ABSTRACT
Background
Little is known about the duration of antibiotic use in hospital settings. We evaluated the duration of hospital antibiotic therapy for four commonly prescribed antibiotics (amoxicillin, co-amoxiclav, doxycycline, and flucloxacillin) including the assessment of COVID-19 impact.
Methods
A repeated, cross-sectional study using the Hospital Electronic Prescribing and Medicines Administration system (January/2019-March/2022). Monthly median duration of therapy/duration categories was calculated, stratified by routes of administration, age, and sex. The impact of COVID-19 was assessed using segmented time-series analysis.
Results
There were significant variations in the median duration of therapy across routes of administration (P < 0.05), with the highest value among those antibiotic courses composed of both oral and IV antibiotics (‘Both’ group). Significantly higher proportions of prescriptions within the ‘Both’ group had a duration of >7 days compared to oral or IV. The duration of therapy differed significantly by age. Some small statistically significant changes in the level/trends of duration of therapy were observed in the post-COVID-19 period.
Conclusions
No evidence for prolonged duration of therapy were observed, even during COVID-19 pandemic. The duration of IV therapy was relatively short, suggesting timely clinical review and consideration of IV to oral switch. Longer duration of therapy was observed among older patients.
1. Introduction
Antimicrobial resistance (AMR) is a global public health threat, complicating the treatment of many infections and leading to longer duration of illness, higher mortality, and increased treatment costs [Citation1]. Unaddressed AMR is predicted to result in 10 million preventable deaths worldwide by 2050, with consequent economic costs of US$100 trillion [Citation2]. Inappropriate uses of antimicrobials, including both overuse and unnecessary prolonged courses of treatment, are modifiable drivers for AMR and as such are a key focus for antimicrobial stewardship programmes (ASPs) [Citation3]. ASPs have been challenged through the COVID-19 pandemic not only because of the negative impact of the pandemic on ASP activities [Citation4] but also due to the increased likelihood of prescribing common respiratory antibiotics in COVID pneumonitis [Citation5]. A recent meta-analysis reported a high prevalence (61.7%) of antibiotic use among hospitalized COVID-19 patients despite low prevalence (5.6%) of bacterial coinfection [Citation6].
A point prevalence survey of antibiotic prescribing in patients with suspected and/or confirmed COVID-19 in Scottish hospitals in 2020 provided data on antibiotic indication, route of administration, and timing of IV to oral switch and proposed duration of therapy but did not provide information on the total duration of inpatient antibiotic exposure [Citation7]. In this survey, antibiotics were most frequently prescribed for suspected respiratory tract infections (RTIs) (73.9%), whereby amoxicillin, doxycycline, and co-amoxiclav collectively accounted for over half of all antibiotics prescribed on the survey day [Citation7].
Antibiotic course duration is a key focus for ASP activity [Citation8] particularly since shorter course therapy is safe and effective for many common infections including lower RTI [Citation9]. Using shorter recommended antibiotic duration is important to minimize the risk of both AMR development and antibiotic related adverse events [Citation10–12]. Despite the importance of antibiotic duration in relation to AMR, data on antibiotic duration in hospital settings are scarce, especially in the COVID-19 era. This study aimed to evaluate the duration of antibiotic therapy across hospitals in Scotland for four commonly prescribed antibiotics, namely amoxicillin, co-amoxiclav, doxycycline, and flucloxacillin (as a proxy for assessing the quality of use of these antibiotics), including the assessment of any impact of COVID-19.
2. Methods
2.1. Study design and data source
A repeated, cross-sectional study was conducted using individual-patient level data from the Hospital Electronic Prescribing and Medicines Administration (HEPMA) system [Citation13] from six out of the 14 health boards in Scotland, covering about 65% (3.58 million out of 5.46 million) of the Scottish population from January 2019 to March 2022. The study period was divided into a pre-COVID-19 period (January 2019 to Febebruary 2020) and a post-COVID-19 period (April 2020 to March 2022). HEPMA contains prescriptions and administrations data for all medicines prescribed for in-patients in acute hospitals including dates (prescribed and administered), drug names, formulations, dosages instructions, and routes of administration.
2.2. Study population/subjects
The study subjects comprised all prescriptions for the four antibiotics of interest that were prescribed and administered (for systemic therapy) to patients admitted to hospital (for any reason) during the study period. The date of first administration of any of the antibiotics of interest was defined as the index date for calculating duration of therapy. We have selected amoxicillin, co-amoxiclav, and doxycycline as they are the most commonly used antibiotics for RTIs in Scottish hospitals. As this includes any potential use for bacterial co-infection in COVID-19 patients, these three drugs work as a reasonable indicator for any potential impact of COVID-19. Flucloxacillin was used as a control because it is rarely indicated for RTIs and hence unlikely to be affected by COVID-19 infection.
2.3. Study outcomes
The primary study outcome was duration of antibiotic therapy, calculated by counting the total days between the index date and last administration date for each antibiotic; an antibiotic with a single dose was assigned a duration of one day. Furthermore, we estimated the proportion of antibiotic prescriptions with the following categories of duration: ≤3 days, >3-5 days, >5-7 days, >7-10 days, and >10 days for amoxicillin, co-amoxiclav, and flucloxacillin and ≤5 days, >5-7 days, >7-10 days, and >10 days for doxycycline. The study outcomes were stratified by sex, age groups, and routes of administration (oral, IV, and ‘Both’) where appropriate. ‘Both’ refers to those courses in which the antibiotic was administered both as IV and oral in any subsequent order, although the majority were IV to oral switch. Doxycycline is only licensed in the UK for oral route administration.
2.4. Data analysis
Duration of therapy was summarized as median (interquartile range, IQR), while categories of duration of therapy were summarized as frequencies and percentages. Median duration of therapy was compared across routes of administration, age groups, and sex using the non-parametric statistical test Kruskal-Wallis [Citation14] and Wilcoxon signed-rank tests [Citation15], respectively, whereas chi-square test was used for the duration of therapy categories.
In order to assess the impact of the first major COVID-19 lockdown (imposed in March 2020), segmented time-series analysis [Citation16] was applied to the monthly median duration of therapy and proportion of prescriptions with >7 days duration (as a proxy for unnecessary prolonged course for most common RTIs), stratified by route of administration (oral, IV, and ‘Both’), age groups, and sex. The baseline trends (β1) in the pre-COVID-19 period and any changes in the levels (β2) and trends in the post-COVID-19 period (β3) following the first COVID-19 lockdown were assessed and presented.
3. Results
3.1. Baseline characteristics
Overall, there were 186,061 prescriptions of the four antibiotics issued for a total of 143,951 patients; amoxicillin accounted for the largest number of prescriptions (39.4%; n = 73,311) followed by co-amoxiclav (30.1%; n = 55,963), with IV route as the mostly commonly used route of administration for each of amoxicillin, co-amoxiclav, and flucloxacillin (). Patients’ mean age differed significantly across the three routes of administration types for amoxicillin, co-amoxiclav, and flucloxacillin (). For amoxicillin and doxycycline, >40% of patients were >70 years except for IV co-amoxiclav and flucloxacillin (). Similarly, sex distributions were significantly different across routes of administration for the four antibiotics ().
Table 1. Baseline characteristics of the 143,951 hospitalized patients who received the 186,061 prescriptions for the four studied antibiotics from January 2019 to March 2022 in Scotland.
3.2. Duration of therapy
Median duration of therapy (days) for amoxicillin, co-amoxiclav, and flucloxacillin prescriptions were significantly different (P < 0.05) across their routes of administration types, with the highest median duration of therapy observed in the ‘Both’ group compared to the lowest median duration in the IV route group (). However, the pattern of median duration of therapy for a particular route across each of amoxicillin, co-amoxiclav, and flucloxacillin was comparable; for example, median duration of therapy for ‘Both’ groups was 5.2 (IQR: 3.2, 6.8), 5.1 (IQR: 2.8, 7.0), and 5.8 (IQR: 3.2, 9.0) days for amoxicillin, co-amoxiclav, and flucloxacillin, respectively, and the IV route consistently had the lowest median duration of therapy (). In terms of duration of therapy categories, again there was a statistically significant difference between the three types of routes for each of amoxicillin, co-amoxiclav, and flucloxacillin (). Similar to the median duration of therapy, a comparable pattern was observed with the duration of therapy categories: the majority of the oral prescriptions for amoxicillin, co-amoxiclav, and flucloxacillin were for ≤3 days (57.6% vs. 65.6% vs. 54.4%, respectively) and similar was seen in the IV route (75.6% vs. 85.5% vs. 68.3%, respectively). However, for the ‘Both’ group, a higher proportion were for a longer duration (>7 days) in comparison to the oral and IV routes for each of amoxicillin, co-amoxiclav, and flucloxacillin (). Noticeably, the proportion of prescriptions with a duration category of >10 days for the ‘Both’ group was significantly higher for flucloxacillin prescriptions (19.7%) compared with each of amoxicillin (4.8%) and co-amoxiclav (6.7%) (). For doxycycline prescriptions, the median duration of therapy was 2.4 days (IQR = 1, 4.7) with the majority of prescriptions falling into the duration category of ≤5 days (79.3%) ().
Table 2. Duration of therapy for the 186,061 prescriptions of the four studied antibiotics from January 2019 to March 2022 in Scotland, stratified by routes of administration (when appropriate).
3.3. Duration of therapy stratified by age groups and sex
Stratifying the median duration of therapy by age groups indicated a statistically significant difference in median duration (). There was a consistent increasing trend in median duration of therapy as age increases for all the four antibiotics, with the longest median duration of therapy observed in those aged ≥71 years old (). In terms of sex, median duration of therapy did not differ significantly between male and female patients except for IV amoxicillin (1.4 vs. 1.3), ‘Both’ co-amoxiclav (5.5 vs. 4.8), and IV and ‘Both’ flucloxacillin (1.2 vs 1.6; 5.8 vs. 6.0, respectively) ().
Table 3. Median duration of therapy (IQR) for the 186,061 prescriptions of the four studied antibiotics from January 2019 to March 2022 in Scotland, stratified by routes of administration (when appropriate).
In terms of duration categories, there was a statistically significant variation in the duration categories across the different age groups (). Although the majority of antibiotic courses has a duration of ≤3 days, there was a trend pattern toward increasing duration of therapy in the older age groups, especially those aged ≥71 years (i.e. as age increased, the proportion of antibiotic courses with a duration of >3 days increased), albeit mostly still within ≤7 days (). This was more pronounced in the ‘Both’ group across each of amoxicillin, co-amoxiclav, and flucloxacillin. Also of note, the proportion of antibiotic courses from oral and IV routes with a duration of ≥7 days was higher among those aged >71 years in comparison to the other age groups (). Similarly, the majority of doxycycline prescriptions were for ≤5 days across the different age groups (85.2% vs. 73.9% in <18 and >80 years old, respectively); however, there was still a clear trend toward increasing duration of therapy with increasing age, even though mostly still within the ≤7 days duration (). In terms of sex, duration categories differed significantly between male and female patients for all the four studied antibiotics except for ‘Both’ amoxicillin, oral co-amoxiclav, and oral flucloxacillin, with the general pattern appearing to be a significantly higher duration of therapy among men compared to women ().
Table 4. Categories of duration of therapy for the 186,061 prescriptions of the four studied antibiotics from January 2019 to March 2022 in Scotland, stratified by routes of administration (when appropriate): A-Amoxicillin; B-Co-amoxiclav; C-Flucloxacillin, and D-Doxycycline.
3.4. Impact of COVID-19 on the duration of antibiotic therapy
Prior to COVID-19, there were statistically non-significant changes (both increasing and decreasing) in the baseline trends for all four antibiotics except for amoxicillin (,). Immediately after the COVID-19 lockdown in March 2020, statistically significant changes (toward increasing duration of therapy) in the level were observed only in amoxicillin ‘Both’ (β2: 0.4, P < 0.05) and flucloxacillin IV (β2: 0.36, P < 0.05). Similarly, statistically non-significant changes were observed in the trend post-COVID-19 lockdown for all four antibiotics except for amoxicillin ‘Both’ (β3: 0.05, P < 0.05) (, ). When stratified by sex, similarly, there were statistically non-significant changes except among female patients for amoxicillin ‘Both’ (β2: 0.56, P < 0.05; β3: 0.07, P < 0.05) and male patients for flucloxacillin ‘Both’ (β3: −0.13, P < 0.05) (, Appendix 1). In terms of stratification by age groups, statistically significant changes were observed only in the level immediately after COVID-19 lockdown among those aged 61–70 (β2: 1.6, P < 0.05), 71–80 (β2: 0.8, P < 0.05), and >80 years (β2: 0.7, P < 0.05), respectively, for those receiving IV flucloxacillin and those aged 61–70 years (β2: −0.7, P < 0.05) for doxycycline (Appendix 2, Supplementary file 1, Supplementary file 2, Supplementary file 3, Supplementary file 4). Furthermore, statistically significant changes were observed in the trends after COVID-19 among those aged 41–50 (β3: −0.04, P < 0.05), >80 years old (β3: −0.02, P < 0.05), respectively, for those received IV amoxicillin, 18–40 years (β3: 0.1, P < 0.05) for those within ‘Both’ amoxicillin, 41–50 years old (β3: 0.05, P < 0.05) within oral co-amoxiclav (Appendix 2, Supplementary file 1, Supplementary file 2, Supplementary file 3, Supplementary file 4).
Figure 1. Median duration of therapy overtime for the 186,061 prescriptions of the four studied antibiotics from January 2019 to March 2022 in Scotland, stratified by routes of administration (when appropriate): A-Amoxicillin; B-Co-amoxiclav; C-Flucloxacillin, and D-Doxycycline.
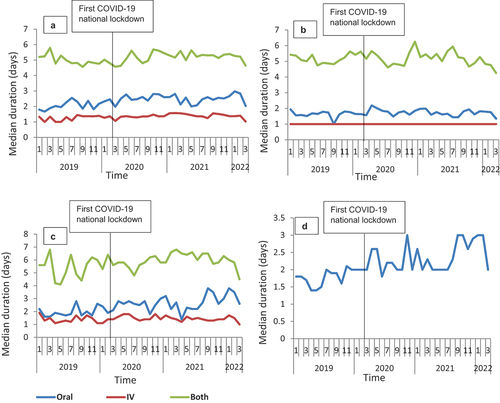
Figure 2. Median duration of therapy overtime for the three routes of administration for amoxicillin from January 2019 to March 2022 in Scotland, stratified by sex: A-Oral; B-IV; C-Both.
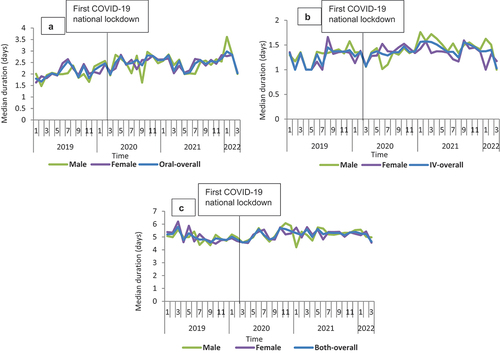
Figure 3. Median duration of therapy overtime for the three routes of administration for co-amoxiclav from January 2019 to March 2022 in Scotland, stratified by sex: A-Oral; B-IV; C-Both.
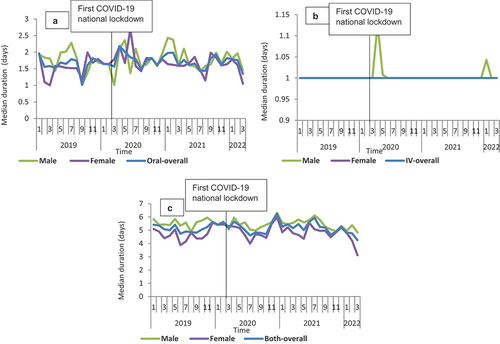
Figure 4. Median duration of therapy overtime for the three routes of administration for flucloxacillin from January 2019 to March 2022 in Scotland, stratified by sex: A-Oral; B-IV; C-Both.
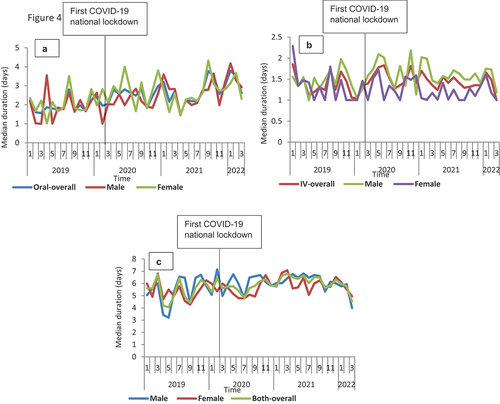
Figure 5. Median duration of therapy overtime for doxycycline from January 2019 to March 2022 in Scotland, stratified by sex.
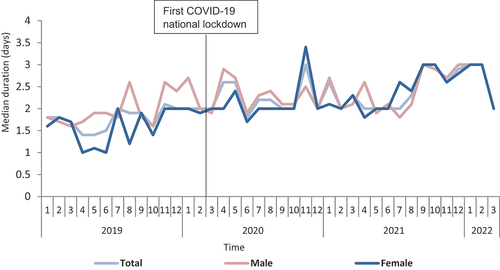
Table 5. Segmented regression analysis on the monthly median duration of therapy and proportion of prescriptions with >7 days duration for the 186,061 prescriptions of the four studied antibiotics from January 2019 to March 2022 in Scotland, stratified by routes of administration (when appropriate).
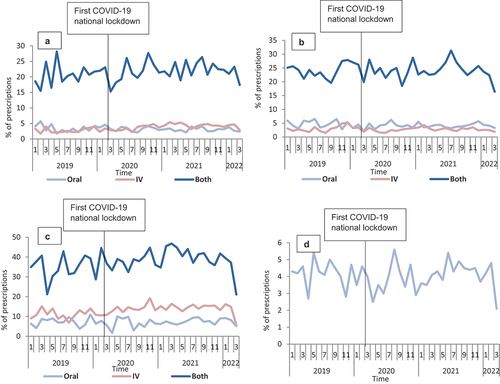
In terms of the impact of COVID-19 on the proportion of prescriptions with >7 days duration, results from the segmented regression analysis indicated an overall lack of any statistically significant impact for all four antibiotics except for amoxicillin IV (β1: 0.13, P < 0.05; β2: −1.42, P < 0.05; β3: −0.12, P < 0.05) (,). Similarly, upon stratification by sex, there was a statistically significant reduction only in the level immediately after COVID-19 lockdown among male patients who received IV co-amoxiclav (, Appendix 3). When stratifying by age groups, statistically significant changes were observed only in the level immediately after COVID-19 lockdown among those aged 71–80 years old (β2: −4.2, P < 0.05), who received oral co-amoxiclav, 51–60 years old (β2: −6.6, P < 0.05), and >80 years (β2: 14.4, P < 0.05) who received oral and ‘Both’ flucloxacillin, respectively (Appendix 4, Supplementary file 5, Supplementary file 6, Supplementary file 7, Supplementary file 8). Likewise, statistically non-significant changes were observed in the trends after COVID-19 lockdown for all the four studied antibiotics except for those aged 71–80 years old (β3: −0.3, P < 0.05; β3: −1.1, P < 0.05) who received oral and ‘Both’ co-amoxiclav, respectively, and 51–60 years old (β3: −0.8, P < 0.05) who received oral flucloxacillin (Appendix 4, Supplementary file 5, Supplementary file 6, Supplementary file 7, Supplementary file 8).
Figure 6. Proportion of prescriptions with >7 days duration overtime for the 186,061 prescriptions of the four studied antibiotics from January 2019 to March 2022 in Scotland, stratified by routes of administration (when appropriate): A-Amoxicillin; B-Co-amoxiclav; C-Flucloxacillin, and D-Doxycycline.
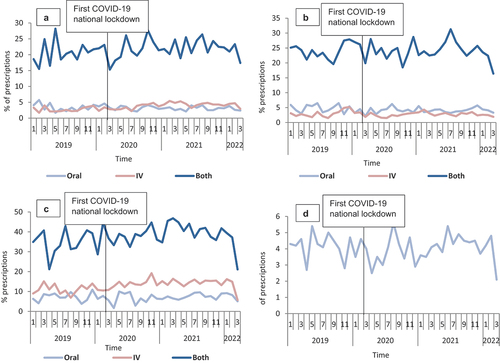
Figure 7. Proportion of prescriptions with >7 days duration overtime for the 186,061 prescriptions of the four studied antibiotics from January 2019 to March 2022 in Scotland, stratified by routes of administration (when appropriate) and sex: A-Amoxicillin; B-Co-amoxiclav; C-Flucloxacillin, and D-Doxycycline.
4. Discussion
4.1. Main findings
The study results indicated statistically significant variations in the median duration of therapy among the four antibiotics with the highest median duration of therapy among those antibiotic courses composed of both oral and IV antibiotics (‘Both’ group), which is clinically expected. IV courses consistently had the lowest duration of therapy and was the most commonly used route of administration (). This could be explained by either patients been prescribed IV emprically but then discontinued because it was realized that antibiotics were not required or potentailly switched to another oral agent, or IV was stopped and patients discharged on oral antibiotic; however, this needs further investigation. Significantly higher proportions of prescriptions within the ‘Both’ group had a duration of >7 days compared to oral and IV (). Although the duration of therapy overall did not differ significantly by sex, it differed significantly by age groups with a consistent pattern of increasing duration of therapy with increasing age, with the longest median duration of therapy observed in those aged >71 years old (); this observation was more substantial among the ‘Both’ group. In terms of the impact of COVID-19, we observed some statistically significant changes in the level and trends of duration of therapy in the post-COVID-19 period; however, these were very small changes (mostly equivalent to a few hours or less than one dosage administration interval) and unlikely to be clinically significant, which is encouraging.
It is encouraging to see that the observed durations of therapy were broadly comparable to the Scottish treatment guidelines for lower RTIs such as pneumonia [Citation17] and infective exacerbations of COPD [Citation18], which are the main indications for the studied antibiotics. Promoting IV to oral switch (IVOST) has been a key focus within the Scottish antimicrobial stewardship programme whereby particular focus was given nationally to review antibiotic therapy and to early IVOST for RTIs during the COVID-19 pandemic, given the overlapping clinical features of bacterial pneumonia and COVID pneumonitis [Citation19]. It was therefore encouraging to see that the duration of IV therapy was relatively short and well maintained throughout the study period and particularly during the peak of the pandemic in the spring of 2020. Shorter duration of IV therapy is particularly important as early removal of vascular devices reduces complications associated with IV administration, including line-related infections and Staphylococcus aureus bacteremia; shorter IV therapy has other significant healthcare benefits including reduction in treatment costs and nursing time for the preparation and administration of therapy and also often enables earlier discharge from hospital [Citation20,Citation21]. Although antibiotic courses that were compromised of both IV and oral (‘Both’ group) had the longest median duration of therapy, the durations were deemed reasonable as they ranged from 5.1 to 5.8 days for co-amoxiclav and flucloxacillin, respectively. These align within the guidelines-recommended durations for these antibiotics especially for lower RTIs as 5 days (including both IV to oral switch) duration course is recommended. Furthermore, our study found a consistent pattern of longer duration of antibiotic therapy for older patients; this could be due to a number of clinical factors including complexity of managing infection attributed to the presence of co-comorbidity, and potentially, diagnostic uncertainty in ‘ruling out’ infection and perception of higher consequences of ‘under treating’ in older, frailer patients, all of which contribute to the overuse and misuse of antibiotics including unnecessary prolonged empiric antibiotic therapy, in this vulnerable population [Citation22,Citation23].
It is reassuring and encouraging that the duration of the studied antibiotics was not affected by the COVID-19 pandemic and that the observed antibiotic durations were consistent and comparable with a previous Scottish point prevalence survey of antibiotic prescribing for RTIs in patients with suspected and proven COVID-19 [Citation24], which reported a median recorded planned duration of oral antibiotic therapy of 5 days (IQR 3–7 days). Patients who had switched from IV to oral did so after a median IV duration of 2 days (IQR 1–4 days). Of those receiving IV therapy, the median duration of IV antibiotics was 2 days (IQR 2–3) [Citation24]. This lack of COVID-19 impact could be explained by the Scottish Antimicrobial Prescribing Group (SAPG) (https://www.sapg.scot/) substantial efforts nationally and locally to promote antibiotic review, shorten antibiotic duration, or stop if COVID-19 confirmed and early IVOST [Citation19]. Findings from another study assessing antimicrobial use among hospitalized COVID-19 patients across the UK [Citation25] further suggest the positive impact of these efforts by SAPG in Scotland to promote the appropriate antibiotic use during the COVID-19 pandemic, in particular, whereby Scotland, in comparison with England and Wales, had not only lower use of broad-spectrum antibiotic but also did not show the similar extent of observed increase in antibiotic use across the other UK regions [Citation25]; in fact, a 3.2% reduction in hospital antibiotic use was reported in Scotland between 2019 and 2020 [Citation26]. This impact of COVID-19 on increasing antibiotic consumption has also been reported in a cross-continent survey [Citation27] of 73 countries whereby more than half of participating countries (63%, 35/63) reported an increase in total antibiotic use during COVID-19, with higher rates reported by low- and middle-income countries compared to high-income countries.
In the future, the use of HEPMA data to describe the duration of therapy may identify areas for antimicrobial stewardship improvement initiatives such as promoting IVOST or reducing total duration (‘Both’) without the need for a labor-intensive, time-consuming clinical manual audit. With a clinical focus of ‘shorter is better’ [Citation3], these types of interventions have been seen as a ‘low-hanging fruit’, selecting the most obtainable targets rather than confronting more complicated management issues [Citation28]. Furthermore, it is important to highlight the fact that shorter antibiotic courses have been found to be as effective as longer antibiotic courses in all clinical trials that compared short-course antibiotics with longer courses across all single bacterial infections studied, which included community-acquired pneumonia (3–5 days vs. 7–10 days) [Citation29,Citation30], nosocomial pneumonia (≤8 days vs. 10–15) [Citation31], pyelonephritis (5–7 days vs. 10–14 days) [Citation32], intra-abdominal infection (4 days vs. 10 days) [Citation33], acute exacerbation of chronic bronchitis and COPD (≤5 days vs. ≥7 days) [Citation34], acute bacterial sinusitis (5 days vs. 10 days) [Citation35], cellulitis (5–6 days vs. 10 days) [Citation36], and chronic osteomyelitis (42 days vs. 84 days) [Citation37].
4.2. Strengths and limitations
To our knowledge, this is the first published study (in the UK and Europe at least) to explore/evaluate the duration of antibiotic therapy in an acute hospital setting using individual patient-level data, although we are aware of other studies which [Citation38,Citation39], unlike our study, presented the duration of antibiotic therapy as a utilization/consumption metric (days of therapy per 1,000 patient days) rather than as a quality indicator proxy. Using HEPMA data enabled the analyses of the actual antibiotic use in clinical practice, describing the duration of antibiotic therapy by routes of administrations (stratified by patient characteristics such as age and sex), conducting a population-based evaluation covering all inpatients from the participating health boards over a prolonged period of time, across all indications and clinical specialties, hence providing comprehensive coverage and insight into this important topic in a very efficient way. Furthermore, a control group (flucloxacillin) was used to separate the impact of COVID-19 from other interventions that may have occurred at the same time, which is a standard approach in interrupted segmented regression [Citation16]. We did, however, not discuss the comparison of duration of therapy between flucloxacillin and the other studied antibiotics because flucloxacillin, unlike the others studied antibiotics, is rarely used for RTIs; hence, any comparison would have been clinically inappropriate.
However, some limitations need to be acknowledged. First, our study only included antibiotic use during patients’ hospital stay and did not include any antibiotic use being prescribed for out-patient use at discharge (these data are not captured within HEPMA); therefore, the observed duration of antibiotic therapy might be an underestimation of the actual duration of therapy. Furthermore, we did not consider the totality of the duration of therapy for infection episodes treated with different types of antibiotics (where the IV antibiotic switched to a different oral antibiotic) because our study aimed to estimate the duration of therapy for the same antibiotic rather than the total duration of antibiotic course (with differing antibiotics) used to treat the same infection episode; therefore, some of the reported results such as for IV antibiotic therapy might not reflect the real total duration of antibiotic therapy. Hence, our findings in terms of comparing duration of therapy across routes of administration should be interpreted with caution. Moreover, it is worth noting that we did not report our resulted days of therapy per 1,000 patient days or defined daily dose per 1,000 patient days because these measures are antibiotic consumption metrics used to quantify the antibiotic use [Citation40], which was not the aim of our study; rather our study was aimed at assessing the quality of antibiotic use, using duration of therapy as a proxy.
Furthermore, we did not stratify the antibiotic duration by indication because data on indication are not available within the data source that we have used in our study (HEPMA) but would require multiple linkage with other national datasets which was out with the scope of the current study. Consequently, some of the studied antibiotics therefore may have been prescribed for non-respiratory tract infections; however, lower RTIs are reported to be the most common indications for antibiotics across hospitals in Scotland [Citation41], with over half of these reportedly treated with either amoxicillin, doxycycline, or co-amoxiclav [Citation7]. This gave us assurance that lower RTIs were the most likely indications for these antibiotics.
4.3. Conclusions
The study showed no evidence for prolonged duration of therapy. As expected, median duration of therapy varied by routes of administration reflecting clinical review and IVOST. The duration of IV therapy was relatively short (median <2 days) throughout the study period, suggesting timely clinical review and consideration of IVOST. Where both IV and oral therapy (most likely reflecting IVOST) had been used, median duration of therapy was about 5 days reflecting national stewardship guidance for lower RTIs. Longer duration of therapy was observed among older patients possibly reflecting greater complexity or clinical uncertainty. There were no clinically significant differences in the duration of IV therapy or total antibiotic course during the period of peak COVID-19 admissions, which may reflect national guidance to limit the overuse of antibiotics in COVID-19 infection. Further work is needed to link prescriptions with indications, evaluating the total duration of antibiotic therapy across the whole infection episode, in particular, the duration of antibiotics for older patients should be considered a priority for stewardship teams.
Declaration of interest
RA Seaton has attended advisory boards and received a professional fee from Advanz pharma (Dalbavancin) and Mundipharma (Rezafungin). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Ethical approval
We can confirm that our study followed the principles of the Declaration of Helsinki. Ethical approval was not required because this was a service evaluation study using anonymized generated during routine care and subject to standard NHS Scotland procedures to ensure no risk of individual patient; hence, no informed consent was required either. No identifiable information has been used for analyses.
Author contributions
Study conception and design: all authors; data collection and management: AK, AM, KG, and TM; data analysis and interpretation: AK, NP, AS, WM, and MB; manuscript writing, drafting, reviewing, and approval: all authors.
Supplemental Material
Download Zip (3 MB)Acknowledgments
Part of this work was presented as an abstract entitled “The impact of the COVID-19 pandemic on duration of antibiotic therapy across hospitals in Scotland: a segmented interrupted time series analysis” at the the 38th ICPE conference meeting, 24–28 August 2022, Bella Centre, Copenhagen, Denemark,
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14787210.2023.2181789
Additional information
Funding
References
- Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One. 2017;12(12):e0189621.
- OECD. Stemming the superbug tide. 2018.
- Spellberg B. The new antibiotic mantra—“shorter is better.” JAMA Intern Med. 2016;176(9):1254–1255.
- Ashiru-Oredope D, Kerr F, Hughes S, et al. Assessing the impact of COVID-19 on antimicrobial stewardship activities/programs in the United Kingdom. Antibiotics. 2021;10(2):110.
- Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27(4):520–531.
- Alshaikh FS, Godman B, Sindi ON, et al. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: a systematic review and meta-analysis. PloS one. 2022;17(8):e0272375.
- Seaton RA, Gibbons CL, Cooper L, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect. 2020;81(6):952–960.
- Antimicrobial Resistance and Healthcare Associated Infection (ARHAI) Scotland. Scottish one health antimicrobial use and antimicrobial resistance in 2020. 2020.
- Pinzone MR, Cacopardo B, Abbo L, et al. Duration of antimicrobial therapy in community acquired pneumonia: less is more. Sci World J. 2014;2014:759138.
- Hanretty AM, Gallagher JC. Shortened courses of antibiotics for bacterial infections: a systematic review of randomized controlled trials. Pharmacother J Human Pharmacol Drug Ther. 2018;38(6):674–687.
- Spellberg B. The maturing antibiotic mantra: “shorter is still better”. J Hosp Med. 2018;13(5):361.362.
- Vaughn VM, Flanders SA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med. 2019;171(3):153–163.
- Health Improvment Scotland. Implementing an electronic prescribing and medicines administration system. 2022. [cited 2022 Oct 11]. Available from: https://www.healthcareimprovementscotland.org/our_work/technologies_and_medicines/electronic_prescribing.aspx
- McKight PE, Najab J. Kruskal‐wallis test I.B. Weiner and W.E. Craighead . In: The corsini encyclopedia of psychology (New York, USA: Wiley). 2010. p. 1.
- Woolson RF. Wilcoxon signed‐rank test. Wiley encyclopedia of clinical trials: John Wiley & Sons, Inc; 2007. p. 1–3.
- Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
- National Institute for Health and Care Excellence (NICE). Pneumonia in adults: diagnosis and management. 2019.
- National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing. 2018.
- Seaton A. COVID-19 and its impact on antimicrobial stewardship. Switzerland: REVIVE; 2020.
- Gray A, Dryden M, Charos A. Antibiotic management and early discharge from hospital: an economic analysis. J Antimicrob Chemother. 2012;67(9):2297–2302.
- Hand KS, Cumming D, Hopkins S, et al. Electronic prescribing system design priorities for antimicrobial stewardship: a cross-sectional survey of 142 UK infection specialists. J Antimicrob Chemother. 2017;72(4):1206–1216.
- Biedron C, Chopra T. Issues surrounding antibiotic use in older adults. Curr Transl Geriatr Exp Gerontol Rep. 2013;2(3):151–158
- Beckett C, Harbarth S, Huttner B. Special considerations of antibiotic prescription in the geriatric population. Clin Microbiol Infect. 2015;21(1):3–9.
- Seaton RA, Cooper L, Gibbons CL, et al. Antibiotic prescribing for respiratory tract infection in patients with suspected and proven COVID-19: results from an antibiotic point prevalence survey in Scottish hospitals. JAC-Antimicrob Resist. 2021;3(2):dlab078.
- Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial usage in hospitalised patients with COVID-19 from the ISARIC WHO CCP-UK study: a prospective, multicentre cohort study. Lancet Microbe. 2021;2(8):e354–65.
- National Services Scotland. Antimicrobial Resistance and Healthcare Associated Infection Scotland (ARHAI). Scotland; 2021.
- Tomczyk S, Taylor A, Brown A, et al. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: a global survey. J Antimicrob Chemother. 2021;76(11):3045–3058.
- Nathwani D, Lawson W, Dryden M, et al. Implementing criteria-based early switch/early discharge programmes: a European perspective. Clin Microbiol Infect. 2015;21:S47–S55.
- El Moussaoui R, De BCA, Van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. British Medical Journal Publishing Group; 2006.
- Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257–1265.
- Chastre J, Wolff M, Fagon J-Y, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. Jama. 2003;290(19):2588–2598.
- Eliakim-Raz N, Yahav D, Paul M, et al. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection—7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183–2191.
- Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372(21):1996–2005.
- El Moussaoui R, Roede BM, Speelman P, et al. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63(5):415–422.
- Falagas ME, Karageorgopoulos DE, Grammatikos AP, et al. Effectiveness and safety of short vs long duration of antibiotic therapy for acute bacterial sinusitis: a meta‐analysis of randomized trials. Br J Clinic Pharmacol. 2009;67(2):161–171
- Hepburn MJ, Dooley DP, Skidmore PJ, et al. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Internal Med. 2004;164(15):1669–1674.
- Duration of Treatment for Spondylodiscitis (DTS) study group; Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385(9971):875–882.
- Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007 Mar 1;44(5):664–670.
- Polk RE, Hohmann SF, Medvedev S, et al. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis. 2011 Dec;53(11):1100–1110.
- Bennett N, Schulz L, Boyd S, et al. Understanding inpatient antimicrobial stewardship metrics. Am J Health Syst Pharm. 2018;75(4):230–238.
- Health Protection Scotland. National point prevalence survey of healthcare associated infection and antimicrobial prescribing. 2016.
