?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
This study was aimed to explore thresholds with interaction effects among antibiotic usage, covariates (alcohol-based hand rub (ABHR)), and their effect on extended-spectrum β-lactamase–producing Klebsiella pneumoniae (ESBL-producing K. pneumoniae) in hospitalized patients.
Methods
Multivariate Adaptive Regression Spline models were used. These considered second-order interactions among antibiotic use and ABHR in addition to potential thresholds that further improve explained variance in the ESBL-producing K. pneumoniae response. The study involved collecting monthly hospital-level data for January 2017–December 2021.
Results
Analysis of the main effects showed that third-generation cephalosporins above 2.00 DDD/100 occupied bed days (OBD) generally increased ESBL-producing K. pneumoniae incidence (cases/100 OBD). Levels of ABHR above 6.61 L/100 OBD were shown to generally decrease ESBL-producing K. pneumoniae incidence. Second-order interactions revealed that when third-generation cephalosporin use was greater than 3.71 DDD/100 OBD, and ABHR was greater than 6.6 L/100 OBD (same as main effect threshold), ABHR partially lost effectiveness in its ability to reduce ESBL-producing K. pneumoniae incidence. This demonstrates the importance of not exceeding the identified thresholds of 3.71 DDD/100 OBD for third-generation cephalosporin use.
Conclusion
The main-effect thresholds in third-generation cephalosporins and ABHR, and the identified interaction between third-generation cephalosporins and ABHR can inform effective hospital antimicrobial stewardship.
1. Background
Antimicrobial resistance (AMR) has become one of the leading public health issues seen worldwide [Citation1–3]. Antibiotic use and overuse can cause antibiotics to become less effective and bacteria more difficult to treat due to AMR, thereby threatening successful patient treatment in modern healthcare systems [Citation4–7]. The size of the AMR threat was summarized in a recent review, predicting an increase in annual deaths caused by drug-resistant infections from 700,000 to 10 million, incurring significant healthcare costs [Citation2]. A recent systematic analysis estimated 4·95 million deaths associated with AMR in 2019, including 1·27 million deaths attributable to bacterial AMR [Citation3]. Klebsiella pneumoniae (K. pneumoniae) is a Gram-negative pathogenic bacterium, that is one of the common causes for serious infections including pneumonia, UTIs, and bloodstream infections [Citation8–12]. Extended-spectrum β-lactamases (ESBLs) are a group of β-lactamase enzymes which hydrolyze and inactivate several types of β-lactam antibiotics, including first-, second-, and third-generation cephalosporins and monobactams [Citation13]. Studies showed that infection with ESBL-producing bacteria is associated with an increased rate of morbidity and mortality, longer hospital stay and increased antibiotic consumption [Citation14–16]. The link between antibiotic use and the subsequent development and spread of AMR has been established, and has been shown in several studies [Citation17–26]. The use of certain antibiotics can provide selection pressure, resulting in the elimination of sensitive strains, thereby decreasing competition and allowing resistant strains to proliferate. The use of cephalosporins and fluoroquinolones has been reported to be associated with ESBL-producing organisms [Citation27–32].
During healthcare delivery, adequate infection control, involving appropriate hand hygiene practice, should be implemented to prevent the cross-transmission of antibiotic-resistant bacteria [Citation33–36]. The value of ABHR in reducing nosocomial infections has been shown in several studies [Citation37–40].
Non-linear modeling methods have been found to be a robust method to use to determine associations between antibiotic use and resistance, and to inform antimicrobial stewardship [Citation17–26]. The use of non-linear methods, including time-series adaptations, to identify thresholds in association with antibiotic use and resistance, has provided quantitative targets that balance access to effective therapies with control of resistance [Citation22–26,Citation41]. Research has focused on employing additive models (i.e. not considering potential interaction between predictors/explanatory variables) to study relationships between antibiotic use and resistance, and to identify relevant thresholds.
In this study, we aimed to explore thresholds with interaction effects among antibiotic usage, covariates (alcohol-based hand rub (ABHR)), and their effect on extended-spectrum β-lactamase – producing K. pneumoniae (ESBL-producing K. pneumoniae). The recently conducted systematic and comprehensive analysis showed that K. pneumoniae is one of the six leading pathogens linked to deaths associated with resistance [Citation3]. Considering the associated morbidity and mortality, being within the WHO priority pathogens list for research and development of new antibiotics, and the lack of studies employing the threshold concept, ESBL-producing K. pneumoniae was selected for this assessment [Citation3,Citation42–44].
2. Methods
2.1. Study design and population
This study was undertaken in Khawlah Hospital in Oman, a 539-bed facility that provides medical, surgical, pediatrics, ICU services, and obstetrics and gynecology with a focus on surgical services (60%). The study involved retrospective data collection that included all the inpatient population. Data were collected for the study period from January 2017 to December 2021. The time-series analysis requires at least 60 monthly observations (5 years) of antibiotic use, covariates, and microbiological data [Citation22,Citation25,Citation26]. The availability of the longest period of consistent outcome and explanatory variables data, i.e. 5-year dataset was used to define the duration of the time series used in this study [Citation22,Citation25,Citation26]. We hypothesized that the use of third-generation cephalosporins, and fluoroquinolones could explain variations in ESBL-producing K. pneumoniae. These antibiotics were identified a priori based on their resistance profiles and their role as risk factors for ESBL-producing K. pneumoniae [Citation28,Citation45–48]. Data on alcohol-based hand rub (ABHR) were also included in the analysis as one of the explanatory variables. The study was approved by the research committee of Khoula and Al-Nahdha Hospitals (Approval number: MOH/DGKH/REC/22/10/26142). Due to the nature of this study and the retrospective anonymized collected hospital-level aggregated dataset and analysis, consenting participants were not required.
2.2. Microbiology and pharmacy data
The standard microbiological procedures were followed in relation to the identification of isolates and antibiotic susceptibility, and were in line with the Clinical and Laboratory Standards Institute (CLSI) guidelines [Citation49,Citation50]. ESBL-producing K. pneumoniae isolates from clinical samples were included in this study, and infection control screening swabs were excluded from the study. An ESBL-producing K. pneumoniae isolate identified within 30 days of a previous isolate from the same patient was considered as the same case. ESBL-producing K. pneumoniae cases were normalized per 100 OBD. Monthly antibiotic use data were expressed as DDD per 100 OBD. The calculated DDD was done following the classification of antimicrobials for systemic use (J01) in the WHO/ATC index [Citation51]. ABHR use (in Liters) was obtained on a monthly basis and was expressed as L/100 OBD. All data were obtained from the Hospital Information Management System (Al Shifa 3 Plus).
2.3. Statistical analysis
Our analysis began by graphing the scatter plots and inspecting the lagged cross-correlation functions of all available predictor variables individually against the ESBL-producing K. pneumoniae response variable. From information learned, we applied multiple linear regression on potential predictor variables at significant lags. A feasible linear regression model was identified and served as a base comparison model prior to considering more complex relationships that may be present in the data.
Next, we examined whether thresholds were present in the predictor variables that produced a nonlinear response; divergent from the straight-line relationship inherent in a linear regression process. The method used was an additive form of the Multivariate Adaptive Regression Splines (MARS) approach [Citation52,Citation53]. Although we included all available predictor variables at multiple lags, the MARS-additive method identified the same predictor variables at the same lags discovered in the multiple linear regression analysis. The difference is that the MARS-additive model identified thresholds associated with both third-generation cephalosporins and ABHR that improved explained variance in the ESBL-producing K. pneumoniae response. It is only when the predictor variables exceeded the identified thresholds that the predictors are associated with an effect on ESBL-producing K. pneumoniae.
The MARS algorithm attempts to approximate the nonlinear function by
where is an additive function of the product basis functions
associated with the s sub-regions
and
is the coefficient for the
product basis function. The MARS procedure can identify the sub-regions under which the coefficients are stable and detect any possible interactions up to a maximum number of interactions controllable by a tuning parameter imposed on the algorithm. In terms of the additive MARS model notation, it is represented by
where is the threshold value (knot) and (∙)+ is the right (+) truncated spline function which takes on the value 0 if the expression inside (∙)+ is negative and its actual value if the expression inside (∙)+ is > 0.
Up to this point, we employed modeling methods that assume that the predictor variables are independent of each other with regard to their effect on the response. Lastly, we examined MARS models that considered second-order interactions among the predictor variables in addition to potential thresholds that further improve explained variance in the ESBL-producing K. pneumoniae response.
The MARS model with a second-order interaction can be expressed as
where defines the interaction term between (x and z).
The residuals for all models were tested for serial correlation using the autocorrelation function (ACF) diagnostic test. No serial correlation was detected in the residuals. Therefore, it was not necessary to consider autoregressive moving average (ARMA) terms in the models [Citation54].
The Adjusted R-squared statistic was used to determine if the added model complexities in the MARS-additive and MARS-interaction approaches justified the incremental improvement in explained variance of the ESBL-producing K. pneumoniae response. The R-squared statistic measures the proportion of the variation in the response variable (ESBL-producing K. pneumoniae) explained by the predictor variables with respect to total variation. Adjusted R-squared adds a penalty in the formula for the number of predictor variables included in the model.
The SCA Statistical System version 8.2 (Scientific Computing Associates Corp., River Forest, Illinois, U.S.A) and R software (R Foundation for Statistical Computing, Vienna, Austria) were used to perform analysis.
3. Results
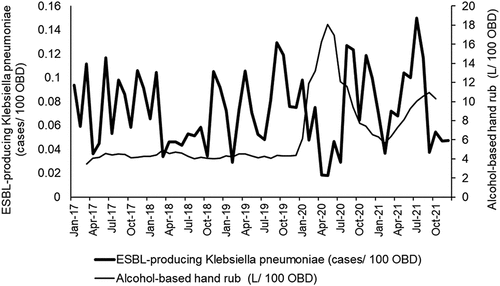
Overall, 632 non-duplicated ESBL-producing K. pneumoniae cases were identified for the entire study period; average monthly incidence rate was 0.07317 cases/100 occupied bed-days (OBD) (range: 0.0179–0.1501). Median for third-generation cephalosporin use was 2.33 defined daily dose (DDD)/100 OBD (Interquartile: 1.77–3.27), and the median ABHR use was 4.89 L/100 OBD (Interquartile: 3.97–7.54). A plot of the relationship between third-generation cephalosporins, alcohol-based hand rub (ABHR), and the incidence of ESBL-producing K. pneumoniae cases is shown in .
Figure 1. Monthly ESBL-producing K. pneumoniae incidence versus use of third-generation cephalosporin (5-month moving averages).
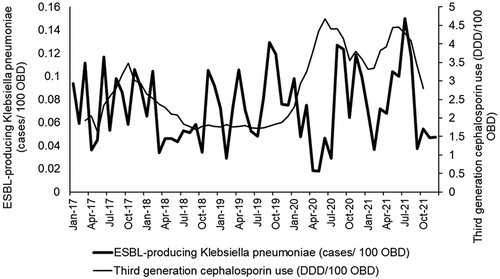
Figure 2. Monthly ESBL-producing K. pneumoniae incidence versus use of alcohol-based hand rub (5-month moving averages).
3.1. Multiple linear regression model
As part of our initial analysis, a multiple linear regression model was identified to be a feasible model, capturing straight-line relationships between the predictor variables and ESBL-producing K. pneumoniae. We also tested whether the COVID−19 pandemic period impacted relationships by including a binary indicator variable in the model. The post COVID−19 period showed that it did not significantly alter the linear relationship; coefficient = −0.0003 (−0.0232 to 0.0225), p-value = 0.976, and was dropped from the final model. In the first few months of the start of the COVID−19 pandemic (March 2020 – July 2020), ABHR levels were high. As a precaution, an indicator variable was also tested in the models to determine its effect on ESBL-producing K. pneumoniae responses. The coefficient was found to be statistically insignificant (coefficient = −0.0293; 95%CI= −0.0621 to 0.0034; p = 0.083), and was also dropped from the final model. provides the estimated results from the multiple linear regression.
Table 1. Linear regression modeling results for ESBL-producing Klebsiella pneumoniae, January 2017 to December 2021; adjusted R-squared = 20.03%.
3.2. Multivariate Adaptive Regression Splines (MARS) - Additive model
We explored the potential of thresholds in antibiotic and ABHR use that could improve the explained relationship between the predictors and ESBL-producing K. pneumoniae. All antibiotic series were once again considered in the additive MARS model. We allowed the MARS algorithm to identify relevant predictors, lags, and thresholds. The MARS approach identified the same predictors and lagged relationships as the directed analysis using multiple linear regression. It also detected thresholds in the predictors. Although a straight-line relationship may be a feasible modeling approach using multiple linear regression, it may not be optimal due to non-linearities that may be present in the data that are not captured through linear regression. The additive MARS model is presented in . As with the multiple linear regression analysis, we estimated the model with a COVID−19 indicator (coefficient= −0.0003; 95%CI= −0.0223 to 0.0178; p = 0.824) and high ABHR indicator (coefficient= −0.0256; 95%CI= −0.0583 to 0.0071; p = 0.129), and found them to be insignificant, therefore dropping them from the final model.
Table 2. MARS (Additive) result in modeling ESBL-producing Klebsiella pneumoniae incidence, January 2017 to December 2021; adjusted R-squared = 22.47%.
A threshold was detected for third-generation cephalosporins at 2.40 with a coefficient of 0.1047 (p-value <0.001). This suggests that when third-generation cephalosporin use is greater than 2.40 DDD/100 OBD, ESBL-producing K. pneumoniae incidence rates increase. ABHR was found to have a threshold at 6.51 L/100 OBD with a negative coefficient of −0.0037 (p-value <0.001). This suggests that ABHR becomes most effective in reducing ESBL-producing K. pneumoniae when more than 6.51 L/100 OBD is used.
3.3. MARS-Interaction model
The MARS summary with second-order interactions is presented in . The model consists of main effects with thresholds for third-generation cephalosporins at Lag 2 (threshold = 2.00, coefficient = 0.0109, p-value = 0.0026) and ABHR at lag 1 (threshold = 6.61, coefficient = −0.0083, p-value <0.001). Statistical interactions with thresholds are also present, implying that the effect of one independent variable on the dependent variable changes depending on the level of another independent variable. We also estimated the model with a COVID−19 indicator (coefficient = 0.0164; 95%CI= −0.0164 to 0.0388; p = 0.156) and high ABHR indicator (coefficient= −0.0165; 95%CI= −0.0498 to 0.0167; p = 0.332), finding them to be insignificant and therefore were dropped from the final model.
Table 3. MARS (Interaction, i = 2) result in modeling ESBL-producing Klebsiella pneumoniae incidence, January 2017 to December 2021; adjusted R-squared = 29.77%.
In this model, levels of third-generation cephalosporins above 2.00 generally increased ESBL-producing K. pneumoniae, and higher levels of ABHR above 6.61 generally decreased ESBL-producing K. pneumoniae. The main effects establish this, however we can also bring together additional information from the interaction terms. For example, when third-generation cephalosporin use at lag 1 is greater than 3.71 (1.71 above the main effect threshold) and ABHR at lag 1 is greater than 6.61 (same as main effect threshold), ABHR loses effectiveness in its contribution to reduce ESBL-producing K. pneumoniae incidence due to the observed increased level of third-generation cephalosporin use (coefficient = 0.0045, p-value = 0.0027; ). We also find an interaction between third-generation cephalosporins at lag 2 and third-generation cephalosporins at lag 1. This interaction suggests that upon satisfying threshold conditions, there is an observed carry-over effect of third-generation cephalosporins use on ESBL-producing K. pneumoniae from one period to the next (coefficient = 0.0026, p-value = 0.0174). The improvement in adjusted R-squared across the three presented models () is shown in
Table 4. Absolute and relative change in determined Adjusted R-squared for the identified models.
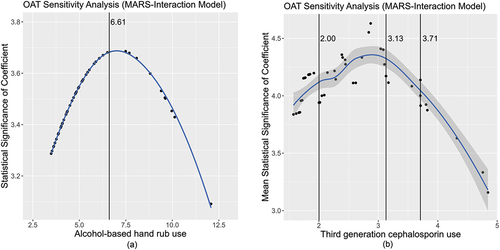
A ‘One At a Time’ (OAT) approach was used to analyze sensitivity around the thresholds identified for third-generation cephalosporins and ABHR. We noted that the confidence range around the thresholds was wide and covered nearly the entire range of third-generation cephalosporins and ABHR usage. This is due to the fact that the linear regression model was shown to be feasible, and if we expressed the linear regression in terms of a threshold model, the threshold values would equal the minimum values in the third-generation cephalosporin and ABHR series with 100% of cases satisfying the threshold condition. The additive and interaction MARS models searched for optimal thresholds in the explanatory variables over the full range of values in the explanatory variables. show the statistical significance (t-values) of the estimated coefficients related to the threshold values from the OAT sensitivity analysis. The solid black vertical lines mark identified thresholds. Three thresholds are associated with third-generation cephalosporin usage and are displayed on the chart. The mean of the t-values associated with the three third-generation cephalosporin threshold coefficients was used to simplify visualization of the sensitivity analysis. A coefficient is considered statistically significant with 95% confidence when the t-value ≥1.97. It is observed that all t-values on the y-axis of the charts exceed 1.97, however, peak significance is shown to occur near the thresholds, supporting the rationale of improved variance reduction through the additive and interaction MARS approaches for this study. It is also evident from the adjusted R-squared statistics that MARS shows improvement and is of higher optimality compared to the multiple linear regression method.
Figure 3. a) Alcohol-based hand rub chart showing the statistical significance (t-values) of the estimated coefficients related to the threshold values from the OAT sensitivity analysis. The solid black vertical lines mark an identified threshold. b) Third-generation cephalosporin chart showing the statistical significance (t-values) of the estimated coefficients related to the threshold values from the OAT sensitivity analysis. The solid black vertical lines mark an identified threshold.
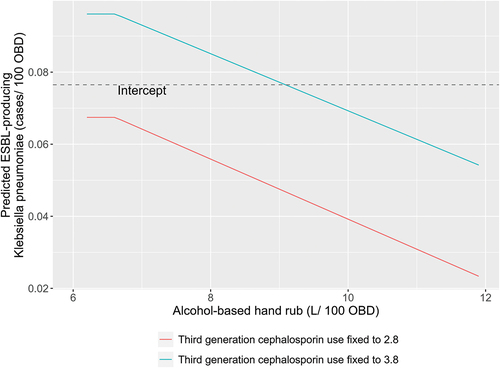
The overall contribution of ABHR use can be studied in . The dashed horizontal line on the charts is the intercept of the model which can be considered as the mean ESBL-producing Klebsiella incidence rate after removing the influence of the predictor variables. The contribution for ABHR is derived by fixing third-generation cephalosporin at a constant value and systematically increasing ABHR usage over a historic range. ABHR exhibits a linearly proportional negative effect on the ESBL-producing Klebsiella incidence rates when third-generation cephalosporin is fixed at a constant value. We also find that third-generation cephalosporin use at a fixed level of 2.8 DDD/100 OBD results in an expected pathogen incidence rate below the intercept level. When third-generation cephalosporin use is at a fixed level of 3.8 DDD/100 OBD, it is not until ABHR is above 9.0 L/100 OBD that expected pathogen incidence rate is below the intercept.
4. Discussion
In this study, we aimed to explore thresholds and interaction effects among antibiotic usage and ABHR, and their effect on ESBL-producing K. pneumoniae. The findings highlighted the value of considering potential interactions when analyzing the association between antibiotic use and pathogen incidence. To our knowledge, this is the first study to assess and demonstrate the concept of interactions when identifying thresholds in antibiotic usage in association with ESBL-producing K. pneumoniae. Our analysis identified the use of third-generation cephalosporins as directly associated with the incidence of ESBL-producing K. pneumoniae in hospitals. These results are consistent with other published research, indicating that prior use of third-generation cephalosporins was significantly associated with ESBL organisms [Citation28,Citation46–48]. Inappropriate antimicrobial use has been shown to contribute to the selection and spread of drug-resistant microorganisms [Citation1,Citation17,Citation55]. Due to the selective pressure imposed by antibiotic agents over bacterial populations, antibiotic consumption has been linked to the increased prevalence of resistant bacterial infections [Citation1,Citation4,Citation17,Citation56].
Antibiotics should be used in ways that encourage the reestablishment of susceptible flora, and this could be achieved if the susceptible micro- organisms were given a chance to ‘come back,’ through removing antibiotic selective pressure [Citation41]. The ‘selection threshold’ concept suggests that that there is a ‘threshold’ level of drug, above which, the persistent selection of the antibiotic will lead to the development and spread of antibiotic resistance [Citation41]. In this context, it was hypothesized that antibiotic resistance will develop and increase if the consumption of specific antibiotics exceeds a specific usage threshold and selection pressures cause the benefits of resistance to outweigh the fitness costs [Citation1,Citation22,Citation41] This is consistent with several published studies that suggest critical thresholds in antibiotic consumption, above which resistance emerges rapidly, thus reducing antibiotic use to below these thresholds may curtail resistance [Citation1,Citation22,Citation57]. Of importance, the role of third-generation cephalosporins in the development of resistant bacteria such as Clostridium difficile-associated diarrhea and meticillin-resistant Staphylococcus aureus has been documented [Citation21,Citation58]. Therefore, optimizing the use of third generation cephalosporins may have a positive impact on tackling the development of antibiotic resistance in other pathogens.
The results of this study showed that ABHR is inversely associated with the incidence of ESBL-producing K. pneumoniae in hospitals. Since the development of resistant infections is recognized as a multifactorial problem, guidelines on controlling healthcare acquired infections emphasize the importance of implementing optimal infection control practices and the prudent use of antibiotics [Citation59–62]. The core principles of infection control include effective and timely hand hygiene and maintenance of a clean environment [Citation63]. In addition, antibiotic stewardship which aims at improving patient outcomes while reducing adverse effects associated with antimicrobial use, has proved to be effective in decreasing antimicrobial use and in reducing healthcare costs [Citation59,Citation60,Citation64]. These practices represent the standard of care that should be used routinely in the care of all patients to minimize the transmission and spread of multi-drug-resistant organisms.
Indirect transmission between patients and healthcare workers’ hands plays a role in the acquisition of ESBL-producing Enterobacterale in hospitals [Citation13]. Hand hygiene was proposed as one of the interventions to limit the transmission of multidrug-resistant organisms (MDROs) in hospitals [Citation65]. The potential impact of ABHR on cross-transmission and reducing nosocomial infections has been shown in other published studies [Citation26,Citation39,Citation40,Citation66–68].
In addition to the main effects of third-generation cephalosporins and ABHR usage as mentioned above, this study found statistically significant interactions between ABHR and third-generation cephalosporins, and between third-generation cephalosporin use in consecutive months. The interactions increase the effectiveness of the predictor variables in explaining the incidence of ESBL-producing K. pneumoniae [] more than the main effects alone. The interaction between third-generation cephalosporins and ABHR suggests ABHR may lose some effectiveness in its ability to reduce ESBL-producing K. pneumoniae incidence in hospitals when third-generation cephalosporin use exceeds a given threshold. From a policy point of view in this study, it is prudent to maintain third-generation cephalosporin use below the 3.71 DDD/100 OBD threshold to fully benefit from the pathogen incidence-reducing effect of ABHR. It can also be quantified using the estimated coefficients of the interaction model. The main effects of the model indicate that for every unit change in third-generation cephalosporin use above the 2.0 DDD/100 OBD level, the pathogen incidence rate increases by 0.0109. This is counter-balanced by ABHR usage which indicates that for every unit change in ABHR above the 6.61 L/100 OBD level, pathogen incidence rate will decrease by −0.0083. ABHR would therefore need to be increased by 1.31 L/100 OBD (0.0109 ÷ 0.0083) for every DDD/100 OBD unit change in third-generation cephalosporin use above the 2.0 DDD/100 OBD threshold. However, when third-generation cephalosporin use exceeds 3.71 DDD/100 OBD, and the additive effect of the interaction with ABHR is taken into account, ABHR would need to be increased above the identified threshold by 1.86 L/100 OBD ((0.0109 + 0.0045) ÷ 0.0083) for every DDD/100 OBD unit change in third-generation cephalosporin above the 3.71 DDD/100 OBD threshold.
The analysis presented in this paper began by modeling ESBL-producing K. pneumoniae using multiple linear regression, which is suited to modeling straight-line relationships when there is an assumed constant rate of change between the predictors, at all levels, and dependent variable. We then compared the results from the multiple linear regression to the modeling results of an Additive Multivariate Adaptive Regression Splines (MARS–Additive) approach. The MARS-Additive approach has the benefit of being able to identify thresholds among the predictor variables in which the response of the dependent variable is nonlinear and diverges from the globally linear relationship. Another benefit of the MARS approach is that the nonparametric model identification algorithm is fairly automated, and can handle a large number of predictor variables while accounting for nonlinearity and multi-colinearity. Importantly, the MARS approach is able to identify interactions among the predictors. We compared the results of multiple linear regression and MARS-Additive model to a MARS approach allowing for second order interactions (MARS–Interactions).
The MARS approach, with second-order interactions and thresholds, allowed us to evaluate if third-generation cephalosporins and ABHR predictors were independently related to ESBL-producing K. pneumoniae. The alternative would be that third-generation cephalosporins and ABHR interact at some level which produce an increased or decreased effect beyond what is observed in the model’s main effects. As we progressed from multiple linear regression to MARS–Interaction methods, we added more complexity in the models and suggested more complex relationships among the predictors and response variables. We considered three feasible models to explain variation in ESBL-producing K. pneumoniae, increasing in structural complexity. Adjusted R-squared was used to evaluate the justification of increased model complexity versus the improvements in goodness of fit. Adjusted R-squared measures how well a set of predictor variables is able to explain the variation in the response variable, adjusted for the number of predictors in a model.
In this assessment, we used rigorous analysis methods and routinely collected data for all hospitalized patients, making selection and information bias unlikely. Nevertheless, the analysis could be improved by inclusion of additional relevant explanatory variables, an example being infection prevention and control activities and proxy measures for changes in patient population and case mix [Citation69,Citation70]. In addition, this work was undertaken in one hospital, and further multi-center assessment is warranted.
In conclusion, we explored the potential of thresholds and interactions in antibiotic usage and ABHR use that could improve the explained relationship between the predictors and the ESBL-producing K. pneumoniae pathogen. The Interaction model increased effectiveness to explain variation of ESBL-producing K. pneumoniae incidence rates while showing the importance of exploring interactions when modelling the relationship between antibiotic use, covariates (ABHR), and resistance in hospitals. Analysis of data showed that MARS approach with second-order interaction was the optimal model providing best goodness of fit in consideration of model complexity. The determined thresholds for third generation cephalosporins and ABHR would be useful in helping to formulate hospital antibiotic guidelines that would result in curbing ESBL-producing K. pneumoniae incidence. Reducing antibiotic use below a specific threshold helps to maintain diversity and access to these antibiotics, along with avoiding the challenge of implementing complete restriction of antibiotics in clinical practice [Citation71]. The determined interaction between third-generation cephalosporins is of importance to inform effective hospital ABHR use. The main-effect thresholds in third-generation cephalosporins and ABHR, and the identified interaction between third-generation cephalosporins and ABHR, can help to inform hospital antimicrobial stewardship activities.
Article highlights
Non-linear modeling methods have been found to be a robust method to identify associations between antibiotic use and resistance.
Research is needed to study relationships between antibiotic use and resistance, and to identify relevant thresholds, while considering potential interaction between predictors.
The Interaction model increased effectiveness to explain variation of ESBL-producing K. pneumoniae incidence rates while showing the importance of exploring interactions between antibiotic use, covariates, and resistance in hospitals.
The identified thresholds and the identified interactions can help to inform effective hospital antimicrobial stewardship.
Author contributions
Conceptualization and design, M. A. Aldeyab and W. Lattyak; methodology, all authors; software, W. Lattyak.; data acquisition and investigation: all authors; formal analysis, M. A. Aldeyab and W. Lattyak; W. Lattyak was the principal analyst; writing – original draft preparation, M. A. Aldeyab and W. Lattyak; writing. Review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Aldeyab M, López-Lozano JM, Gould IM. Global antibiotics use and resistance. In: Babar ZUD, editor. Global pharmaceutical policy. Singapore: Palgrave Macmillan; 2020. p. 331–344. doi: 10.1007/978-981-15-2724-1_13
- O’Neill J Antimicrobial Resistance: tackling a crisis for the health and wealth of nations. The Review on Antimicrobial Resistance. 2014. (cited 6 Aug 2022). Available online: https://amr-review.org/Publications.html
- Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0
- Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(S12):S122–S129. doi: 10.1038/nm1145
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10
- Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg Infect Dis. 2002;8(4):347–354. doi: 10.3201/eid0804.010312
- Tomson G, Vlad I. The need to look at antibiotic resistance from a health systems perspective. Ups J Med Sci. 2014;119(2):117–124. doi: 10.3109/03009734.2014.902879
- Martin RM, Bachman MA. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front Cell Infect Microbiol query. 2018;8:4. doi: 10.3389/fcimb.2018.00004
- Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801
- Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi: 10.1128/CMR.11.4.589
- Gupta A, Ampofo K, Rubenstein D, et al. Extended spectrum β lactamase-producing klebsiella pneumoniae infections: a review of the literature. J Perinatol. 2003;23(6):439–443. doi: 10.1038/sj.jp.7210973
- Becker L, Fuchs S, Pfeifer Y, et al. Whole genome sequence analysis of CTX-M-15 producing Klebsiella isolates allowed dissecting a polyclonal outbreak scenario. Front Microbiol. 2018;9:322. doi: 10.3389/fmicb.2018.00322
- Paterson DL, Bonoma RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005
- Ramphal R, Ambrose PG. Extended-Spectrum β-Lactamases and clinical outcomes: current data. Clin Infect Dis. 2006;42(Supplement_4):164–187. doi: 10.1086/500663
- Rodríguez-Baño J, Picón E, Gijón P, et al. Community-onset bacteremia due to extended-spectrum β-lactamase–producing escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010;50(1):40–48. doi: 10.1086/649537
- Kritsotakis EI, Tsioutis C, Roumbelaki M, et al. Antibiotic use and the risk of carbapenem-resistant extended-spectrum- -lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: results of a double case-control study. J Antimicrob Chemother. 2011;66(6):1383–1392. doi: 10.1093/jac/dkr116
- Jirjees FJ, Al-Obaidi HJ, Sartaj M, et al. Antibiotic use and resistance in hospitals: time-series analysis strategy for determining and prioritising interventions. Hosp Pharm Eur. 2020;95:13–19. Available online: https://hospitalpharmacyeurope.com/news/reviews-research/antibiotic-use-and-resistance-in-hospitals-time-series-analysis-strategy-for-determining-and-prioritising-interventions/
- Monnet DL, López-Lozano JM, Campillos P, et al. Making sense of antimicrobial use and resistance surveillance data: application of ARIMA and transfer function models. Clin Microbiol Infect. 2001;7(Suppl 5):29–36. doi: 10.1046/j.1469-0691.2001.00071.x
- López-Lozano JM, Monnet DL, Yagüe A, et al. Modelling and forecasting antimicrobial resistance and its dynamic relationship to antimicrobial use: a time series analysis. Int J Antimicrob Agents. 2000;14(1):21–31. doi: 10.1016/S0924-8579(99)00135-1
- Monnet DL, MacKenzie FM, López-Lozano JM, et al. Antimicrobial drug use and methicillin-resistant staphylococcus aureus , aberdeen, 1996–2000. Emerg Infect Dis. 2004;10(8):1432–1441. doi: 10.3201/eid1008.020694
- Aldeyab MA, Monnet DL, López-Lozano JM, et al. Modelling the impact of antibiotic use and infection control practices on the incidence of hospital-acquired methicillin-resistant Staphylococcus aureus: a time-series analysis. J Antimicrob Chemother. 2008;62(3):593–600. doi: 10.1093/jac/dkn198
- Lopez-Lozano JM, Lawes T, Nebot C, et al. A nonlinear time-series analysis approach to identify thresholds in associations between population antibiotic use and rates of resistance. Nat Microbiol. 2019;4(7):1160–1172. doi: 10.1038/s41564-019-0410-0
- Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis. 2015;15(12):1438–1449. doi: 10.1016/S1473-3099(15)00315-1
- Lawes T, Lopez-Lozano JM, Nebot C, et al. Turning the tide or riding the waves? Impacts of antibiotic stewardship and infection control on MRSA strain dynamics in a Scottish region over 16 years: non-linear time series analysis. BMJ Open. 2015;5(3):e006596. doi: 10.1136/bmjopen-2014-006596
- Hayajneh WA, Al-Azzam S, Yusef D, et al. Identification of thresholds in relationships between specific antibiotic use and carbapenem-resistant Acinetobacter baumannii (CRAb) incidence rates in hospitalized patients in Jordan. J Antimicrob Chemother. 2021;76(2):524–530. doi: 10.1093/jac/dkaa463
- Al-Hashimy ZS, Conway BR, Al-Yaqoobi M, et al. Identifying Targets for antibiotic use for the management of carbapenem-resistant acinetobacter baumannii (CRAb) in hospitals-a multi-centre nonlinear time-series study. Antibiotics (Basel). 2022;11(6):775. doi: 10.3390/antibiotics11060775
- Colodner R, Rock W, Chazan B, et al. Risk factors for the development of extended spectrum beta lactamase producing bacteria in non-hospitalised patients. Eur J Clin Microbiol Infect Dis. 2004;23(3):163–167. doi: 10.1007/s10096-003-1084-2
- Graffunder EM, Preston KE, Evans AM, et al. Risk factors associated with extended-spectrum β-lactamase-producing organisms at a tertiary care hospital. J Antimicrob Chemother. 2005;56(1):139–145. doi: 10.1093/jac/dki180
- Vibet MA, Roux J, Montassier C, et al. Systematic analysis of the relationship between antibiotic use and extended spectrum beta-lactamase resistance in enterobacteriaceae in a French Hospital: a time series analysis. Eur J Clin Microbiol Infect Dis. 2015;34(10):1957–1963. doi: 10.1007/s10096-015-2437-3
- Kaier K, Frank U, Hagist C, et al. The impact of antimicrobial drug consumption and alcohol-based hand rub use on the emergence and spread of extended-spectrum -lactamase-producing strains: a time-series analysis. J Antimicrob Chemother. 2009;63(3):609–614. doi: 10.1093/jac/dkn534
- Vernaz N, Huttner B, Muscionico D, et al. Modelling the impact of antibiotic use on antibiotic resistant Escherichia coli using population based data from a large hospital and surrounding community. J Antimicrob Chemother. 2011;66(4):928–935. doi: 10.1093/jac/dkq525
- Aldeyab MA, Harbarth S, Vernaz N, et al. The impact of antibiotic use on the incidence and resistance pattern of extended spectrum beta-lactamase producing bacteria in primary and secondary healthcare settings. Br J Clin Pharmacol. 2012;74(1):171–179. doi: 10.1111/j.1365-2125.2011.04161.x
- Coia JE, Duckworth GJ, Edwards DI, et al. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect. 2006;63 Suppl 1:S1–44. Erratum in: J Hosp Infect. 2006; 64(1):97-8. doi: 10.1016/j.jhin.2006.01.001
- Ventola CL. The antibiotic resistance crisis: part 2: management strategies and new agents. P T. 2015;40(5):344–352.
- Lushniak BD. Antibiotic resistance: a public health crisis. Public Health Rep. 2014;129(4):314–316. doi: 10.1177/003335491412900402
- Mathur P. Hand hygiene: back to the basics of infection control. Indian J Med Res. 2011;134(5):611–620. doi: 10.4103/0971-5916.90985
- Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305–315. doi: 10.1016/j.jhin.2009.04.019
- Lotfinejad N, Peters A, Tartari E, et al. Hand hygiene in health care: 20 years of ongoing advances and perspectives. Lancet Infect Dis. 2021;21(8):e209–e221. doi: 10.1016/S1473-3099(21)00383-2
- Barrera L, Zingg W, Mendez F, et al. Effectiveness of a hand hygiene promotion strategy using alcohol-based handrub in 6 intensive care units in Colombia. Am J Infect Control. 2011;39(8):633–639. doi: 10.1016/j.ajic.2010.11.004
- Kingston L, O’Connell NH, Dunne CP. Hand hygiene-related clinical trials reported since 2010: a systematic review. J Hosp Infect. 2016;92(4):309–320. doi: 10.1016/j.jhin.2015.11.012
- Levy SB. Balancing the drug-resistance equation. Trends Microbiol. 1994;2(10):341–342. doi: 10.1016/0966-842X(94)90607-6
- Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3
- Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. doi: 10.1128/MMBR.00078-15
- Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228–236. doi: 10.1016/S1473-3099(09)70054-4
- Wiener J, Quinn JP, Bradford PA, et al. Multiple antibiotic–resistant klebsiella and escherichia coli in nursing homes. JAMA. 1999;281(6):517–523. doi: 10.1001/jama.281.6.517
- Lee SO, Lee ES, Park SY, et al. Reduced use of third-generation cephalosporins decreases the acquisition of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2004;25(10):832–837. doi: 10.1086/502304
- Lautenbach E, Patel JB, Bilker WB, et al. Extended-spectrum -lactamase-producing escherichia coli and klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32(8):1162–1171. doi: 10.1086/319757
- Nham E, Huh K, Cho SY, et al. Characteristics and clinical outcomes of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteremia in cancer patients. Infect Chemother. 2020;52(1):59–69. doi: 10.3947/ic.2020.52.1.59
- Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Sixth Edition: m100. Wayne PA USA: CLSI; 2016.
- WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment, 2023. Oslo, 2022. cited 1 Apr 2023. https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/
- Friedman J. Multivariate adaptive regression splines. Ann Statist. 1991;19(1):1–67. doi: 10.1214/aos/1176347963.
- Hastie T, Tibshirani R. Generalized additive models. London: Chapman & Hall; 1990.
- Liu L-M. Time Series Analysis and Forecasting. 2nd edn ed. River Forest IL USA: Scientific Computing Associates Corp; 2009.
- Hecker MT, Aron DC, Patel NP, et al. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978. doi: 10.1001/archinte.163.8.972
- Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2(2): CD003543. doi: 10.1002/14651858.CD003543.pub4
- Haber M, Levin BR, Kramarz P. Antibiotic control of antibiotic resistance in hospitals: a simulation study. BMC Infect Dis. 2010;10(1):254. doi: 10.1186/1471-2334-10-254
- Aldeyab MA, Harbarth S, Vernaz N, et al. Quasiexperimental study of the effects of antibiotic use, gastric acid-suppressive agents, and infection control practices on the incidence of Clostridium difficile-associated diarrhea in hospitalized patients. Antimicrob Agents Chemother. 2009;53(5):2082–2088. doi: 10.1128/AAC.01214-08
- Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9
- Dellit TH, Owens RC, McGowan JE, et al. Infectious diseases society of america and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393
- Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362–386. doi: 10.1086/502213
- Lemmen SW, Lewalter K. Antibiotic stewardship and horizontal infection control are more effective than screening, isolation and eradication. Infection. 2018;46(5):581–590. doi: 10.1007/s15010-018-1137-1
- Wilson J. Infection control in clinical practice. 3rd ed. London: Bailliere Tindall; 2006.
- Ya K Z, Win PTN, Bielicki J, et al. Association between antimicrobial stewardship programs and antibiotic use globally: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(2):e2253806. doi: 10.1001/jamanetworkopen.2022.53806
- Goto M, O’Shea AMJ, Livorsi DJ, et al. The effect of a nationwide infection control program expansion on hospital-onset gram-negative rod bacteremia in 130 veterans health administration medical centers: an interrupted time-series analysis. Clin Infect Dis. 2016;63(5):642–650. doi: 10.1093/cid/ciw423
- Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme Lancet. 2000;356(9238):1307–1312. doi: 10.1016/S0140-6736(00)02814-2
- Gordin FM, Schultz ME, Huber RA, et al. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infect Control Hosp Epidemiol. 2005;26(7):650–653. doi: 10.1086/502596
- Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641–652. doi: 10.1016/S1473-3099(06)70600-4
- Aldeyab MA, McElnay JC, Scott MG, et al. Hospital antibiotic use and its relationship to age-adjusted comorbidity and alcohol-based hand rub consumption. Epidemiol Infect. 2014;142(2):404–408. doi: 10.1017/S0950268813001052
- Aldeyab MA, McElnay JC, Scott MG, et al. A modified method for measuring antibiotic use in healthcare settings: implications for antibiotic stew-ardship and benchmarking. J Antimicrob Chemother. 2014;69(4):1132–1141. doi: 10.1093/jac/dkt458
- Conlon-Bingham GM, Aldeyab M, Scott M, et al. Effects of antibiotic cycling policy on incidence of healthcare-associated MRSA and clostridioides difficile infection in secondary healthcare settings. Emerg Infect Dis. 2019;25(1):52–62. doi: 10.3201/eid2501.180111