed with conventional fluoroscopy to compare with FOSS results.
4. Discussion
A systematic review of studies describing a current or potential radiation-reducing technique used during aortic endovascular procedures resulted in 23 studies identifying 5 different instruments that alone or in combination may contribute to the reduction of radiation dose during endovascular procedures. Image fusion is the most widely described radiation exposure limiting tool in the included articles. Particularly in complex endovascular aortic procedures such as fenestrated or bifurcated endovascular aneurysm repair (FEVAR/BrEVAR), this instrument showed a trend toward reductions in cumulative radiation dose and contrast volume. In contrast, fluoroscopy time remained generally similar in both groups. This is most likely because the use of image fusion during navigation reduces the need to create DSA with contrast medium for navigational purposes, as the use of image fusion allows navigation based on projection of a preoperatively acquired CTA onto real-time 2D fluoroscopy display. In addition, the chosen registration method plays an important role in achieving radiation reduction. While the use of a 3D registration method often produces a more accurate image fusion result compared to the 2D method, CBCT necessary for the 3D registration method accounts for a significant portion of the radiation exposure [Citation38,Citation39]. Therefore, it is quite possible that this explains why in case of image fusion is used for example more simple EVAR procedures, the results show a trend rather than a significant reduction in radiation exposure.
Although in many cases, image fusion leads to a decrease in radiation exposure, this tool in itself is still completely dependent on the use of radiation during the visualization of endovascular procedures. The four other (potential) radiation-limiting instruments described, on the other hand, can be used almost completely radiation-free, whether or not in combination with image fusion. This is especially true for IVUS, as it is an intraluminal imaging technique that can visualize the vessel and surrounding structures. In addition, IVUS can also be used to compare neck aneurysm characteristics, such as diameter, with preoperative values obtained during the procedure and to accurately assess the position of the stent. In contrast, the other three techniques find their application in the reduction of radiation during the navigation phase of endovascular procedures. However, the use of these technologies also has limitations. The use of RNS, for example, has the great advantage that the devices have greater maneuverability and stability, but on the other hand the disadvantage that virtually no information is available about the course of the device without using fluoroscopy. Using EM tracking and FOSS technologies, the positioning and movement of the devices can be more accurately visualized, but the systems are prone to inaccuracy caused by disturbances in the magnetic field or optical signal. Moreover, there are currently only limited devices available for these three techniques and setting up the technology during procedures and the associated learning curve currently often extends the procedure time. While these techniques have the potential to reduce radiation exposure, they generally still increase radiation exposure at this stage.
4.1. Limitation of outcome parameters
In order to make an optimal comparison between all characteristics of radiation exposure and cannulation task, the conditions under which the data were obtained should be as similar as possible. However, the data compared in this review are highly heterogeneous, significantly increasing the potential for bias. For example, the amount of radiation exposure depends on multiple factors and achieving radiation reduction often involves addressing more than one of these factors. The X-ray settings selected during the procedure, such as the fluoroscopy protocol and the number of frames per second, the desired position and orientation of the C-arm and the application of the ALARA principle, influence the radiation exposure results [Citation40,Citation41]. In addition, the complexity of the procedure performed and the operator’s experience also affect radiation exposure results, such as cannulation task characteristics, procedure, and fluoroscopy time. For a fair comparison with the smallest possible chance of bias, the characteristics in both groups should be matched to create homogeneous groups. However, most factors, such as X-ray and contrast protocol, operator experience, and complexity of the procedure, are only mentioned to a limited extent in the included studies. This makes it difficult to determine the exact degree of difference between the included studies and the impact on the outcomes.
4.2. Study limitations
Nine of the fourteen included clinical studies have a retrospective design of which eight studies [Citation13,Citation14,Citation23–28] reported outcomes of the use of IF and one study [Citation33] of IVUS. Most of these studies describe the comparison made with a cohort composed of procedures performed with older technology. For a fair and unbiased comparison between the parameter outcomes of both groups, the properties such as co-morbidities, complexity, and conditions of the procedure in these groups should be matched as closely as possible. However, due to the current design of the included studies, it is quite possible that outcomes based on tasks performed will lead to different outcomes compared to the presented outcomes per procedure.
In addition to all 12 included IF studies [Citation13,Citation14,Citation23–32], only three other clinical studies describing EM [Citation21], IVUS [Citation33] and FOSS [Citation17] were included. This distribution of radiation-reducing devices in clinical studies makes it difficult to make a good comparison between the technologies, as it is based on one study for three technologies. Moreover, the comparison between outcomes found in clinical trials and in vivo studies should be done with extreme care, as in vivo studies can never mimic all factors of a clinical setting and will therefore always provide a simplified representation of reality. Thus, the in vivo results only show the potential for exposure reduction, while the clinical results confirm or refute this.
The purpose of this review is to provide an overview of new radiation-reducing instruments. It was therefore decided to emphasize radiation reduction in the design of the search strategy. By emphasizing radiation exposure reduction, articles describing potential radiation exposure-reducing tools, but not focusing on this goal, may have fallen outside the scope of this review. Many studies describing navigation methods are more focused on demonstrating the accuracy and safety of the devices used. The potential for radiation reduction is briefly mentioned in the discussion section, but no further data is given to support this. In these cases, the search strategy we choose usually excludes the article, so that it was ultimately not included in the analysis.
5. Conclusion
In this systematic review, five different tools are presented to reduce potential radiation exposure. At present, these studies provide evidence that image fusion leads to a greater degree of reduction in radiation exposure with increasing complexity of the procedure. The remaining instruments have the potential to make a specific contribution to reducing radiation exposure during endovascular procedures. However, due to factors such as limited availability of devices or long preparation time, these tools are currently not yet of added value in limiting radiation exposure.
6. Expert opinion
Vascular surgery has undergone enormous development over the past 30 years from open to endovascular surgery. These developments offer the possibility to perform increasingly complex procedures as minimally invasive as possible. Besides the fact that more and more patients can be treated adequately, it also reduces the risks of unwanted complications, such as infection, during or after these procedures. However, the disadvantage of the increasing number of endovascular procedures of increasing complexity is that the radiation exposure of performing physicians especially also increases. Because of the increased health risk associated with long-term radiation exposure, it is therefore important to keep radiation use as low as possible during these procedures. There is therefore a great need to develop techniques that can achieve the same results with less or no radiation exposure than used in conventional endovascular procedures.
As discussed in this review, the use of image fusion in particular is currently of added value in reducing radiation exposure in more complex procedures. This technology is widely available and provides the user with insight during procedures by projecting the anatomy onto real-time 2D fluoroscopy images, eliminating the need for DSA with contrast agent to facilitate navigation and reduce radiation exposure. Moreover, the implementation of this technique in current practice does not require much deviance from the current procedure, so that the first step toward radiation reduction can be taken in an easy and fast way. However, the limitation of this technology at present is that the anatomical roadmap created does not adapt to live changes during the procedure. As a result, it is sometimes necessary to adapt the registration made to the live situation, which in turn requires the use of radiation. In addition, with the standalone use of this technology, the need to visualize devices by means of fluoroscopy remains necessary. As a result, the technology contributes to a reduction in radiation, but will never lead to radiation-free procedures.
Technologies such as IVUS or FOSS and EM tracking, possibly in combination with robot navigation systems, offer great potential for realizing the next step toward radiation-free endovascular interventions. These techniques can be applied autonomously and virtually radiation-free. In addition, using these technologies it is possible to visualize the position and orientation of the devices relative to, for example, a cannulation target. The colorized display of these devices makes it easier to distinguish position from the background, which is often gray. This provides the user with more information and better discrimination while navigating compared to navigation with conventional 2D fluoroscopy. An additional advantage of IVUS is that this technology can provide direct information about the neck of the aneurysm, the exact origin of target vessels or the position of the expanded stent graft. This allows IVUS to be deployed during both the navigational and monitoring phases of the procedure, further reducing the need to use radiation.
Although these navigation techniques potentially have a lot to offer in reducing radiation exposure, they are still minimally applied in practice. The main problem is that the advantages of these techniques do not yet outweigh the limitations. For example, relatively few commercial devices are available and many of the setups are still experimental. In addition, the preparation of the set-up of the technology currently still takes a lot of time and in many cases, this even extends the procedure time. Add to this the often high purchase value of the technology with the necessary equipment and you quickly fall back on the current conventional fluoroscopy method. Nevertheless, increasing awareness of the risks of long-term radiation exposure can to lead to more widespread adoption of these technologies in the coming years. The increasing demand for radiation reduction methods during endovascular procedures will lead to a greater focus of the medical industry on developing existing options and expanding the number of available devices. As a result, it is not a question of whether, but when all operating rooms will be equipped with these radiation-reducing technologies. Ultimately, performing endovascular interventions without requiring any form of radiation is certainly the future.
Article highlights
This review presents overview of possible radiation dose reducing tools used during endovascular aortic procedures
Image fusion is currently the most widely adopted technology for reducing radiation dose during endovascular aortic procedures.
Fiber Optic shape-sensing Technology, Intravascular Ultrasound and Electromagnetic Tracking have high potential to contribute to further reduction of radiation exposure during endovascular aortic procedures
Declaration of interest
JA van Herwaarden has served as a consultant for Philips Medical Systems B.V. Netherlands. CEVB. Hazenberg has served as a consultant for Philips Medical Systems B.V. Netherlands. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (14.8 KB)Supplementary material
Supplemental data for this article can be accessed here
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Volodos NL, Karpovich IP, Troyan VI, et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa Suppl. 1991;33. 93–95.
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(6):491–499.
- Swerdlow NJ, Wu WW, Schermerhorn ML. Open and Endovascular Management of Aortic Aneurysms. Circ Res. 2019;124(4):647–661.
- Behrendt C-A, Sedrakyan A, Rieß HC, et al. Short-term and long-term results of endovascular and open repair of abdominal aortic aneurysms in Germany. J Vasc Surg. 2017;66(6):1704–1711.
- Edwards ST, Schermerhorn ML, O’Malley AJ, et al. Comparative effectiveness of endovascular versus open repair of ruptured abdominal aortic aneurysm in the Medicare population. J Vasc Surg. 2014;59(3):575–582.
- Son S-A, Jung H, Cho JY. Long-term outcomes of intervention between open repair and endovascular aortic repair for descending aortic pathologies: a propensity-matched analysis. BMC Surg. 2020;20:266.
- Ko S, Kang S, Ha M, et al. Health effects from occupational radiation exposure among fluoroscopy-guided interventional medical workers: a systematic review. J Vasc Interv Radiol. 2018;29:353–366.
- Tang FR, Loganovsky K. Low dose or low dose rate ionizing radiation-induced health effect in the human. J Environ Radioact. 2018;192:32–47.
- Ketteler ER, Brown KR. Radiation exposure in endovascular procedures. J Vasc Surg. 2011;53(1):35S–38S.
- Zoli S, Trabattoni P, Dainese L, et al. Cumulative radiation exposure during thoracic endovascular aneurysm repair and subsequent follow-up. Eur J Cardio Thoracic Surg. 2012;42:254–260.
- Dindyal S, Rahman S, Kyriakides C. Review of the use of ionizing radiation in endovascular aneurysm repair. Angiology. 2015;66(7):607–612.
- Goudeketting SR, Heinen SGH, Ünlü Ç, et al. Pros and cons of 3D image fusion in endovascular aortic repair: a systematic review and meta-analysis. J Endovasc Ther. 2017;24:595–603.
- Ahmad W, Hasselmann H-C, Galas N, et al. Image fusion using the two-dimensional-three-dimensional registration method helps reduce contrast medium volume, fluoroscopy time, and procedure time in hybrid thoracic endovascular aortic repairs. J Vasc Surg. 2019;69(4):1003–1010.
- McNally MM, Scali ST, Feezor RJ, et al., Three-dimensional fusion computed tomography decreases radiation exposure, procedure time, and contrast use during fenestrated endovascular aortic repair. J Vasc Surg. 61(2): 309–316. 2015.
- de Ruiter QMB, Moll FL, van Herwaarden JA. Current state in tracking and robotic navigation systems for application in endovascular aortic aneurysm repair. J Vasc Surg. 2015;61:256–264.
- Schwein A, Kramer B, Chinnadurai P, et al. Flexible robotics with electromagnetic tracking improves safety and efficiency during in vitro endovascular navigation. J Vasc Surg. 2017;65:530–537.
- van Herwaarden JA, Jansen MM, Vonken EPA, et al. First in human clinical feasibility study of endovascular navigation with Fiber Optic RealShape (FORS) technology. Eur J Vasc Endovasc Surg. 2021;61:317–325.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535–b2535.
- Cochennec F, Riga C, Hamady M, et al. Improved catheter navigation with 3D electromagnetic guidance. J Endovasc Ther. 2013;20:39–47.
- Condino S, Calabrò EM, Alberti A, et al. Simultaneous tracking of catheters and guidewires: comparison to standard fluoroscopic guidance for arterial cannulation. Eur J Vasc Endovasc Surg. 2014;47:53–60.
- Manstad-Hulaas F, Tangen GA, Dahl T, et al. Three-dimensional electromagnetic navigation vs. fluoroscopy for endovascular aneurysm repair: a prospective feasibility study in patients. J Endovasc Ther. 2012;19(1):70–78.
- Tystad Lund K, Tangen GA, Manstad-Hulaas F. Electromagnetic navigation versus fluoroscopy in aortic endovascular procedures: a phantom study. Int J Comput Assist Radiol Surg. 2017;12(1):51–57.
- Ahmad W, Obeidi Y, Majd P, et al. The 2D-3D registration method in image fusion is accurate and helps to reduce the used contrast medium, radiation, and procedural time in standard EVAR procedures. Ann Vasc Surg. 2018;51:177–186.
- Dias NV, Billberg H, Sonesson B, et al. The effects of combining fusion imaging, low-frequency pulsed fluoroscopy, and low-concentration contrast agent during endovascular aneurysm repair. J Vasc Surg. 2016;63:1147–1155.
- Dijkstra ML, Eagleton MJ, Greenberg RK, et al. Intraoperative C-arm cone-beam computed tomography in fenestrated/branched aortic endografting. J Vasc Surg. 2011;53:583–590.
- Hiraoka A, Shiraya S, Chikazawa G, et al. Feasibility of three-dimensional fusion imaging with multimodality roadmap system during endovascular aortic repair. J Vasc Surg. 2018;68:1175–1182.
- Stangenberg L, Shuja F, Carelsen B, et al. A novel tool for three-dimensional roadmapping reduces radiation exposure and contrast agent dose in complex endovascular interventions. J Vasc Surg. 2015;62:448–455.
- Tacher V, Lin M, Desgranges P, et al. Image guidance for endovascular repair of complex aortic aneurysms: comparison of two-dimensional and three-dimensional angiography and image fusion. J Vasc Interv Radiol. 2013;24:1698–1706.
- De Beaufort LM, Nasr B, Corvec Le T, et al. Automated image fusion guidance during endovascular aorto-iliac procedures: a randomized controlled pilot study. Ann Vasc Surg. 2021;75:86–93.
- Hertault A, Maurel B, Sobocinski J, et al. Impact of hybrid rooms with image fusion on radiation exposure during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2014;48:382–390.
- Rolls AE, Rosen S, Constantinou J, et al. Introduction of a team based approach to radiation dose reduction in the enhancement of the overall radiation safety profile of FEVAR. Eur J Vasc Endovasc Surg. 2016;52:451–457.
- Sailer AM, de Haan MW, Peppelenbosch AG, et al. CTA with fluoroscopy image fusion guidance in endovascular complex aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2014;47:349–356.
- Pecoraro F, Bracale UM, Farina A, et al. Single-center experience and preliminary results of intravascular ultrasound in endovascular aneurysm repair. Ann Vasc Surg. 2019;56:209–215.
- Shi C, Tercero C, Wu X, et al. Real-time in vitro intravascular reconstruction and navigation for endovascular aortic stent grafting. Int J Med Robot Comput Assist Surg. 2016;12:648–657.
- Riga CV, Bicknell CD, Hamady MS, et al. Evaluation of robotic endovascular catheters for arch vessel cannulation. J Vasc Surg. 2011;54:799–809.
- Schwein A, Kramer B, Chinnadurai P, et al. Electromagnetic tracking of flexible robotic catheters enables “assisted navigation” and brings automation to endovascular navigation in an in vitro study. J Vasc Surg. 2018;67:1274–1281.
- Jäckle S, Eixmann T, Schulz-Hildebrandt H, et al. Fiber optical shape sensing of flexible instruments for endovascular navigation. Int J Comput Assist Radiol Surg. 2019;14(12):2137–2145.
- Mohapatra A, Greenberg RK, Mastracci TM, et al. Radiation exposure to operating room personnel and patients during endovascular procedures. J Vasc Surg. 2013;58(3):702–709.
- Van Den Berg JC. Three-dimensional image overlay to assist endovascular procedures. Vasc Dis Manag. 2013;10:E179–E184.
- de Ruiter QM, Gijsberts CM, Hazenberg CE, et al. Radiation awareness for endovascular abdominal aortic aneurysm repair in the [hybrid operating room. an instant patient risk chart for daily practice. J Endovasc Ther. 2017;24:425–434.
- Stecker MS, Balter S, Towbin RB, et al. Guidelines for patient radiation dose management. J Vasc Interv Radiol. 2009;20(7):S263–S273.