ABSTRACT
Introduction
Heart failure (HF) is considered as a chronic long-term and lethal disease and will continue to be a major public health problem. Studying (circulating) biomarkers is a promising field of research and could be the first step toward HF tailored prognostic strategies as well as understanding the response to HF drugs in CHF patients.
Areas covered
In literature, there has been considerable research on elevated biomarker levels that are related to a poor prognosis for HF. Since biomarker levels change over time, it is important to study serial (repeated) biomarker measurements which may help us better understand the dynamic course of HF illness. However, the majority of research focuses predominantly on baseline values of biomarkers. Additionally, remote monitoring devices, like sensors, can be used to link hemodynamic information to freshen biomarker data in order to further ameliorate the management of HF.
Expert opinion
Novel biomarkers and additional scientific insights with hemodynamic feedback strongly aid in the prognostication and risk prediction of chronic HF.
Graphical abstract
1. Introduction
Heart failure (HF) is with a worldwide incidence of 1 to 20 cases per 1,000 persons and a 5-year death rate ranging 50% to 75%, considered as a lethal disease comparable to many cancers [Citation1]. HF is a long-term, chronic cardiac clinical illness that occurs from the heart muscle’s inability to pump adequately, leading to deprivation of supply of blood to organs, preventing them from receiving oxygen and nutrients they need to thrive [Citation2].
In recent decades, it has been evident that the rising incidence of cardiovascular diseases, particularly HF, will continue to be a major public health problem due to the longer life expectancy and rising prevalence of comorbidities. Furthermore, because HF patients frequently need to be hospitalized, the illness has a significant economic impact on health care expenses [Citation1,Citation3]. For these reasons, there is an increasing need for novel, creative solutions to enhance HF diagnosis, prognosis, and prevention.
According to the most recent HF guidelines released by the American Heart Association, American College of Cardiology, and Heart Failure Society of America, it is critical to focus on early indicators of the condition in order to prevent and manage HF in addition to its diagnosis, prognosis, and treatment [Citation4]. In literature, there has been extensive research on elevated biomarker levels that are related to a poor prognosis for HF [Citation5–13]. Since biomarker levels change over time, we advocate that serial (repeated) biomarker measurements may help us better understand the dynamic nature of HF illness. However, the majority of research focuses primarily on baseline data of biomarkers.
Several indicators that suggest higher filling pressures are associated with HF hospitalization and death [Citation14]. The (invasive) cardioMEMS sensor measures pulmonary artery pressures as a surrogate of filling pressures and with proactive intervention it can significantly reduce HF hospitalizations [Citation15]. The trend in filling pressures is an indirect measure of the fluid state in patients.
We advocate that the prognostication and risk stratification of individuals with HF can be improved by investigating serial biomarker patterns in connection to serial hemodynamic feedback provided by these innovative novel sensors. With these new developments, we believe the field of biomarkers can make significant steps to improve and provide a better guidance of HF therapy.
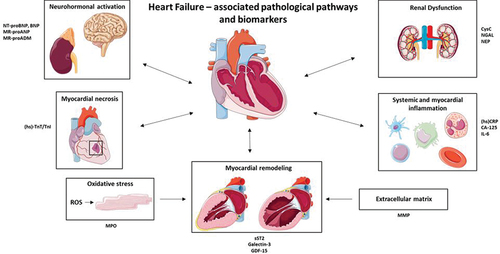
In this review, we summarize the current evidence on the association of several (promising) novel and established primary targets of biomarkers to HF hospitalization and mortality, with regard to prognosis, risk stratification, and hemodynamics. Additionally, we highlight several pathological pathways that provide us with comprehensive knowledge on HF disease as summarized in . We will discuss the value of temporal (repeated) measurements of serial biomarkers, in addition to the solely baseline measures. This review will offer a unique opportunity to provide more insight into the promising features of biomarker monitoring, which can be further improved with the hemodynamic integration of data from remote sensors like CardioMEMS that can better reflect the dynamic state of the disease. While sensors will be concentrated on a selected patient group taking into account costs, biomarkers can play a role in all HF patients. This could be the next step in developing tailored intervention strategies to reduce HF-related burden worldwide and focus resources on those patients most likely to benefit from a biomarker guided strategy.
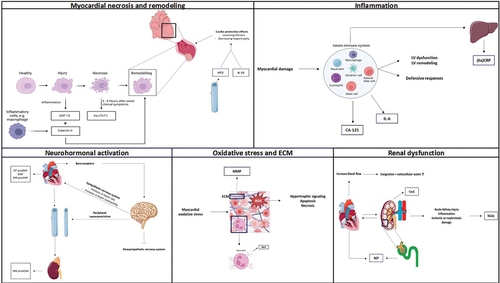
Figure 1. Main pathological pathways involved in heart failure and a selection of associated biomarkers. NT-proBNP, N-terminal pro hormone BNP. BNP, Brain natriuretic peptide. MR-proANP, Mid-regional pro atrial natriuretic peptide. MR-proADM, Mid-regional proadrenomedullin. (hs)-TnT/TnI, (high sensitivity) – troponin T/I. MPO, myeloperoxidase. sST2, soluble suppression of tumorigenesis 2. GDF-15, Growth-differentiation factor 15. MMP, Matrix metalloproteinase. (hs)CRP, (high sensitivity) C-reactive protein. CA-125, Cancer antigen 125. NGAL, Neutrophil gelatinase-associated lipocalin. Parts of the figure were drawn by using pictures from Servier Medical Art licensed under a Creative Commons Attribution 3.0 unported license.
2. Heart failure: definition and subtypes
HF is defined as a progressive, chronic cardiac clinical disorder, in which the heart muscle is unable to pump blood sufficiently, depriving vital organs of oxygen and nourishment. As a consequence, the inadequate cardiac output results in an increase in hemodynamic load with sodium and water retention leading to congestion or fluid overload, a classical symptom of HF [Citation2]. The connection between cardiac and renal dysfunction may be a crucial point in understanding the course and prognosis of HF disease and will be discussed in more detail later on this review [Citation16]. In line with congestion, HF patients may present themselves with dyspnea, fatigue, and edema.
HF is generally classified into acute HF (AHF) and chronic HF (CHF). In this review, we only discuss the biomarker findings of CHF because this type of HF is reported to be most relevant for biomarker focused research. The European Society of Cardiology (ESC) has classified HF patients into three categories based on their Left Ventricular Ejection Fraction (LVEF) values: those with preserved LVEF (>50%, HF with preserved ejection fraction (HFpEF)), mid-range LVEF (40–49%, HF with mid-range ejection fraction (HFmrEF)), and reduced LVEF (<40%, HF with reduced ejection fraction (HFrEF)) [Citation4]. However, LVEF has its pitfalls and cannot be utilized as a substitute for cardiac output [Citation17]. This further implies the need of additional parameters, such as biomarkers and hemodynamic information, in order to optimize our current diagnosis, treatment, and prognostication of HF disease.
3. Clinically relevant (cardiac) biomarkers in CHF
3.1. The neurohormonal activation pathway
The pathophysiology of HF is greatly influenced by the activation of the neurohormonal system. In order to keep the cardiovascular system in a state of homeostasis, the regulation of the blood volume in circulation is closely controlled by the neurohormonal system. The aorta and carotid sinus contain what are known as baroreceptors, which are able to detect changes in the arterial blood volume of the peripheral circulation. While the carotid sinus’s baroreceptors detect high pressure, those in the aortic arch operate as low-pressure cardiopulmonary mechanoreceptors. When engaged, the baroreceptors have an inhibitory impact on the central nervous system and suppress systemic circulation and outflow to the heart. Changes in the heart’s ability to pump blood, the amount of blood circulating in the body, or both cause a drop-in baroreceptor activity. Parasympathetic tone decreases as a result, and sympathetic tone is reflexively enhanced [Citation18,Citation19]. This causes an increase in heart rate and contractility as well as peripheral vasoconstriction. Additionally, neurohormonal stimulation can result in renal salt retention, which can result in hypervolemia. The effects of diuretics are exacerbated, and their effectiveness is decreased when renal impairment is present [Citation20]. Therefore, the extent of neurohormonal activity in HF has been found to be correlated with the severity of the condition and the clinical prognosis [Citation21].
Relevant biomarkers in the neurohormonal activation pathway:
3.1.1. Natriuretic peptides
The diagnostic and prognostic value of natriuretic peptides (NP) in patients with CHF has already been thoroughly investigated in several large studies, with a focus on N-terminal pro-B-type natriuretic peptide (NT-proBNP), B-type natriuretic peptide (BNP), and Midregional proANP (MR-proANP), an overview is given in . As a result of intracardiac volumes and filling pressures, NP are specifically released by cardiac tissue and reflect wall stress. A variety of pathophysiological mechanisms, including diuresis, vasodilation, and natriuresis, can be activated when NP bind to their receptors, which are present in many tissues throughout the body. In this review, we will focus mainly on the prognostic value of NP in CHF. Numerous studies have demonstrated a correlation between changes in plasma NT-proBNP levels and cardiovascular mortality as well as HF hospitalization in HF patients [Citation22–27]. NT-proBNP levels correlate with several other circulating biomarkers, including nitric oxide (NO) metabolism markers, the carboxy-terminal fragment of insulin-like growth factor binding protein 4 (CT IGFBP 4), and IGFBP 7 making them useful in a multi-biomarker approach [Citation28–30]. Moreover, values of NT-proBNP may be of use to prognosticate the course of HF. For example, a study by Zile, MR. et al. analyzed the data of the PARADIGM-HF trial to address the prognostic value of changes in NP. They discovered that individuals with a decline in NT-proBNP value to less than 1,000 pg/ml had a 59% reduced risk of hospitalization for HF and cardiovascular mortality than patients without such a fall (HR (95% CI) 0.41 (0.29 to 0.58), P < 0.0001) [Citation31]. The Valsartan Heart Failure Trial (Val-HeFT) found similar findings for the cardiac biomarker BNP. In this study, the researchers discovered that the incidence of all-cause mortality and first morbid events was significantly higher for patients with baseline BNP values above the median than for those with values below the median. For BNP levels above vs. below the median, the relative risks for all-cause mortality and the first morbid events were (RR (95% CI), 2.1 (1.79 to 2.42) and 2.2 (1.98 to 2.52)), respectively [Citation32]. The importance of plasma BNP as a predictor of HF-related events is emphasized by this and other research [Citation25,Citation33]. Finally, the more stable MR-proANP has been brought to attention, due to its superior long-term prognostic value as compared to other NP like NT-proBNP and BNP [Citation34–36]. This was highlighted by the research by von Haehling, S. and colleagues, who evaluated the predictive significance of MR-proANP in CHF patients. They discovered that elevated MR-proANP levels were associated with poor survival (RR (95% CI), 1.35 (1.17 to 1.57), P = 0.0061) [Citation35]. These findings confirm the importance of the clinical use of NP in the prognostic validation of CHF. It should be mentioned, nonetheless, that there are several considerations with regard to the clinical application of NP, such as the fact that serial measurements of NP yield more prognostic information as compared to baseline tests. Furthermore, results from cardiac biomarkers such as NP should always be evaluated in light of other clinical indicators, like hemodynamic information.
Table 1. Neurohormonal activity pathway.
3.1.2. Adrenomedullin
Another cardiac biomarker involved in neurohormonal activation and promising in prognostication of CHF is adrenomedullin (ADM). Mid Regional Pro-Adrenomedullin (MR-proADM), a stable inactive protein fragment, has been shown to reflect ADM levels more closely in blood. Therefore, in this review, we decided to pay particular attention to the prognostic ability of MR-proADM in CHF patients. The potential value of MR-proADM in HF disease is described in . The potential of MR-proADM to serve as a prognostic biomarker has been proved by the study of von Haehling, S. et al. [Citation37]. In this study, the researchers found that increasing levels of MR-proADM were a predictor of mortality in patients with CHF (HR (95% CI), 1.82 (1.24 to 2.66), P = 0.002). Interestingly, they also found that MR-proADM added prognostic value to NT-proBNP (P = 0.00094). Another research measured plasma MR-proADM in CHF outpatient and demonstrated a significant positive predictive value (HR (CI 95%), 1.41 (1.30 to 1.54), P < 0.001). Additionally, the Kaplan–Meier analysis revealed higher mortality for patients with MR-proADM plasma levels above the median (P < 0.001, log-rank test) [Citation38]. These findings have also been confirmed by a more recent study of Feng, Z. et al., in which they found that MR-proADM scored the best for predicting a Major Adverse Cardiovascular Event (MACE) and HF hospitalization in CHF patients [Citation39]. Elevated MR-proADM levels have been proved to predict mortality and HF hospitalization, and they can improve the predictive power of currently available biomarkers like BNP and other clinical indicators. This makes MR-proADM a promising biomarker that may be applied in a biomarker-guided treatment approach.
3.2. The myocardial necrosis pathway
Myocardial necrosis, is a biological process in which irreversible myocardial damage causes the intracellular components of the irreparably injured cardiac myocytes to flow into the myocardial extracellular space. After an inflammatory reaction, the necrotic myocardial zone will go through a restorative or remodeling phase, where the necrotic tissue is destroyed, resorbed, and ultimately replaced by cardiac scar tissue [Citation40]. Myocardial necrosis has also been acknowledged to play a significant part in the phenotype of individuals with CHF as a result of left ventricular remodeling, which also involves loss of myocyte cells [Citation20,Citation41].
Relevant biomarkers in the myocardial necrosis pathway:
3.2.1. High-sensitivity cardiac troponin T/I
High-sensitivity cardiac Troponin T (hs-cTnT) and Troponin I (hs-cTnI), two well-known cardiac biomarkers, are acknowledged for their cardio-specific diagnostic and prognostic utility in CHF (). Four to six hours after the onset of clinical symptoms, cardiac troponins – which are released upon myocardial necrotic damage – are discovered in the plasma [Citation42]. For this reason, their potential to serve as HF biomarkers has prompted their inclusion in the current European (ESC) and American (American college of Cardiology/American Heart Association, ACC/AHA) guidelines [Citation4]. Numerous studies have revealed promising results regarding the prognostic significance of hs-cTnT and hs-cTnI in terms of their ability to forecast a worse course of CHF [Citation9,Citation43–47]. Aimo, A. and colleagues’ investigation on the prognostic value of hs-TnT in CHF revealed HR (CI 95%) values of 1.48 (1.41 to 1.55) for all-cause mortality, 1.40 (1.33 to 1.48) for cardiovascular death, and 1.42 (1.36 to 1.49) for hospitalization for cardiovascular reasons (P < 0.001). Moreover, these results were not affected by NT-proBNP, sex, or age [Citation9]. Similar conclusions were drawn by the study of deFilippi, CR. et al., the researchers concluded that cTnT levels >12.94 pg/mL in older individuals (aged 65 years or older) were associated with the highest risk of new onset HF and cardiovascular death (HR (CI 95%), 2.48 (2.04 to 3.00)) [Citation47]. It is also interesting to note that they discovered that low cTnT concentrations regularly vary over time. This stresses the necessity of monitoring serial patterns of biomarkers in order to precisely predict the course of (non-)symptomatic HF disease. This will later be emphasized in the section clinical relevance of serial biomarker analyses.
Table 2. Myocardial necrosis and remodeling pathways.
3.3. The myocardial remodeling pathway
The evolution of the clinical course of HF includes an important recognized process known as myocardial remodeling. The hemodynamic load and neurohormonal activation both have an impact on changes that occur in the heart’s cellular and molecular components. Since remodeling of the left ventricle (LV) of the heart is most associated with myocardial remodeling, we will concentrate on LV remodeling in this review. The ventricle tends to grow, the general form of the ventricle becomes more globular and less elliptical, the muscular wall of the ventricle frequently thins, and finally symptoms of HF become apparent. The ejection fraction (EF), a key indicator of mortality in HF, diminishes if this remodeling process is allowed to continue and the changes in the ventricle’s size and shape become more prominent [Citation48,Citation49]. The prognostic significance of the cardiac remodeling biomarkers, soluble suppressor of tumorigenicity 2 (sST2), growth differentiation factor-15 (GDF-15), and galectin-3 (Gal-3), will be described below, and a summary is provided in .
Relevant biomarkers in the myocardial remodeling pathway:
3.3.1. Soluble suppressor of tumorigenicity 2 (sST2)
Another promising cardiac biomarker is sST2, which is released upon stretching of cardiomyocytes, where it eventually neutralizes its ligand interleukin (IL)-33. IL-33ʹs relation with the ST2 receptor has cardio-protective effects on the myocardium by lowering fibrosis, increasing survival, and decreasing hypertrophy [Citation50]. Elevated circulating concentrations of sST2 have been linked with adverse clinical outcomes, with an ability to predict prognosis in CHF [Citation10,Citation51,Citation52]. This has been under investigation in a meta-analysis of Aimo, A. and colleagues and shown that sST2 is a predictor of both all-cause and CV death in CHF outpatients (HR (95% CI) 1.75 (1.37 to 2.22) for all-cause death and 1.79 (1.22 to 2.63) for CV death (P < 0.001)) [Citation10]. Similar findings were reached by Emdin, M. et al.’s study, who discovered that the risk of all-cause and CV mortality, and HF hospitalization increased by 26%, 25%, and 30%, respectively, with each doubling of sST2 [Citation51]. When focusing on the elderly CHF population, ST2 also emerged as the strongest predictor in both univariate and multivariate analysis for all-cause mortality or HF-related hospitalization [Citation52]. Considering their temporal pattern, the serial measurement of sST2 has been found to offer more prognostic information and reflect changes in LV remodeling over time, making the biomarker potentially useful for HF evaluation and management [Citation53]. The idea of employing sST2 as a biomarker for the prognostication of HF to lessen the burden associated with the condition is raised by this and other research.
3.3.2. Growth differentiation factor 15 (GDF-15)
Inflammation, cardiac damage, and tissue injury all cause the expression of GDF-15 in a variety of tissues. HF development and unfavorable cardiac remodeling are both associated with a substantial rise in GDF-15 in the presence of cardiovascular damage [Citation54]. Moreover, HF mortality and hospitalization are linked to elevated levels of GDF-15 [Citation11,Citation55,Citation56]. A recent meta-analysis that examined the relationship between circulating GDF-15 levels and the prognosis of CHF discovered a 6% increase in the risk of all-cause death (HR (CI 95%) 1.06 (1.03 to 1.10), P < 0.001) [Citation56]. That GDF-15 offers predictive information on CHF was demonstrated by a study of Kempf, T. et al. The study’s findings included a graded association between GDF-15 levels and all-cause mortality, which were both considerably elevated in CHF patients. Outstandingly, GDF-15 significantly enhanced LVEF and NYHA functional class by adding predictive information [Citation11]. Same conclusions were drawn by Fernandez, A.B.M. et al., who found that the ability of GDF-15 to predict death increased by 3.3%; HFmrEF and HFpEF patients in the highest tertile (GDF-15 level >4330 ng/L) had the poorest 5-year survival, at 16%, whereas the lowest tertile (GDF-15 level <1625 ng/L) had the best survival, of 78% (P < 0.001) [Citation55]. The potential of GDF-15 as a biomarker in CHF to predict the risk of death and/or hospitalization is great and could provide new prognostic information when combined with other clinical markers.
3.3.3. Galectin-3
Recent research has demonstrated the increasing significance of galectin-3 in the pathogenesis of HF. Macrophages and other inflammatory cells release galectin-3, which causes additional inflammatory cytokines and growth factors to be secreted. Galectin-3, however, is also known to be a key player in myocardial remodeling [Citation57]. In light of this, galectin-3 may be a useful biomarker for HF prognostication, this has been demonstrated in several studies [Citation12,Citation27,Citation58–61]. A substudy of the coordinating study reviewing the results of the Advising and Counseling in Heart Failure (COACH) trial by de Boer, RA et al. has demonstrated that galectin-3 is a potent and independent predictive biomarker, particularly in HF patients with preserved LVEF. Specifically, a doubling of galectin-3 levels was linked to a hazard ratio (HR (CI 95%)) of 1.97 (1.62 to 2.42) for all-cause death and HF hospitalization (P < 0.001) [Citation58]. This was enhanced even further by a pooled analysis of data from three clinical studies, which demonstrated the usefulness of galectin-3 in predicting patients with HF who would likely require a short-term hospital stay again. Galectin-3 concentrations of more than 17.8 ng/mL were shown to be substantially associated with increased risk of HF rehospitalization when compared to individuals with concentrations less than this value (P < 0.001) [Citation59]. Together, the aforementioned studies and others imply that galectin-3 may have a significant role in determining a patient’s prognosis for CHF.
3.4. The renal dysfunction pathway
A strong connection exists between the kidney and the heart. The control of the body’s salt and water balance by the kidneys is directly dependent on the heart, and the kidneys are directly reliant on the heart’s production of blood flow and blood pressure. This is particularly true for conditions like HF that are associated with higher levels of congestion and extracellular water. As renal impairment eventually develops in virtually all patients with CHF, it has become clear over the past few decades that there is a substantial association between renal dysfunction and CHF. Higher morbidity and mortality accompany this [Citation62,Citation63]. Renal biomarkers should thus be included when developing HF patient preventive and therapy plans. We will concentrate on the potential of the biomarkers, cystatin C (CysC), neprilysin (NEP), and neutrophil gelatinase-associated lipocalin (NGAL), in this review, and an overview is provided in .
Table 3. Renal dysfunction pathway.
Relevant biomarkers in the renal dysfunction pathway:
3.4.1. Cystatin C
CysC is another promising sensitive renal marker that might be a helpful biomarker in HF. CysC, a cysteine protease inhibitor, regulates cathepsins S and K in particular and acts as a marker of renal function in the pathophysiology of human vascular systems [Citation64]. There is already strong evidence that CysC is associated with CVD risk [Citation65]. Furthermore, there is growing proof that CysC may be able to predict worse outcomes in CHF [Citation66,Citation67]. According to the research of Dupont, M. et al., CysC levels higher than 1.41 mg/L were linked to an HR (CI 95%) of 3.02 (2.01 to 4.67), P < 0.001. This indicated that CysC is an independent predictor of adverse outcomes in CHF. Curiously, they discovered that CysC levels had a predictive significance in individuals with maintained renal function precisely because of their substantial correlation with creatinine (r = 0.73) [Citation66]. More recently, research by Wang, C. and colleagues examined the utilization of serum CysC/prealbumin ratio in order to determine its prognostic value on the long-term prognosis in patients with CHF. The Cys-C/PAB ratio was found to be a significant independent predictor of both cardiovascular mortality (HR (95% CI) 1.12 (1.15 to 1.23), P < 0.01) and all-cause mortality (HR (95% CI) 1.19 (1.13 to 1.24), P < 0.01) by multivariable Cox analysis. Even better prognostic prediction was made for HF patients in the long term when the CysC/PAB ratio and NT-proBNP were coupled [Citation67]. In light of the complex etiology and clinical nature of HF illness, it has been proposed that a multimarker biomarker model combining multiple potential biomarkers may be more useful in determining prognosis in patients with CHF.
3.4.2. Neprilysin
The well-known enzyme NEP, often referred to as membrane metallo-endopeptidase (MME), is expressed in a variety of tissues, including vascular smooth muscle cells, cardiac myocytes, and is most abundant in the proximal tubules of nephrons [Citation68]. The PARADIGM-HF trial illuminated the potential of NEP in HF; researchers discovered remarkable clinical advantages from the combination of an angiotensin receptor blocker (ARB) and a neprilysin inhibitor (angiotensin receptor neprilysin inhibitor (ARNi)) over those that receive standard care with an angiotensin-converting enzyme (ACE) inhibitor in HFrEF patients. NEP has also been researched for its potential predictive, prognostic usefulness [Citation69,Citation70]. Increased NEP levels were shown to be substantially positively associated with CV mortality and HF hospitalization in one of the studies carried out by Bayés-Gens, A. et al. Hospitalization for HF or CV death had an HR (95% CI) of 1.37 (1.11 to 1.69), P = 0.003 [Citation69]. This was further strengthened by a more recent study by Gommans, D.H.F. and colleagues, who found a correlation between clinical outcomes and classification of CHF patients based on circulating NEP and corin concentrations. HF hospitalization and CV mortality were also substantially correlated with soluble NEP concentrations (HR (95% CI) 1.13 (1.04 to 1.24), P = 0.005) [Citation70]. Important pathobiological insights into the HF syndrome are provided by the discovery of circulating NEP levels in patients with HF and the favorable correlation of NEP with CV prognosis. Therefore, evaluating this biomarker in CHF patients may enable us to gather stratified data on which particular individuals are more likely to experience, for instance, an HF hospitalization.
3.4.3. Neutrophil gelatinase-associated lipocalin (NGAL)
According to literature, individuals with acute kidney injury (AKI), inflammation, and ischemic or nephrotoxic damage had significantly higher levels of the promising renal biomarker NGAL, also known as lipocalin-2. Furthermore, it has been discovered that increased NGAL expression is associated with CV disorders, including HF. That increased NGAL levels are linked to renal impairment in patients with CHF has been demonstrated by the study of Damman, K and colleagues. The median urine NGAL levels in CHF patients were significantly higher than controls, at 175 vs. 37 μg/gCr, respectively (P < 0.0001) [Citation71]. Many research have examined the predictive usefulness of NGAL in CHF patients, including the study by Villacorta, H et al., who discovered that NGAL was a potent predictor of clinical outcomes including CV mortality and HF hospitalizations (HR (95% CI) 1.0035 (1.0019 to 1.0052), P < 0.0001). Interestingly, NGAL appeared even to outperform several clinical measures of renal function, including creatinine and eGFR, as well as the conventional HF biomarker BNP [Citation72]. This was further strengthened by Bolignano, D et al., who found that elderly CHF patients with baseline NGAL concentrations >783 ng/mL had a substantially increased risk of mortality (p = 0.001; log-rank test), with an HR (95%) of 4.08 (1.29 to 12.96) for death [Citation73]. The promising NGAL, a biomarker of kidney injury, has the potential to be a reliable predictive predictor of prognosis in CHF patients.
3.5. The inflammation pathway
Inflammation is recognized to be a key player in the pathophysiology of HF. The stimulation of the innate and adaptive immune system in patients with HFrEF has been shown to have a significant impact. The adaptive immune system, which is mediated by B cells and T cells, offers a highly targeted defense against infections or tissue injury in contrast to the innate immune system’s nonspecific protection. Following myocardial damage, the innate immune system’s subsequent inflammatory response upregulates defensive responses, giving the heart a momentary adaptability to elevated stress. The hallmarks of HFrEF, LV dysfunction, and LV remodeling might be caused by this inflammatory response, which can become dysregulated and culminate in chronic inflammation [Citation74,Citation75]. It has also been acknowledged that there exists a connection between serum lipid profiles and the transition from AHF to CHF, ultimately leading to further progression and development of HF [Citation76]. The potential of inflammatory biomarkers, such as Carbohydrate Antigen 125 (CA-125), High-sensitivity C-reactive Protein (hsCRP), and interleukin-6 (IL-6), which are elevated in patients with HF, to assist in the prognostication of HF illness will be reviewed below. A summary of their importance in HF disease can be found in .
Table 4. Inflammation, oxidative stress, and ECM pathways.
Relevant biomarkers in the inflammation pathway:
3.5.1. Carbohydrate antigen 125
The big mucin glycoprotein CA-125, which is produced by mesothelial cells, is already a commonly used tumor biomarker for ovarian cancer screening, diagnosis, and follow-up. CA-125, however, has been hypothesized to be up-regulated in HF patients and may have the potential as a new marker for congestion, prognosis, and risk classification of HF disease [Citation77–79]. According to our knowledge, one of the first investigations into the behavior of CA-125 in CHF patients discovered that mean CA-125 serum levels were above normal not only in patients with moderate-to-severe CHF (NYHA classes III/IV) but also in individuals with modest symptoms (class II), as in those with asymptomatic LV failure (class I). Further, the researchers discovered a relationship between CA-125 and filling pressures as well as a decrease in CA-125 serum levels in a subgroup of patients following an improvement in clinical condition [Citation79]. The study by D’Aloia, A et al., which found that increased CA-125 levels in CHF patients were associated with clinical status, (non-)invasive hemodynamic anomalies, and indicative of a poorer prognosis in short-term follow-up, provided more support for this [Citation78]. Fluctuations in CA-125 biomarker levels may therefore offer additional data on the hemodynamic fluid condition of CHF patients, have prognostic significance, and potentially propose HF medication modifications.
3.5.2 (High-sensitivity) C-reactive protein (hs)CRP
The inflammatory HF biomarker (hs)CRP is another one of interest since it appears to rise in response to the pathophysiological mechanisms behind progressive ventricular remodeling. Patients with HF are found to have higher (hs)CRP levels, and higher (hs)CRP levels are associated with symptoms of more severe HF. Plasma (hs)CRP is also independently correlated with mortality and morbidity [Citation27]. A post hoc analysis of the Val-HeFT database showed a direct relationship between elevated plasma CRP and the progression of HF. Increased CRP levels in comparison to the lowest CRP quartile were associated with an increased risk of death and the occurrence of the first morbid event, with respective HRs (CI 95%) of 1.51 (1.2 to 1.9) and 1.53 (1.28 to 1.84), P < 0.001. Additionally, a link between poorer hemodynamic state and increased CRP concentrations was established [Citation80]. This was further supported by a recent study of Pellicori, P et al., in which CHF patients with hsCRP ≥10 mg/L had a higher risk of all-cause mortality (HR (CI 95%) 2.49 (2.19 to 2.84), P < 0.001) than those with hsCRP levels <2 mg/L [Citation81]. Although individual biomarkers have demonstrated their relevance in HF mortality risk prediction and prognosis, a multimarker strategy’s function requires additional investigation. Interestingly, the use of a three-biomarker combination of heat shock protein 27 (HSP27), sST2, and hsCRP is an independent predictor of cardiovascular mortality and unnecessary HF-related hospitalization and may improve risk stratification [Citation82].
3.5.3. Interleukin-6
The proinflammatory cytokine biomarker, IL-6, plays a key role in spreading the immune and inflammatory responses and is crucial for the initiation and progression of cardiac remodeling [Citation83,Citation84]. Additionally, IL-6 promotes the synthesis of C-reactive protein. It has been shown that HF induces long-term IL-6 production, and that the level of expression rises as CHF severity increases [Citation85–90]. The STABILITY trial found that IL-6 was significantly linked to an increased risk of HF hospitalization (HR (95% CI) 3.51 (2.4 to 5.09), P < 0.0001) [Citation90]. In a more recent study, Chia, Y.C. and colleagues discovered that elevated levels of IL-6 were linked to an increased risk of developing HF, and more particularly, HFpEF [Citation91]. This observation was further strengthened by the BIOSTAT-CH study [Citation92]. However, this was in contrast to the DIAST-CHF study who found that IL-6 showed higher expression especially in HFrEF patients [Citation93]. This draws attention to IL-6ʹs potential as a powerful prognostic indicator in HF patients. This could aid in the risk classification of the HF population and help identify certain target patient groups that require closer monitoring.
3.6. The oxidative stress and extracellular matrix pathways
Myocardial oxidative stress occurs in the failing heart and is associated with LV failure. Reactive oxygen species (ROS) induce hypertrophic signaling, apoptosis, and necrosis, which have detrimental effects on the myocardium’s ability to handle calcium, result in arrhythmias, and lead to cardiac remodeling [Citation94]. Therefore, it is interesting to investigate how myocardial necrosis indicators relate to HF prognosis; in this review, we will concentrate on the oxidative stress marker myeloperoxidase (MPO). The expansion of the cardiac extracellular matrix (ECM) is strongly associated with a poor prognosis in patients with HF, according to growing body of clinical data [Citation95]. Matrix metalloproteinases (MMP) play a crucial part in cardiac remodeling; hence, it is important to comprehend their pathophysiological process and their potential to be used as biomarkers in CHF prognostication ().
Relevant biomarkers in these pathways:
3.6.1. Myeloperoxidase
The enzyme known as MPO, which is mostly expressed in neutrophils and monocytes, belongs to the superfamily of heme peroxidases. By taking part in phagocytosis, MPO-derived reactive species are principally responsible for the neutrophil antimicrobial activity and human defense against a variety of infections. Oxidative stress is more prevalent when MPO levels are elevated in the blood [Citation96]. The possible involvement of MPO in predicting the prognosis of CHF patients as well as their relationship to structural and functional cardiac indicators and the course of the disease have received attention in a number of studies [Citation97–100] (). So has the study of Tang, W H W et al., who found that patients with plasma MPO above median levels (>303pM) showed a 2.9-fold greater risk of adverse clinical outcomes compared to those with lower plasma MPO levels (<303pM) ((95% CI, 1.2–8.1), P < 0.05) [Citation101]. For the clinical advantages of ‘biomarker-guided’ surveillance, MPO may carry a potential in risk stratification and therefore may be customized to individuals with increased levels of this or other biomarkers.
3.6.2. Matrix metalloproteinases
All elements of the ECM are broken down by the zinc-dependent proteolytic enzyme family known as MMP. The upregulation of MMP, a family of downstream proteases, occurs in conjunction with HF. It is now clear that these compounds play a key role in the unfavorable remodeling of the myocardium during HF [Citation102]. The myocardium has elevated cytokine and MMP levels, which have the potential to spread to the rest of the body. Collagenases (MMP-1 and -8), gelatinases (MMP-2 and -9), and stromelysins (MMP-3) are the MMP that have been evaluated in HF clinical investigations. MMP are mainly regulated by the tissue-type inhibitors of matrix metalloproteinases (TIMP). Jordán, A. and colleagues examined the function of MMP in the prognostication of CHF and found that MMP-1 levels were independently predictive of CV events (P = 0.039) [Citation103]. Similar findings were found for MMP-2 and their association with congestive HF. Researchers discovered that individuals with MMP-2 mean serum levels over 352 ng/mL had substantially worse prognoses for all-cause mortality and HF hospitalization, with correlation coefficients of r = 0.41, P < 0.001, and r = 0.38, P < 0.001, respectively [Citation104]. In comparison to Galectin-3, MMP-2ʹs predictive strength contributes much more to the clinical outcome prediction in HF patients [Citation105]. By using MMP as biomarkers, we may be able to distinguish between subgroups of CHF patients whose ECM deterioration has a detrimental effect on their prognosis.
3.7. Other promising biomarkers
There are several other promising biomarkers that carry the potential to aid in the prognostication of HF [Citation106–108]. AXL, a member of the receptor tyrosine kinase subfamily, has been found to be correlated with higher mortality in HF patients [Citation106]. The same findings were reached for microRNAs (miRNAs), which have the capacity to predict HF-related events [Citation107].
4. Clinical relevance of serial biomarker analyses
Most of the studies have assessed biomarkers at baseline in relation to a potential HF-related event. This assessment is usually based on the baseline value of the biomarker, which is a random moment and usually stable in the course of HF disease. However, since HF is characterized by its dynamic course, baseline values are not able to evaluate the biomarker performance over time. This review will therefore highlight the importance of serial measurement of biomarkers, which provides longitudinal information to predict HF prognosis. A joint model of longitudinal and survival data allows us to investigate the relationship between a repeatedly measured biomarker and the time to an event of interest, such as HF hospitalization. This approach is useful because this data provides predictive information on the prognosis and risk stratification of HF [Citation109].
In a study of van Boven, N et al., the detailed temporal patterns of NT-proBNP, hs-cTnT, and CRP were evaluated for their predictive value in patients with CHF. According to the authors, these biomarkers were each individually linked to a poor prognosis and especially elevated in the frame toward an upcoming event, and both individual patterns and the combination of many biomarkers might be useful for HF prognostication [Citation109]. Given that most patients in prior trials are observed for just short periods of time (mostly 6 months), the researchers’ evaluation of the aforementioned biomarkers over a median of 27 months makes the study even more valuable. The Val-HeFT trial of Anand, I.S. et al. found that percent changes in BNP and norepinephrine (NE) over time strongly predicted the adverse clinical outcomes of patients with HF. Hereby, they collected the baseline sample together with a sample 4, 12 and 24 months thereafter. The researchers discovered that the quartiles with the largest percent rise in BNP and NE from baseline to 4 months displayed the highest mortality, whereas those with the greatest fall demonstrated the lowest mortality [Citation32]. Similar conclusions were drawn by deFilippi, CR et al., who discovered that changes in the risk of both new-onset HF and CV mortality are independently correlated with serial changes in extremely low levels of cTnT [Citation47]. Consequently, repeated measurement of the cardiac biomarker cTnT instead of a single baseline value may aid in more accurate forecasting and representation of the dynamic change associated with the course of HF illness. In a recent study, Gürgöze, M.T. et al, investigated the prognostic value of serially measured levels of GDF-15, NT-proBNP, ST2, galectin-3, cTnI, and creatinine. The researchers discovered that GDF-15 dynamically and independently predicts the composite risk of all-cause death or HF rehospitalization over the course of a year. In addition, they found that a multi-marker model integrating GDF-15, NT-proBNP, and cTnI offers a promising risk discrimination [Citation110]. Recent findings thus far imply that repeated monitoring of (multiple) cardiac biomarkers may be a successful management technique for HF disease. Elevations of biomarkers detected during acute decompensations are related to an increased risk of negative clinical outcomes, such as HF hospitalization. Therefore, it would be informative to repeatedly evaluate biomarkers and more closely monitor CHF patients who have elevated levels throughout the early stages of acute decompensation and even in the absence of symptoms. This will help to minimize HF-related morbidity and mortality in an efficient manner.
5. Promising aspects of hemodynamic and biomarker monitoring
As was previously noted, HF is most often characterized by an increase in hemodynamic load, which causes the classical symptom of HF: fluid overload. Since fluid overload is the primary cause of hospitalizations for HF, the main goals of HF management are fluid status surveillance and early symptom monitoring. Physicians may monitor patient health and response to therapy outside of the clinical setting with the use of medical equipment like wearables and sensors, and they can intervene early if needed. It is anticipated that clinical monitoring of the fluid balance and intracardiac filling pressures would improve the management and prognosis of HF [Citation15]. Remote monitoring of the pressures with the CardioMEMS sensor is a new way of targeted filling pressures and one of the most important innovations to focus patient care outside the hospital [Citation15]. Such information can be valuable for biomarker research.
Before any novel biomarker can become a cornerstone of HF care, we are aware that these potential biomarkers need to be prospectively verified and thoroughly assessed. Results from cardiac biomarkers such as NP and TnTs should always be evaluated in light of other clinical indicators, such as hemodynamic information. By using remote monitoring devices, hemodynamic information can be linked for evaluation of biomarker data. It is established that changes in BNP or NT-proBNP levels throughout hospitalization and just before discharge significantly predict poor outcomes [Citation32,Citation109]. Given that HF treatments cause a decline in biomarker concentration that coincides with an improvement in outcome, it stands to reason that a strategy that includes a significant decline in biomarkers as part of the discharge criteria would be preferable to a strategy without such biomarker guidance. Additionally, by using this approach, a baseline is established for ongoing observation in the outpatient situation, where, for example, NP assessment may still be continued. It also enables individualizing decisions about follow-up timing, frequency, and intensity. For example, patients with a significant drop in NP concentration following acute HF treatment are more likely to experience a favorable early post-discharge course, than patients with higher or non-falling concentrations who may require close follow-up, including possible remote monitoring [Citation111]. By doing so, physicians will have more comprehensive knowledge of the HF condition and will get a deeper understanding of the pathological processes. Additionally, by optimizing the use of biomarkers, this enables the identification of patients at high risk and with a poor prognosis to focus on and may increase the survival rate of patients with CHF.
Several biomarkers mentioned in this review are studied in relation to their potential to direct HF therapy. For instance, meta-analyses have proved that, as compared to conventional therapy, NP- and BNP-guided treatment is linked with a substantial decrease in all-cause mortality and HF-related hospitalization in CHF patients [Citation112–114]. Although they have a great deal of potential to direct HF therapy, the current HF recommendations from the European Society of Cardiology have not yet adopted this strategy due to the paucity of conclusive data. This review attempts to highlight the necessity of repeated biomarker measurement, which might offer additional prognostic value by including hemodynamic data to better understand the study levels of biomarkers. This would offer essential knowledge for enhancing their potential in HF guided therapy.
6. Conclusion
There is an immediate urge to develop improved methods for predicting, screening, and prognosticating HF disease. Studying (circulating) biomarkers is the most simple and promising step toward HF tailored prognostic strategies as well as understanding the treatment response to HF drugs in CHF patients. This review aimed to discuss the potential of several novel and established biomarkers to prognosticate the course of HF disease. This might be the first step toward tailored biomarker-guided therapy, since it will not only show us which patients are at highest risk, but also provide us deeper understanding of the pathobiological processes driving HF. Moreover, persistently monitoring (multiple) cardiac biomarkers may be an effective management strategy for HF disease. By using remote monitoring devices which are now available, hemodynamic information can be linked for further refreshment and evaluation of biomarker data, which has the potential to further optimize HF treatments.
7. Expert opinion
The dynamic nature of the HF, which necessitates recurrent hospitalization for HF patients, makes management of the condition challenging. Moreover, with the rising life expectancy of the world’s population, it is expected that HF will remain a major public health concern. HF hospitalization and mortality are preceded by various factors reflecting elevated filling pressures [Citation14]. Therefore, it is anticipated that clinically monitoring the fluid balance and intracardiac filling pressures may improve the effectiveness of HF therapy.
In recent years, there has been a lot of attention in using biomarkers to diagnose, predict, and treat patients with HF. Studying serial biomarkers provides new insights into HF prognosis, response to treatment and fluid status in relation to daily hemodynamics in a patient, which can advance HF research and HF therapeutic targets in the future.
The use of (serial) biomarkers with the integration of hemodynamic feedback can be more beneficial in forecasting HF risk in the general population and in clarifying prognosis in patients with HF due to the complicated etiology and pathological process of HF disease. (Non)-invasive monitoring devices, like the CardioMEMS, have a potential in HF prognostication and risk stratification and could increase clinical efficacy to aid health care professionals [Citation15]. High levels of type III procollagen peptide (P3P), for instance, may indicate hepatic failure due to volume overload during the acute stage of HF, indicating its potential for use as a predictive biomarker [Citation115]. Such information can be valuable for biomarker research. The intriguing aspects of hemodynamic feedback data integration, however, still needs to be further confirmed in a future study.
By early detection of patients who are at a high risk of experiencing an HF-related incident, biomarker monitoring has the potential to reduce the total hospital burden of HF. Since the development of congestion leading to HF decompensation is a potent predictor of a poor patient prognosis, it is critical to more effectively identify and track congestion [Citation14,Citation15,Citation116]. To improve the prognostic utility of already existing biomarker models, this can be achieved, for instance, by focusing on the monitoring of biomarkers that can forecast congestion such as bioactive adrenomedullin (bio-ADM) [Citation117,Citation118]. In addition, compared to (non)-invasive monitoring which may be saved for patients who most need intense advanced HF monitoring, biomarker monitoring is also a reasonably easy and affordable approach. Important progress has been made in the identification of high-risk HF patients. Well-established and commonly used simple risk biomarkers, such as NT-proBNP and troponins, already have a predictive accuracy, while novel biomarkers and the incorporation of hemodynamic feedback are allowing for a more precise prediction of the course of HF disease. However, there still exist important challenges. More research is needed to improve the understanding on how novel biomarkers should be integrated into the prognostication and risk stratification of HF disease. Future research must also develop methods to integrate the repeated serial measurement of biomarkers. Overall, the future of HF patient management lies in a biomarker-guided monitoring approach where the ability to identify high-risk patients is improved. This approach will help to ensure that the relatively small subgroup of HF patients who are most likely to benefit is given the limited and potentially expensive monitoring resources.
Article highlights
Heart failure (HF) is a major contributor to health difficulties including congestion that are associated with increased risk of morbidity and mortality.
By studying biomarkers, we get more insight into the pathobiological pathways that are affected by HF.
Several novel and established biomarkers have the potential to predict the risk of HF-related events and to guide in risk stratification of HF.
Repeated monitoring of (multiple) serial cardiac biomarkers may be a successful management approach for HF disease.
(Non-)invasive monitoring devices, like the cardioMEMS sensor, have a potential in HF prognostication and risk stratification and could increase clinical efficacy to aid health care professionals.
The next step in HF biomarker research for prognostication and risk stratification of HF, is to proceed from a single baseline marker to multiple serial biomarkers with integrated hemodynamic connection of data.
Declaration of interest
JJ Brugts has received research grant and speaker fees from Abbott. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovascular Research. 2022 Feb; p. 12.
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016 Jun;13(6):368–378.
- Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol. 2014 Feb 15;171(3):368–376.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: executive Summary: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):E876–E894.
- Fonarow GC, Stevenson LW, Peacock WF, et al. Admission B-type natriuretic peptide levels in acute decompensated heart failure predict in-hospital mortality: an analysis of 48,629 hospitalizations in ADHERE. J Card Fail. 2005 Aug;11(6):S167–S167.
- Doust JA, Pietrzak E, Dobson A, et al. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. Br Med J. 2005 Mar 19;330(7492):625–627.
- Masson S, Latini R, Anand IS, et al. Direct comparison of B-Type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (Val-HeFT) data. Clin Chem. 2006 Aug;52(8):1528–1538.
- Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. New Engl J Med. 2004 Feb 12;350(7):655–663.
- Aimo A, Januzzi JL Jr., Vergaro G, et al. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: an Individual Patient Data Meta-Analysis. Circulation. 2018 Jan 16;137(3):286–297.
- Aimo A, Vergaro G, Passino C, et al. Prognostic Value of Soluble Suppression of Tumorigenicity-2 in Chronic Heart Failure: a Meta-Analysis. JACC Heart Fail. 2017 Apr;5(4):280–286.
- Kempf T, von Haehling S, Peter T, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007 Sep 11;50(11):1054–1060.
- French B, Wang L, Ky B, et al. Prognostic Value of Galectin-3 for Adverse Outcomes in Chronic Heart Failure. J Card Fail. 2016 Apr;22(4):256–262.
- Imran TF, Shin HJ, Mathenge N, et al. Meta-Analysis of the Usefulness of Plasma Galectin-3 to Predict the Risk of Mortality in Patients With Heart Failure and in the General Population. Am J Cardiol. 2017 Jan 1;119(1):57–64.
- Stevenson LW, Zile M, Bennett TD, et al. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 2010 Sep;3(5):580–587.
- Brugts JJ, Radhoe SP, Aydin D, et al. Clinical Update of the Latest Evidence for CardioMEMS Pulmonary Artery Pressure Monitoring in Patients with Chronic Heart Failure: a Promising System for Remote Heart Failure Care. Sensors (Basel), Vol. 21. 2021. p. 7.
- Metra M, Cotter G, Gheorghiade M, et al. The role of the kidney in heart failure. Eur Heart J. 2012 Sep;33(17):2135–+.
- Mele D, Nardozza M, Ferrari R. Left ventricular ejection fraction and heart failure: an indissoluble marriage? Eur J Heart Fail. 2018 Mar;20(3):427–430.
- Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017 Jan;14(1):30–38.
- Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci. 2016 Jan 1;130(2):57–77.
- Metra M, Teerlink JR. Heart failure. Lancet. 2017 Oct 28;390(10106):1981–1995.
- Francis GS, Benedict C, Johnstone DE, et al. Comparison of Neuroendocrine Activation in Patients with Left-Ventricular Dysfunction with and without Congestive-Heart-Failure - a Substudy of the Studies of Left-Ventricular Dysfunction (Solvd). Circulation. 1990 Nov;82(5):1724–1729.
- Gardner RS, Ozalp F, Murday AJ, et al. N-terminal pro-brain natriuretic peptide - A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003 Oct;24(19):1735–1743.
- Hartmann F, Packer M, Coats AJS, et al. NT-proBNP in severe chronic heart failure: rationale, design and preliminary results of the COPERNICUS NT-proBNP substudy. Eur J Heart Fail. 2004 Mar 15;6(3):343–350.
- Groenning BA, Raymond I, Hildebrandt PR, et al. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma N-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population. Heart. 2004 Mar 1;90(3):297–303.
- Savarese G, Musella F, D’Amore C, et al. Changes of Natriuretic Peptides Predict Hospital Admissions in Patients With Chronic Heart Failure A Meta-Analysis. Jacc-Heart Fail. 2014 Apr;2(2):148–158
- Bettencourt P, Azevedo A, Pimenta J, et al. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004 Oct 12;110(15):2168–2174.
- Mohebi R, Murphy S, Jackson L, et al. Biomarker prognostication across Universal Definition of Heart Failure stages. ESC Heart Fail. 2022 Dec;9(6):3876–3887.
- Bracun V, van Essen B, Voors AA, et al. Insulin-like growth factor binding protein 7 (IGFBP7), a link between heart failure and senescence. Esc Heart Failure. 2022 Sep; p. 11.
- Piatek K, Feuerstein A, Zach V, et al. Nitric oxide metabolites: associations with cardiovascular biomarkers and clinical parameters in patients with HFpEF. ESC Heart Fail. 2022 Dec;9(6):3961–3972.
- Konev AA, Kharitonov AV, Rozov FN, et al. CT-IGFBP-4 as a novel prognostic biomarker in acute heart failure. ESC Heart Fail. 2020 Apr;7(2):434–444.
- Zile MR, Claggett BL, Prescott MF, et al. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J Am Coll Cardiol. 2016 Dec 6;68(22):2425–2436.
- Anand IS, Fisher LD, Chiang YT, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation. 2003 Mar 11;107(9):1278–1283.
- Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002 May 21;105(20):2392–2397.
- Seronde MF, Gayat E, Logeart D, et al. Comparison of the diagnostic and prognostic values of B-type and atrial-type natriuretic peptides in acute heart failure. Int J Cardiol. 2013 Oct 9;168(4):3404–3411.
- von Haehling S, Jankowska EA, Morgenthaler NG, et al. Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in predicting survival in patients with chronic heart failure. J Am Coll Cardiol. 2007 Nov 13;50(20):1973–1980.
- Moertl D, Berger R, Struck J, et al. Comparison of Midregional Pro-Atrial and B-Type Natriuretic Peptides in Chronic Heart Failure Influencing Factors, Detection of Left Ventricular Systolic Dysfunction, and Prediction of Death. J Am Coll Cardiol. 2009 May 12;53(19):1783–1790.
- von Haehling S, Filippatos GS, Papassotiriou J, et al. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail. 2010 May;12(5):484–491.
- Adlbrecht C, Hulsmann M, Strunk G, et al. Prognostic value of plasma midregional pro-adrenomedullin and C-terminal-pro-endothelin-1 in chronic heart failure outpatients. Eur J Heart Fail. 2009 Apr;11(4):361–366.
- Feng ZK, Akinrimisi OP, Gornbein JA, et al. Combination Biomarkers for Risk Stratification in Patients With Chronic Heart Failure Biomarkers Prognostication in HF. J Card Fail. 2021 Dec;27(12):1321–1327.
- Peet C, Ivetic A, Bromage DI, et al. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020 May 1;116(6):1101–1112.
- Chapman AR, Adamson PD, Mills NL. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart. 2017 Jan;103(1):10–18.
- Bel MS, Soldevila JG, Llanos JO. Biological markers of myocardial necrosis. Rev Esp Cardiol. 2003 Jul;56(7):703–720.
- McEvoy JW, Chen Y, Ndumele CE, et al. Six-Year Change in High-Sensitivity Cardiac Troponin T and Risk of Subsequent Coronary Heart Disease, Heart Failure, and Death. JAMA Cardiol. 2016 Aug 1;1(5):519–528.
- Gravning J, Askevold ET, Nymo SH, et al. Prognostic effect of high-sensitive troponin T assessment in elderly patients with chronic heart failure: results from the Corona trial. Circ Heart Fail. 2014 Jan;7(1):96–103.
- Myhre PL, O’Meara E, Claggett BL, et al. Cardiac Troponin I and Risk of Cardiac Events in Patients With Heart Failure and Preserved Ejection Fraction. Circ Heart Fail. 2018;Nov;11(11):e005312.
- Yan I, Borschel CS, Neumann JT, et al. High-Sensitivity Cardiac Troponin I Levels and Prediction of Heart Failure: results From the BiomarCaRE Consortium. JACC Heart Fail. 2020 May;8(5):401–411.
- deFilippi CR, de Lemos JA, Christenson RH, et al. Association of Serial Measures of Cardiac Troponin T Using a Sensitive Assay With Incident Heart Failure and Cardiovascular Mortality in Older Adults. Jama-J Am Med Assoc. 2010 Dec 8;304(22):2494–2502.
- Cohn JN, Ferrari R, Sharpe N, et al. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000 Mar 1;35(3):569–582.
- Burchfield JS, Xie M, Hill JA. Pathological Ventricular Remodeling: mechanisms: part 1 of 2. Circulation. 2013 Jul 23;128(4):388–400.
- Mueller T, Jaffe AS. Soluble ST2–analytical considerations. Am J Cardiol. 2015 Apr 2;115(7 Suppl):8B–21B.
- Emdin M, Aimo A, Vergaro G, et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J Am Coll Cardiol. 2018 Nov 6;72(19):2309–2320.
- Pacho C, Domingo M, Nunez R, et al. Predictive biomarkers for death and rehospitalization in comorbid frail elderly heart failure patients. BMC Geriatr. 2018 May 9;18(1):109.
- Gaggin HK, Szymonifka J, Bhardwaj A, et al. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. 2014 2;Feb(1):65–72.
- Wesseling M, Jhc DP, de Jager SCA. Growth differentiation factor 15 in adverse cardiac remodelling: from biomarker to causal player. ESC Heart Fail. 2020 Aug;7(4):1488–1501.
- Fernandez ABM, Ferrero-Gregori A, Garcia-Osuna A, et al. Growth differentiation factor 15 as mortality predictor in heart failure patients with non-reduced ejection fraction. ESC Heart Fail. 2020 Oct;7(5):2223–2229.
- Luo JW, Duan WH, Song L, et al. A Meta-Analysis of Growth Differentiation Factor-15 and Prognosis in Chronic Heart Failure. Front Cardiovasc Med.2021 Nov;5:8.
- de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010 Mar;7(1):1–8.
- de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011 Feb;43(1):60–68.
- Meijers WC, Januzzi JL, deFilippi C, et al. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: a pooled analysis of 3 clinical trials. Am Heart J. 2014 Jun;167(6):853–U117.
- Wu C, Lv Z, Li X, et al. Galectin-3 in Predicting Mortality of Heart Failure: a Systematic Review and Meta-Analysis. Heart Surg Forum. 2021 Mar 30;24(2):E327–E332.
- Barutaut M, Fournier P, Peacock WF, et al. sST2 adds to the prognostic value of Gal-3 and BNP in chronic heart failure. Acta Cardiol. 2020 Dec;75(8):739–747.
- Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015 Jun 14;36(23):1437–+.
- Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure - Systematic review and meta-analysis. J Am Coll Cardiol. 2006 May 16;47(10):1987–1996.
- Angelidis C, Deftereos S, Giannopoulos G, et al. Cystatin C: an Emerging Biomarker in Cardiovascular Disease. Curr Top Med Chem. 2013 Jan;13(2):164–179.
- van der Laan SW, Fall T, Soumare A, et al. Cystatin C and Cardiovascular Disease A Mendelian Randomization Study. J Am Coll Cardiol. 2016 Aug 30;68(9):934–945.
- Dupont M, Wu YP, Hazen SL, et al. Cystatin C Identifies Patients With Stable Chronic Heart Failure at Increased Risk for Adverse Cardiovascular Events. Circ-Heart Fail. 2012 Sep;5(5):602–609
- Wang CH, Han S, Tong F, et al. Predictive Value of the Serum Cystatin C/Prealbumin Ratio in Combination With NT-proBNP Levels for Long-Term Prognosis in Chronic Heart Failure Patients: a Retrospective Cohort Study. Front Cardiovasc Med. Vol. 14. 2021 Jul; p. 8.
- Bayes-Genis A, Morant-Talamante N, Neprilysin LJ. Natriuretic Peptide Regulation in Heart Failure. Curr Heart Fail Rep. 2016 Aug;13(4):151–157.
- Bayes-Genis A, Barallat J, Galan A, et al. Soluble Neprilysin Is Predictive of Cardiovascular Death and Heart Failure Hospitalization in Heart Failure Patients. J Am Coll Cardiol. 2015 Feb 24;65(7):657–665.
- Gommans DHF, Revuelta-Lopez E, Lupon J, et al. Soluble Neprilysin and Corin Concentrations in Relation to Clinical Outcome in Chronic Heart Failure. Jacc-Heart Fail. 2021 Feb;9(2):85–95
- Damman K, van Veldhuisen DJ, Navis G, et al. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;Oct;10(10):997–1000.
- Villacorta H, Santos RA, Marroig MA, et al. Prognostic value of plasma neutrophil gelatinase-associated lipocalin in patients with heart failure. Rev Port Cardiol. 2015 Jul-Aug;34(7–8):473–478.
- Bolignano D, Basile G, Parisi P, et al. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res. 2009 Feb;12(1):7–14.
- Mann DL. The Emerging Role of Innate Immunity in the Heart and Vascular System For Whom the Cell Tolls. Circ Res. 2011 Apr 29;108(9):1133–U201.
- Adamo L, Rocha-Resende C, Prabhu SD, et al. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020 May;17(5):269–285.
- Gowda SGB, Gowda D, Hou F, et al. Temporal lipid profiling in the progression from acute to chronic heart failure in mice and ischemic human hearts. Atherosclerosis. 2022;363:30–41.
- Vizzardi E, Nodari S, D’Aloia A, et al. CA 125 tumoral marker plasma levels relate to systolic and diastolic ventricular function and to the clinical status of patients with chronic heart failure. Echocardiography. 2008 Oct;25(9):955–960.
- D’Aloia A, Faggiano P, Aurigemma G, et al. Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short-term prognosis. J Am Coll Cardiol. 2003 May 21;41(10):1805–1811.
- Nagele H, Bahlo M, Klapdor R, et al. CA 125 and its relation to cardiac function. Am Heart J. 1999 Jun;137(6):1044–1049.
- Anand IS, Latini R, Florea VG, et al. C-reactive protein in heart failure - Prognostic value and the effect of valsartan. Circulation. 2005 Sep 6;112(10):1428–1434.
- Pellicori P, Zhang J, Cuthbert J, et al. High-sensitivity C-reactive protein in chronic heart failure: patient characteristics, phenotypes, and mode of death. Cardiovasc Res. 2020 Jan 1;116(1):91–100.
- Traxler D, Zimmermann M, Simader E, et al. The inflammatory markers sST2, HSP27 and hsCRP as a prognostic biomarker panel in chronic heart failure patients. Clin Chim Acta. 2020;510:507–514.
- Gonzalez GE, Rhaleb NE, D’Ambrosio MA, et al. Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin II-high salt-induced hypertension. J Hypertens. 2015 Jan;33(1):144–152.
- Fontes JA, Rose NR, Cihakova D. The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine. 2015 Jul;74(1):62–68.
- Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998 Feb;31(2):391–398.
- TorreAmione G, Kapadia S, Benedict C, et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the studies of left ventricular dysfunction (SOLVD). Journal of the American College of Cardiology. 1996 Apr;27(5):1201–1206.
- Kubota T, Miyagishima M, Alvarez RJ, et al. Expression of proinflammatory cytokines in the failing human heart: comparison of recent-onset and end-stage congestive heart failure. J Heart Lung Transpl. 2000 Sep;19(9):819–824.
- MacGowan GA, Mann DL, Kormos RL, et al. Circulating interleukin-6 in severe heart failure. Am J Cardiol. 1997 Apr 15;79(8):1128.
- Gwechenberger M, Hulsmann M, Berger R, et al. Interleukin-6 and B-type natriuretic peptide are independent predictors for worsening of heart failure in patients with progressive congestive heart failure. J Heart Lung Transpl. 2004 Jul;23(7):839–844.
- Held C, White HD, Stewart RAH, et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc. 2017;6(10):Oct.
- Chia YC, Kieneker LM, van Hassel G, et al. Interleukin 6 and Development of Heart Failure With Preserved Ejection Fraction in the General Population. J Am Heart Assoc. 2021;10:11.
- Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019 Aug;21(8):965–973.
- Eidizadeh A, Schnelle M, Leha A, et al. Biomarker profiles in heart failure with preserved vs. reduced ejection fraction: results from the DIAST-CHF study. ESC Heart Fail. 2022 Oct 2;10(1).
- van der Pol A, van Gilst WH, Voors AA, et al. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019 Apr;21(4):425–435.
- Frangogiannis NG. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ Res. 2019 Jun 21;125(1):117–146.
- Myeloperoxidase NG. - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta. 2019 Jun;493:36–51.
- Tang WHW, Tong W, Troughton RW, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007 Jun 19;49(24):2364–2370.
- Tang WHW, Brennan ML, Philip K, et al. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol. 2006 Sep 15;98(6):796–799.
- Bouwens E, Schuurman AS, Akkerhuis KM, et al. Associations of serially measured PCSK9, LDLR and MPO with clinical outcome in heart failure. Biomark Med. 2021 Jan;15(4):247–255.
- Avaliani T, Talakvadze T, Tabagari S. Prognostic Value of Plasma Myeloperoxidase Level’s and Echocardiographic Determinants in Chronic Heart Failure Patients. Georgian Med News. 2019 Mar;288:55–60.
- Tang WH, Shrestha K, Troughton RW, et al. Integrating plasma high-sensitivity C-reactive protein and myeloperoxidase for risk prediction in chronic systolic heart failure. Congest Heart Fail. 2011 May-Jun;17(3):105–109.
- Deardorff R, Spinale FG. Cytokines and matrix metalloproteinases as potential biomarkers in chronic heart failure. Biomark Med. 2009 Oct;3(5):513–523.
- Jordan A, Roldan V, Garcia M, et al. Matrix metalloproteinase-1 and its inhibitor, TIMP-1, in systolic heart failure: relation to functional data and prognosis. J Intern Med. 2007 Sep;262(3):385–392.
- George J, Patal S, Wexler D, et al. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am Heart J. 2005 Sep;150(3):484–487.
- Chang YY, Chen AR, Wu XM, et al. Comparison the Prognostic Value of Galectin-3 and Serum Markers of Cardiac Extracellular Matrix Turnover in Patients with Chronic Systolic Heart Failure. Int J Med Sci. 2014;11(11):1098–1106.
- Liu Y, Wang X, Pan X, et al. Prognostic value of plasma sAXL in patients with heart failure: insights from the DRAGON-HF trial. ESC Heart Fail. 2022 Nov; p. 27.
- Rincon LM, Rodriguez-Serrano M, Conde E, et al. Serum microRNAs are key predictors of long-term heart failure and cardiovascular death after myocardial infarction. ESC Heart Fail. 2022 Oct;9(5):3367–3379.
- Roy C, Lejeune S, Slimani A, et al. Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2020 Oct;7(5):2494–2507.
- van Boven N, Battes LC, Akkerhuis KM, et al. Toward personalized risk assessment in patients with chronic heart failure: detailed temporal patterns of NT-proBNP, troponin T, and CRP in the Bio-SHiFT study. Am Heart J. 2018 Feb;196:36–48.
- Gurgoze MT, van Vark LC, Baart SJ, et al. Multimarker Analysis of Serially Measured GDF-15, NT-proBNP, ST2, GAL-3, cTnI, Creatinine, and Prognosis in Acute Heart Failure. Circ Heart Fail. 2022 Nov 21;16(1):e009526.
- Maisel A, Januzzi J, Xue Y, et al. Post-acute care: the role of natriuretic peptides. Congest Heart Fail. 2012;Sep-Oct;18(Suppl 1):S14–6.
- Felker GM, Hasselblad V, Hernandez AF, et al. Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J. 2009 Sep;158(3):422–430.
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J. 2014 Jun 14;35(23):1559–1567.
- Savarese G, Trimarco B, Dellegrottaglie S, et al. Natriuretic peptide-guided therapy in chronic heart failure: a meta-analysis of 2,686 patients in 12 randomized trials. PLoS One. 2013;8(3):e58287.
- Shirakabe A, Okazaki H, Matsushita M, et al. Type III procollagen peptide level can indicate liver dysfunction associated with volume overload in acute heart failure. ESC Heart Fail. 2022 Jun;9(3):1832–1843.
- Boorsma EM, JM TM, Damman K, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nature Reviews Cardiology. 2020 Oct;17(10):641–655
- Egerstedt A, Czuba T, Bronton K, et al. Bioactive adrenomedullin for assessment of venous congestion in heart failure. ESC Heart Fail. 2022 Oct;9(5):3543–3555.
- Nunez J, De la Espriella R, Rossignol P, et al. Congestion in heart failure: a circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Euro J Heart Fail. 2022 Sep 7;24(10).
