ABSTRACT
Background
The potential of ursodeoxycholic acid (UDCA) in inhibiting angiotensin-converting enzyme 2 was demonstrated. However, conflicting evidence emerged regarding the association between UDCA and COVID-19 outcomes, prompting the need for a comprehensive investigation.
Research design and methods
Patients diagnosed with COVID-19 infection were retrospectively analyzed and divided into two groups: the UDCA-treated group and the control group. Kaplan–Meier recovery analysis and Cox proportional hazards models were used to evaluate the recovery time and hazard ratios. Additionally, study-level pooled analyses for multiple clinical outcomes were performed.
Results
In the 115-patient cohort, UDCA treatment was significantly associated with a reduced recovery time. The subgroup analysis suggests that the 300 mg subgroup had a significant (adjusted hazard ratio: 1.63 [95% CI, 1.01 to 2.60]) benefit with a shorter duration of fever. The results of pooled analyses also show that UDCA treatment can significantly reduce the incidence of severe/critical diseases in COVID-19 (adjusted odds ratio: 0.68 [95% CI, 0.50 to 0.94]).
Conclusions
UDCA treatment notably improves the recovery time following an Omicron strain infection without observed safety concerns. These promising results advocate for UDCA as a viable treatment for COVID-19, paving the way for further large-scale and prospective research to explore the full potential of UDCA.
1. Introduction
Ursodeoxycholic acid (UDCA) is a naturally occurring bile acid that makes up a minor fraction of the human bile acid pool, which has been used to treat various liver diseases, including primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), intrahepatic cholestasis, and other liver disease [Citation1,Citation2].
Brevini and colleagues reported in Nature in 2022 that the Farnesoid X receptor (FXR) plays a crucial role in regulating the expression of angiotensin-converting enzyme 2 (ACE2) and that this regulation can be effectively inhibited in vitro using organoids and ex-situ on a pair of human lungs by UDCA, and observed retrospectively a significant association between UDCA treatment and improved clinical outcomes following SARS-CoV-2 infection in liver transplant recipients (24 UDCA users versus 95 non-users) [Citation3]. Since then, a number of conflicting observations on the association between the use of UDCA and COVID-19 outcomes were presented [Citation4–10], making the real-world effect of UDCA for COVID highly controversial. On one hand, there is little data on previously healthy patients without preexisting condition and with fully vaccinated against SARS-CoV-2. On the other hand, there is neither a randomized controlled trial nor a meta-analysis of existing observational studies. Therefore, we are committed to providing new insights and partially filling the gap in the real-world effects of UDCA in COVID-19 prevention and early treatment.
From 10 December 2022, to 30 December 2022, a series of patients infected with SARS-CoV-2 were admitted to Nanjing University Hospital, shortly following the end of the Chinese national zero-COVID strategy. Some of them received treatment with UDCA. Here, we present retrospective analyses of this cohort to explore the efficacy of UDCA in treating Chinese patients with non-severe COVID-19, as well as examine the impact of different daily doses of UDCA. Moreover, to provide up-to-date evidence, we synthesized data of existing cohort studies together with our cohort.
2. Patients and methods
2.1. Study design
We accessed and reviewed the de-identified data retrospectively through the Electronic Data Capture system of Nanjing University Hospital in January 2023. This study was exempt from institutional review board approval or the need for informed consent because of the nature of retrospective analyses using de-identified data exclusively.
2.2. Study population
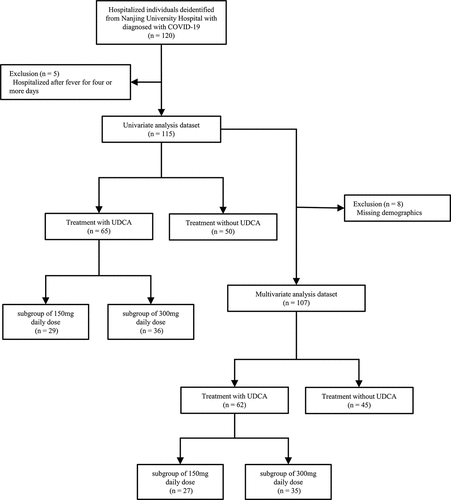
The analysis cohort consisted of 115 patients who met the inclusion criteria (). These patients had sufficient individual data, including baseline demographic information and clinical outcome data. They also had a documented diagnosis of COVID-19 between 10 December 2022, and 30 December 2022, following the end of the Chinese national zero-COVID strategy. Inpatients who experienced fever due to COVID-19 for four or more days were excluded [Citation11].
2.3. Exposures and outcomes
None of the patients had a previous history of UDCA before hospitalization. Upon admission, patients were divided into two groups: the UDCA group, where patients were initiated on UDCA treatment on the day of admission, and the no UDCA group, where patients did not treat with UDCA throughout their hospitalization. The individual patient data collected various demographic characteristics, including gender, age, and BMI. Also, clinical baseline information was collected, such as days from fever onset to hospitalization, body temperature measured on admission, anti-SARS-CoV-2 vaccination status, and any comorbidities present. Concomitant use of medications was further documented, involving non-steroidal anti-inflammatory drugs (NSAIDs) as well as other symptomatic drugs recommended by the Chinese National Diagnosis and Treatment of Novel Coronavirus Pneumonia (Version 9) for alleviating symptoms. The primary outcome was the time to body temperature recovery, which was defined as the duration it took for body temperature to return to 37.3°C or lower after admission. If a patient did not experience temperature recovery by the time of discharge, the outcome was censored on that day. In cases where patients experienced recurrent fever, we used the last recorded time of body temperature recovery for all analyses. The final body temperature for the analysis was the measured axillary temperature +0.5°C, as documented previously.
2.4. Statistical analysis
Baseline characteristics were presented as median (interquartile range, IQR), unless noted. The differences between the UDCA and no UDCA groups in categorical and continuous variables were compared using chi-square tests and Wilcox tests, respectively. All statistical comparisons were two-sided, with significance specified at α = 0.05.
The univariate Kaplan–Meier approach was initially used to characterize recovery curves for the two exposure groups and the subgroups based on the daily dose of UDCA (150 mg or 300 mg). The median recovery time for the two exposure groups and dosage subgroups, along with associated 95% confidence intervals (95% CI), was estimated accordingly. To initially compare the recovery curves between the UDCA and no UDCA groups, as well as the differences between each dosage subgroup (i.e. 150 mg or 300 mg) and the no UDCA group, log-rank tests were employed. P values for subgroup analyses were adjusted for multiplicity using the Benjamini & Hochberg method, whenever necessary. Prior to the log-rank test, the assumption of proportional hazards was examined.
To assess the impact of UDCA versus no UDCA on the time to body temperature recovery quantitatively, the primary analysis was conducted using the Cox proportional hazard model with maximal adjustment for all measured covariates. The measured covariates for adjustment include demographical factors, such as sex, age, and BMI; concomitant use of NSAIDs; clinical baseline on admission, such as days from fever onset to hospitalization and body temperature on admission. To minimize potential biases related to the selection of covariates, we performed sensitivity analyses involving not adjusting for covariates, minor adjustments for only demographical features, concomitant use of NSAIDs, and one of the clinical baselines on admission (either days from fever onset to hospitalization or body temperature on admission). The continuous covariates were normalized to the 0–1 range. The same analysis strategy was applied to investigate the differences between each dosage subgroup (150 mg or 300 mg) and the no UDCA group, whose corresponding p values were adjusted for multiplicity using the Benjamini & Hochberg method. Similar to the initial log-rank tests, the assumption of proportional hazards was examined before Cox proportional hazard analyses [Citation12].
In order to handle missing data, we employed the multiple imputations by chained equations method with 10 imputed datasets and 10 iterations. The missing covariates were allowed a maximal fraction of missing information (FMI)/m ≤ 10% (Supplementary Table S1). For each covariate with missing data, a random forest model was utilized to impute the missing values. This method was adopted under the assumption that the missing observations for covariates were missing at random. The validity of the imputations was assessed by comparing the distribution of recorded and imputed values for all measurements through visual inspection. To further evaluate the robustness of our results, we performed a sensitivity analysis on both samples with complete covariates and those with imputed data. The imputation was performed using the mice package. All statistical analyses were performed using R (version 4.2.1).
2.5. Synthesis of existing evidence
This data synthesis practice was prospectively registered with the PROSPERO (CRD42023495522). To comprehensively evaluate the clinical efficacy of repurposing Ursodeoxycholic Acid (UDCA) for the treatment and prevention of COVID-19, a systematic literature search via PubMed, Embase, Web of Science, and MedRxiv was conducted for cohort studies from inspection to December 2023. Keywords and their MeSH terms related to ‘Ursodeoxycholic Acid,’ ‘COVID-19,’ and ‘SARS-CoV-2’ were used. We followed the PRISMA guidelines. Two authors (H.-L.Y. and J.-S.X. under the supervision of G.-F.L.) independently screened cohort studies for eligibility and extracted relevant information. Inclusion criteria comprised studies that were 1) cohort studies and 2) reported either the SARS-CoV-2 infection rate among patients receiving UDCA therapy in comparison to usual care, or outcomes of interest in COVID-19 patients with UDCA therapy versus usual care. The Newcastle-Ottawa Scale was employed to assess the risk of bias in included cohort studies, all of which demonstrated a low risk of bias (Supplementary Table S2) [Citation13]. Various characteristics, including presenters, timing of presentation, country/region, comorbidities, sample size, age, sex proportion, event numbers, and effect estimates, were extracted and tabulated from each retrieved paper.
Dichotomous results were presented as odds ratios (OR) or hazard ratios (HR) with 95% confidence intervals (CI). Calculated ORs based on event numbers were employed for pooling when effect sizes were not provided. In cases where both OR and HR were available for a single outcome across different studies, a concurrent conversion into Risk Ratios (RR) was performed using established methods [Citation14,Citation15]. Risk differences were utilized for pooling studies with no deaths in either the UDCA treatment or control group. Summary estimates of mean differences for continuous outcomes were generated, converting the original median and interquartile range (IQR) of outcomes into mean and standard deviation (SD) [Citation16]. A random-effects model with the inverse-variance method was employed due to expected heterogeneity across cohort studies. Pooling analysis was typically conducted for at least three studies. A P-value <0.05 was deemed statistically significant. Heterogeneity was assessed using I2 measure, with a value exceeding 50% indicating substantial heterogeneity. Sensitivity analyses were conducted by separating adjusted effect estimates from unadjusted estimates whenever possible and appropriate, and/or excluding one study at a time to identify potential influences [Citation17].
3. Results
3.1. Cohort study
3.1.1. Cohort characteristics
During the study period, 120 patients were diagnosed with COVID-19, of whom 5 were not hospitalized after fever for four or more days. Following exclusion criteria, a total of 115 patients were included in the study, with 65 patients having a claim for ursodeoxycholic acid (UDCA) and 50 patients not. All included patients were young individuals with no comorbidities and had received at least two doses of the COVID-19 vaccine.
The UDCA group was divided into two subgroups based on the daily dose, with 29 (44.6%) and 36 (55.4%) patients receiving 150 mg and 300 mg, respectively. The baseline characteristics of the patients, including age, sex, BMI (calculated as weight in kilograms divided by height in meters squared), body temperature on admission, and days from fever onset to hospitalization, showed no significant difference between the two groups, with a p value of more than 0.05 (). However, there was a significant imbalance in the temperature on admission of clinical baseline among subgroups divided by daily dose (p < 0.001), as demonstrated in Supplementary Table S3.
Table 1. Demographic and clinical characteristics by treatment cohort for UDCA.
Given the retrospective nature of our study, with exposure factors beyond our control, the medications for NSAIDs, which comprised ibuprofen, acetaminophen, and compound preparations that primarily contain either ibuprofen or acetaminophen, varied between the two groups. The proportion of patients using NSAIDs was higher in the UDCA group (98.5%) than in the no-UDCA group (76.0%). There was no notable disparity between the two groups in terms of whether or not to use symptomatic drugs. Specifically, the proportion of patients not using these drugs in the UDCA group accounted for only 1.5% (1 patient) with that in no-UDCA group to be 6.0% (3 patients).
3.1.2. Univariate Kaplan–Meier recovery curves for the primary endpoint
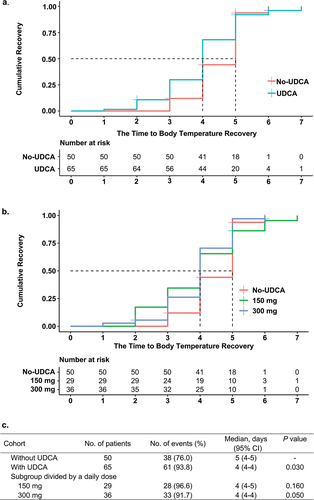
In the two groups of patients who received UDCA and those who did not, 61 patients (93.8%) and 38 patients (76.0%), respectively, had their body temperature returned to 37.3°C or lower before their discharge. Moreover, the median recovery time for the UDCA group was 4 days (95% CI, 4–4), compared to 5 days (95% CI, 4–5) for the no-UDCA group. Meanwhile, a significant difference was observed between the distributions of recovery time in the two groups (p = 0.030). After classifying the patients who received UDCA into two subgroups based on daily dose, the multiplicity-adjusted p value showed that patients in the 300 mg subgroup recovered faster than those in the no-UDCA group with statistical significance, while the difference in recovery time between patients in the 150 mg subgroup and no-UDCA group showed no significance ().
3.1.3. Major adjusted, unadjusted, and minor adjusted cox proportional models by treatment cohort for UDCA
In the primary analysis, after adjusting for covariates (age, sex, BMI, concomitant drug use of NSAIDs, days from fever onset to hospitalization, and temperature on admission) in the Cox proportional hazards models, the use of UDCA was found to be significantly associated with a 63% higher benefit on the speed of Body Temperature Recovery (HR 1.63, 95% CI 1.01–2.60) compared with the no-UDCA group (). The hazard ratio of the UDCA group referring to the no-UDCA group was 1.87 (95% CI, 1.06–3.32) in the unadjusted analysis, suggesting a higher risk of faster recovery in the UDCA group with reasonable confidence, as the baseline is 1. Furthermore, the minor adjusted results of the Cox proportional hazards regression model were consistent with the unadjusted analysis and primary analysis, even after different degrees of adjustments for various covariates, indicating the potential utility of UDCA in improving recovery time with a significant level of confidence.
Table 2. Major adjusted, unadjusted, and minor adjusted Cox proportional models by treatment cohort for UDCA and subgroups divided by daily dose.
3.1.4. Major adjusted, unadjusted, and minor adjusted cox proportional models by UDCA subgroup divided by daily dose
The primary analysis was further conducted using Cox proportional models in the 150 mg and 300 mg subgroups of the UDCA group. Results revealed that the adjusted hazard ratio for the 300 mg subgroup was significantly associated with a shortened time to Body Temperature Recovery ([HR], 1.86 [95% CI, 1.10–3.10]) when compared to the no-UDCA group (), while the 150 mg subgroup did not demonstrate such association. Additionally, under multiplicity adjustment, minor adjustment methods yielded similar results to the primary analysis with major adjustment. Moreover, the estimated hazard ratios of both 150 mg and 300 mg subgroups in the unadjusted analysis did not show significant differences from the baseline no-UDCA group with multiplicity adjustment.
To mitigate the influence of missing data on imputation covariates including age and BMI, a sensitivity analysis was performed, the findings of which were comparable to those of all other analyses, thus ensuring the validity of our results in both groups and further subgroups (Supplementary Tables S4 and S5).
3.2. Data synthesis of state-of-art evidence
3.2.1. Study selection and characteristics of included cohort studies
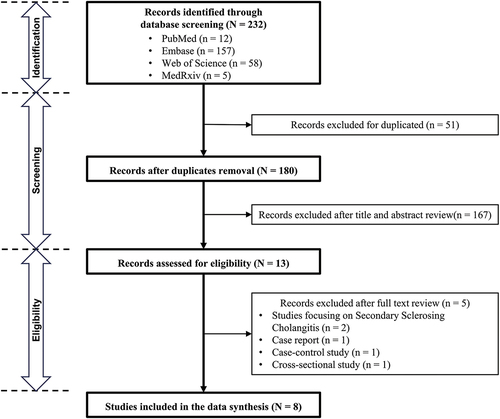
A total of 232 records were identified through systematic database searches. Following title/abstract screening and subsequentially full-text review, eight cohort studies were ultimately included in the evidence synthesis, as delineated in . provides a comprehensive overview of the key characteristics of these studies and the current cohort study. The studies exhibited considerable heterogeneity in terms of patient populations.
Table 3. Selected characteristics of the 9 included cohort studies.
Five of the studies predominantly focused on patients with distinct liver pathologies, encompassing liver transplantation, cirrhosis, chronic liver disease, and cholestatic liver disease. In contrast, the remaining four studies directly targeted hospitalized COVID-19 patients. Notably, aside from this latter subset of our research, patients reported previously presented with diverse comorbidities.
The primary outcomes were risk of severely or critically ill COVID-19 and mortality, which were consistent with most included studies. However, it is crucial to highlight the variance in methodologies employed to control confounding factors across these investigations. More specifically, several studies adopted propensity score matching techniques to mitigate intergroup differences, while others adjusted the effect size based on the baseline conditions of patients.
3.2.2. Evidence synthesis of adverse outcomes of COVID-19
In the examination of seven studies reporting outcomes of severe/critical COVID-19, a notable homogeneity was observed (Pheterogeneity = 0.09, I2 = 46%). Utilizing a random-effects model for pooling, the pooling analysis revealed a significant reduction in the risk of severe/critical COVID-19 among UDCA users compared to UDCA non-users, with a OR of 0.68 (95% CI, 0.50 to 0.94; p = 0.02) (). To mitigate potential bias introduced by effect size transformations, an additional pooling analysis based on the unadjusted OR from the event occurrences was conducted, demonstrating a consistent decrease in risks of severe/critical COVID-19 (OR: 0.49, 95% CI, 0.31 to 0.76; p < 0.01) (Supplementary Figure S1).
Figure 4. Pooled Association between UDCA treatment and clinical outcomes of COVID-19 patients. The outcome included (a) Severe/critical rate and (b) Mortality rate. *Due to the outcome being rare (less than 10%), the RR converted by HR can be approximated as OR, to keep consistent with other studies.
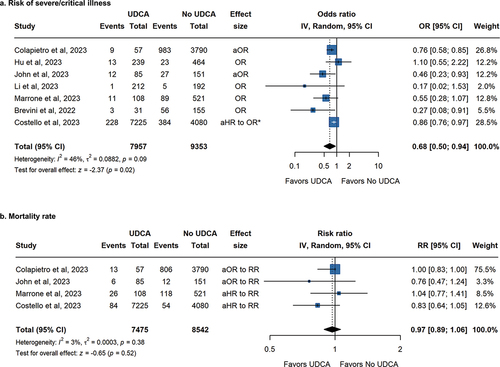
In the evaluation of mortality rates, four studies reported adjusted effect sizes with minimal heterogeneity (Pheterogeneity = 0.38, I2 = 3%). The pooled results indicated no significant difference between the UDCA users and non-users, with an adjusted OR of 0.97 (95% CI, 0.89 to 1.06; p = 0.52) (). Furthermore, two studies reported event occurrences in both groups, with the presence of zero events in some instances. The final effect size, assessed through a combination of six studies using Risk Difference, concluded that UDCA treatment did not exhibit a statistically significant reduction in mortality rates among COVID-19 patients (RD: −0.01, 95% CI, −0.04 to 0.02; p = 0.45) (Supplementary Figure S2).
Sensitivity analyses showed that the removal of either John et al. 2023 or Marrone et al. 2023 would change the pooled OR results for risks of severe or critical illness from significance to non-significance, while removal of any individual study for mortality rate would not yield a differently pooled result (Supplementary Figure S3) [Citation7,Citation9].
3.2.3. Evidence synthesis of COVID-19 recovery
In addition to adverse outcomes of COVID, a total of five studies provided insights into endpoints associated with the recovery of COVID-19. Two of the five studies focused on the length of hospitalization, while our research and two others focused on the duration of recovery. The primary distinction between the two categories lies in the consideration of the length of hospitalization for deceased patients in the former. To ensure consistency between the data in our cohort study and other studies, the recovery time of patients not censored in our study was further used for pooling.
Given that all studies utilized days as the unit of measurement, the mean difference (MD) was used. Regarding the length of hospital stay, results from two studies exhibited minimal heterogeneity (Pheterogeneity = 0.21, I2 = 36%), favoring no use of UDCA, albeit statistically non-significance, (MD: 0.81, 95% CI, −0.92 to 2.53; p = 0.36) (). Three studies showed considerable heterogeneity in recovery times (Pheterogeneity < 0.01, I2 = 92%), and there was not enough data to support a meaningful effect of UDCA on COVID-19 recovery (MD: −0.73, 95% CI, −2.10 to 0.64; p = 0.30) ().
Figure 5. Pooled Association between UDCA treatment and recovery of COVID-19 patients. The outcome included (a) Length of hospital and (b) Recovery time.
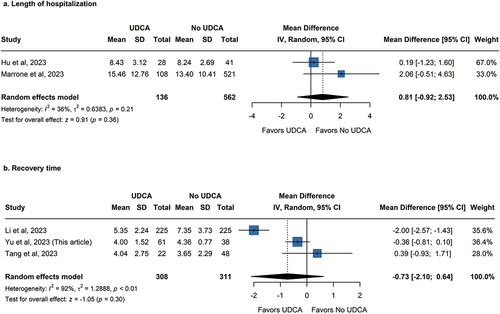
Upon scrutiny of this outcome, it becomes evident that only the study of Li et al. included symptoms other than fever in its calculation of recovery time [Citation8]. This suggests that variations in outcome definition appear to be one of the causes of considerable heterogeneity.
3.2.4. Evidence synthesis of prevention of COVID-19
Three of the nine studies that were examined in-depth investigated the prevalence of COVID-19 in patients with underlying liver diseases. Adjusted odds ratios were reported in these studies to quantify the association between routine use of UDCA and lower susceptibility to SARS-CoV-2. All three studies yielded positive results, indicating a preventive effect of UDCA against COVID-19 in patients with liver diseases receiving routine UDCA treatment. Pooling analysis of these studies revealed a consistent adjusted OR of 0.53 (95% CI, 0.36 to 0.77; p < 0.01; Supplementary Figure S4).
4. Discussion
In this retrospective real-world cohort study of 115 hospitalized COVID-19 patients, we have shown that treatment with UDCA is significantly associated with a reduced time to Body Temperature Recovery after admission. This effect is especially pronounced at a relatively high daily dose.
To our knowledge, this is one of the first retrospective studies evaluating the real-world effectiveness of UDCA in previously healthy, fully vaccinated young patients without preexisting conditions, who were infected with SARS-CoV-2 during the Omicron era. This study provides new data on the treatment of Omicron infection with UDCA and offers additional insights into preventing Omicron subvariants through pretreatment with UDCA. In this cohort study, we found that UDCA significantly shortened the duration of fever caused by SARS-CoV-2 infection in Chinese patients with non-severe COVID-19, without any measurable adverse effects. These promising results warrant further investigation into UDCA as a potential treatment for COVID-19. Both the primary and sensitivity analyses indicated that patients in the UDCA group generally experienced a faster recovery to normal body temperature, regardless of the degree of covariate adjustment. Subgroup analysis showed that patients using 300 mg of UDCA had a significantly shorter duration of fever compared to UDCA nonusers, although there was no significant difference in the duration of fever between users of 150 mg of UDCA and nonusers.
Since the prevention and treatment of COVID-19 with UDCA is currently an off-label use, available data is limited to retrospective analyses in patients with cholestasis or progressive liver disease. These analyses are constrained by numerous expected or unexpected confounding factors. FXR, a key receptor in regulating bile acid synthesis, is influenced by diverse types of bile acids. Importantly, FXR pathway regulates ACE2 in various organs, including the liver, bile ducts, lungs, and bloodstream. This regulation is essential for UDCA in modulating ACE2, which controls the entry of SARS-CoV-2 into host cells. In different liver diseases, FXR expression and serum bile acid levels exhibited significant variation [Citation22]. FXR expression can be affected by gene mutations, such as those seen in progressive familial intrahepatic cholestasis type 2 (PFIC2) [Citation23]. Additionally, the composition and levels of bile acids vary among cholestasis patients with different underlying causes [Citation24–27]. There is no denying that the mechanism of FXR regulated by different endogenous bile acids is complicated, with UDCA just being only one of those bile acids. It has been established that the bile acid composition after UDCA treatment differs between healthy individuals and PBC patients [Citation28,Citation29]. Therefore, it is crucial to evaluate the therapeutic effect of UDCA in healthy individuals to eliminate the influence of disease-related confounding factors.
Our cohort study supports the role of UDCA in the treatment of COVID-19, particularly in previously healthy individuals without comorbidities. The primary endpoint of our study is the duration of fever, as fever is one of the most common symptoms in patients with COVID-19 infection and serves as the main criterion for hospital admission and discharge in the healthcare facilities where our investigation is being carried out [Citation21,Citation30]. We excluded subjects who were not hospitalized after fever for four or more days for two reasons: firstly, the self-limiting characteristics are common in most non-severe COVID-19 cases; secondly, the pharmacological mechanism of UDCA is more geared toward preventing COVID-19 infection. The protective effects of UDCA against COVID-19 infection have been clarified [Citation3]. Specifically, it has been demonstrated that the potential of UDCA to modulate ACE2 provides efficacy as both primary and secondary prophylaxis against COVID-19, as it impedes cellular uptake of SARS-CoV-2 by reducing its binding to membrane-bound ACE2 during the early phase of infection [Citation31]. Likewise, the effectiveness of human recombinant soluble ACE, engineered to specifically bind with SARS-CoV-2 as a substitute for endogenous ACE2, has been proven to significantly reduce viral load [Citation32]. Clinical studies have also found that the use of ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) by veterans of COVID-19 is associated with a lower risk of ICU events and mortality [Citation33].
There was a significant difference between the two groups regarding NSAIDs treatment, which must be considered in our primary analysis. Although it has been suggested that NSAIDs use in COVID-19 patients does not increase the risk of unfavorable outcomes [Citation34], there is no conclusive evidence to indicate NSAIDs can prolong the duration of fever in non-severe COVID-19 patients. Given that the effect of symptomatic drugs on the primary endpoint is negligible and there is a moderate correlation between NSAID use and other symptomatic drugs in this analysis (Supplementary Figure S5), we have chosen to only consider NSAID treatment as an important concomitant medical condition in multivariate adjusted regression. Due to the limited sample size and the impracticality of conducting propensity score matching, we incorporated demographic variables (sex, age, and BMI) as main covariates for adjustment to mitigate the resulting deviation caused by individual variances.
While the overall outcome of our study is positive and clinically significant, its weaknesses must be considered. Due to the short observation period, the small sample size, the relatively restricted patient population characteristics, and the lack of biochemical parameters in medical records, the scope and applicability of this investigation are limited. The short observation period limits our ability to assess the long-term effects and potential late-emerging outcomes of UDCA treatment in COVID-19 patients. Moreover, subgroup analyses of our cohort should be viewed as hypothesis-generated [Citation35]. Additionally, like most other observational studies, we did not formally estimate the sample size priori instead we included all eligible patients on our site. Furthermore, relatively restricted patient population characteristics, such as age, comorbidities, and vaccination status, may not necessarily accurately represent the diverse demographics of all COVID-19 patients. Although there might be unmeasured confounding, sensitivity analyses did not find significant covariate effects on our results [Citation36]. To minimize these limitations and validate the effectiveness of UDCA in the treatment of COVID-19 and overcome the inherent shortcomings of a single study, subsequential pooling analysis was used to provide state-of-art synthesis evidence.
The pooling analysis presented herein represents the most comprehensive assessment and synthesis of studies exploring the association between UDCA and COVID-19 outcomes, which used robust data and methodologies [Citation37–39]. The lack of significant differences in the mortality rates between UDCA users and nonusers might be attributed to the imprecise classifications of COVID-19-induced mortality as opposed to all-cause mortality, even though removal of each of them would not change the significance of pooled estimates [Citation3,Citation4,Citation9]. Another possible reason for the lack of significant difference in mortality between UDCA users and non-users might be the further downregulation of ACE2 in patients with comorbidities, leading to adverse outcomes. ACE2 does play a dual role in COVID-19, acting as an entry route for the virus while also providing protective effects against severe disease. Inhibition of ACE2 can reduce viral entry, but further downregulation of ACE2 in patients who already have low ACE2 levels may lead to increased morbidity and mortality due to enhanced inflammation and thrombosis [Citation40]. Additionally, preexisting renin-angiotensin-aldosterone system (RAAS) dysregulation in the elderly and in patients with heart disease, hypertension, chronic diseases, diabetes, and obesity, as well as large vessel injury caused by SARS-CoV-2 May contribute to adverse outcomes in COVID-19 [Citation41,Citation42]. Therefore, the use of UDCA in patients with these comorbidities requires careful evaluation, which may explain the lack of significant difference in mortality between UDCA users and non-users.
Our study unequivocally indicates that UDCA has a positive impact on the recovery of COVID-19 patients. However, the limited number of studies and the challenges in harmonizing effect sizes in the current pooling analysis hindered the consideration of baseline patient variations in the combined results. Multifactorial influences create significant heterogeneity in recovery rates, complicating the achievement of statistical significance. These nuanced findings underscore the need for further exploration through randomized controlled trials and the standardization of outcome measurement methodologies across studies to improve comparability and yield more definitive conclusions.
Furthermore, considering the pharmacological mechanisms of UDCA, it appears to be better suited as a prophylactic intervention for COVID-19 [Citation43]. The pooling analysis of three studies addressing this question yielded affirmative findings, collectively supporting the potential preventative value of UDCA, especially in individuals with underlying liver disorders. However, the use of UDCA for long-term prevention in healthy individuals or those without preexisting liver conditions necessitates further investigation using different data sources and methods including pharmacovigilance [Citation44], with careful consideration of the balance between benefits and risks.
Overall, this is one of the very first studies conducted in previously healthy, vaccinated patients to evaluate the therapeutic effect of UDCA against SARS-CoV-2. Notably, our study pioneered the use of UDCA in treating COVID-19 infections during the peak of the Omicron pandemic in China. The results are promising, demonstrating that UDCA administration positively impacted therapeutic outcomes in individuals without comorbidities. Specifically, UDCA treatment significantly reduced the time required for Body Temperature Recovery following an Omicron strain infection without observed safety concerns. Furthermore, our cohort study along with other studies reported in the literature were included in the data synthesis to provide the state-of-the-art evidence on associations between UDCA use and COVID-19 outcomes. The findings of this study present novel treatment options for COVID-19, paving the way for further large scale, clinically controlled, and prospective research to explore the full potential of UDCA repurposing for COVID or even long COVID.
5. Conclusion
This study revealed that UDCA treatment notably improves the recovery time following Omicron infection. Subsequently, pooling analysis also supported the use of UDCA and favorable COVID outcomes.
Abbreviations
UDCA, Ursodeoxycholic Acid; COVID-19, Corona Virus Disease 2019; aHR, adjusted Hazard Ratio; OR, Odds Ratio, aOR, adjusted Odds Ratio; RR, Risk Ratio; MD, Mean Difference; CI, Confidence Interval; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; ACE2, Angiotensin-Converting Enzyme 2; FXR, Farnesoid X Receptor; BMI, Body Mass Index; NSAIDs, Nonsteroidal Anti-inflammatory Drugs; CPAP, Continuous Positive Airway Pressure; MV, Mechanical Ventilation; NR, Not Report; PSM, Propensity Score Matching; IQR, Interquartile Range.
Author contributions
Guo YU, Yang Yu, De-Chuan Zhan: conception of cohort study; Guo YU, Guo-Fu Li: design of the study; Jian Li, Shao-Qiu Tang, Lu-Yao Han: Resources and data curation for cohort study; Yang Yu, Guo Yu, Lu-Yao Han, Zhi-Long Zhang, Tian-Shuo Liu: Investigation of cohort study; Guo-Fu Li: Conception of evidence synthesis and meta-analytical methodology; Lu-Yao Han, Shu-Xin Jiao, Yu-Wei Qiao, Na Zhang: Systematic review and meta-analysis; Lu-Yao Han, Guo-Fu Li, Guo Yu: interpretation of the results; Lu-Yao Han, Guo-Fu Li, Guo Yu: drafting of the manuscript; Guo-Fu Li, Guo Yu: critical revision of the manuscript for important intellectual content.
Declaration of interest
The following financial interests may be considered potential competing interests: Y. Yu is the controlling shareholder of Polixir Technologies Co., Ltd. Zhi-Long Zhang and T.S. Liu declare receipt of employment by Polixir Technologies Co., Ltd. The research sponsor, Polixir Technologies Co., Ltd., did not play any role in research design, data collection, analysis and interpretation, report writing, and the decision to submit this paper for publication.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary materials.docx
Download MS Word (639.1 KB)Acknowledgments
The recovery analysis section was presented in a poster at the 81st FIP world congress of pharmacy and pharmaceutical sciences located at Brisbane on September 27, 2023, titled “UDCA May Promote COVID-19 Recovery: A Cohort Study with AI Aided Analysis”, and it was also publicly available in the medRxiv preprint (doi: https://doi.org/10.1101/2023.05.02.23289410). Guo-Fu Li (ORCID: 0000-0002-4628-9941) is also an adjunct professor of clinical pharmacology in Subei People’s Hospital.
Data availability statement
Data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14787210.2024.2376153
Additional information
Funding
References
- Pedersen MR, Greenan G, Arora S, et al. Ursodeoxycholic acid decreases incidence of primary biliary cholangitis and biliary complications after liver transplantation: a meta-analysis. Liver Transplant. 2021 Jun;27(6):866–875. doi: 10.1002/lt.25935
- Bosch A, Dumortier J, Maucort-Boulch D, et al. Preventive administration of UDCA after liver transplantation for primary biliary cirrhosis is associated with a lower risk of disease recurrence. J Hepatol. 2015 Dec;63(6):1449–1458. doi: 10.1016/j.jhep.2015.07.038
- Brevini T, Maes M, Webb GJ, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2023 Mar 2;615(7950):134–142. doi: 10.1038/s41586-022-05594-0
- Colapietro F, Angelotti G, Masetti C, et al. Ursodeoxycholic acid does not improve COVID-19 outcome in hospitalized patients. Viruses-Basel. 2023 Aug;15(8):1738. doi: 10.3390/v15081738
- Costello RE, Waller KMJ, Smith R, et al. Ursodeoxycholic acid and severe COVID-19 outcomes in people with liver disease: a cohort study using the OpenSAFELY platform. medRxiv. 2023:2023.12.
- Hu L, Zhang H, Huang C, et al. Effect of ursodeoxycholic acid on preventing SARS-CoV-2 infection in patients with liver transplantation: a multicenter retrospective cohort study. QJM Int J Med. 2023 Nov 9;117(5):339–347. doi: 10.1093/qjmed/hcad254
- John BV, Bastaich D, Webb G, et al. Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis. J Intern Med. 2023 May;293(5):636–647. doi: 10.1111/joim.13630
- Li Y, Zhu N, Cui X, et al. Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease. Front Cell Infect Microbiol. 2023 May 3;13. doi: 10.3389/fcimb.2023.1178590
- Marrone G, Covino M, Merra G, et al. Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: a retrospective study of propensity score-matched cohorts. Liver Int. 2023;44(1):83–92. doi: 10.1111/liv.15736
- Tianjiao T, Huatao L, Bibo M, et al. Clinical efficacy of ursodeoxycholic acid in the treatment of COVID-19. Chin J Clin Infect Dis. 2023;16(4):289–292,298.
- Zhu CC, Zhu J. The effect of self-limiting on the prevention and control of the diffuse COVID-19 epidemic with delayed and temporal-spatial heterogeneous. BMC Infect Dis. 2021 Nov 9;21(1):1145. doi: 10.1186/s12879-021-06670-y
- Yu G, Zhang N, Guan AJ, et al. Validity of meta-analytical estimates and hazard ratios quantifying survival benefit of immunochemotherapy in low PD-L1-Expressing esophageal squamous cell carcinoma. J Clin Oncol. 2023 May 20;41(15):2862–2864. doi: 10.1200/JCO.23.00057
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.2013
- VanderWeele TJJE. On a square-root transformation of the odds ratio for a common outcome. Epidemiology. 2017;28(6):e58–e60. doi: 10.1097/EDE.0000000000000733
- VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183
- Li GF, An XX, Yu Y, et al. Do proton pump inhibitors influence SARS-CoV-2 related outcomes? A meta-analysis. Gut. 2021 Sep;70(9):1806–1808. doi: 10.1136/gutjnl-2020-323366
- Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–e745. doi: 10.1016/S2352-3026(20)30251-9
- Brusasco C, Corradi F, Dazzi F, et al. The use of continuous positive airway pressure during the second and third waves of the COVID-19 pandemic. ERJ Open Res. 2023;9(2):00365–2022. doi: 10.1183/23120541.00365-2022
- Clinical spectrum of SARS-CoV-2 infection. 2022. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- China updates COVID-19 diagnosis, treatment protocol [Internet]. 2023 [cited 2023]. Available from: http://english.nmpa.gov.cn/2023-01/10/c_861471.htm
- Chiang JYL, Ferrell JM. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol. 2022 May 15;548:111618. doi: 10.1016/j.mce.2022.111618
- Gomez-Ospina N, Potter CJ, Xiao R, et al. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun. 2016 Feb 18;7(1):10713. doi: 10.1038/ncomms10713
- Zhu P, Zhang J, Chen Y, et al. Analysis of human C24 bile acids metabolome in serum and urine based on enzyme digestion of conjugated bile acids and LC-MS determination of unconjugated bile acids. Anal Bioanal Chem. 2018 Aug;410(21):5287–5300. doi: 10.1007/s00216-018-1183-7
- Ambros-Rudolph CM, Glatz M, Trauner M, et al. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143(6):757–762. doi: 10.1001/archderm.143.6.757
- Burkard I, von Eckardstein A, Rentsch KM. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Nov 5;826(1–2):147–159. doi: 10.1016/j.jchromb.2005.08.016
- Henriksen NL, Hansen SH, Lycas MD, et al. Cholestasis alters brain lipid and bile acid composition and compromises motor function in neonatal piglets. Physiol Rep. 2022 Jul;10(13):e15368. doi: 10.14814/phy2.15368
- Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012 Jul;57(1):133–140. doi: 10.1016/j.jhep.2012.02.014
- Dyson JK, Hirschfield GM, Adams DH, et al. Novel therapeutic targets in primary biliary cirrhosis. Nat Rev Gastro Hepat. 2015 Mar 1;12(3):147–158. doi: 10.1038/nrgastro.2015.12
- Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLOS ONE. 2020;15(6):e0234765. doi: 10.1371/journal.pone.0234765
- Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021 Apr;27(4):668–676. doi: 10.1038/s41591-021-01310-z
- Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 May 14;181(4):905–913 e7. doi: 10.1016/j.cell.2020.04.004
- Rizk JG, Wenziger C, Tran D, et al. Angiotensin-converting enzyme inhibitor and angiotensin receptor blocker use associated with reduced mortality and other disease outcomes in US veterans with COVID-19. Drugs. 2022 Jan 1;82(1):43–54. doi: 10.1007/s40265-021-01639-2
- Kragholm K, Torp-Pedersen C, Fosbol E. Non-steroidal anti-inflammatory drug use in COVID-19. Lancet Rheumatol. 2021 Jul;3(7):e465–e466. doi: 10.1016/S2665-9913(21)00144-2
- Qiao Y, Yu G, Li G. Overall Survival Benefit with Sacituzumab Govitecan in Metastatic Breast Cancer: A Post Hoc Interaction Analyses of a Randomized Controlled Trail. Clin Drug Investig. 2024;44(6):455–457. doi: 10.1007/s40261-024-01367-x
- Li GF, Qiao YW, Yu G. Helicobacter pylori therapy and risk of gastric cancer after endoscopic resection of dysplasia: a sensitivity analysis assessing impact of unmeasured confounding. Gastroenterology. 2024 Mar 15;167(2):417–418. doi: 10.1053/j.gastro.2024.03.010
- Wu DN, Yu G, Li GF. Validity of meta-analytical data on cutaneous adverse events with phosphoinositide 3-kinase inhibitors. JAMA Oncol. 2023 Oct 1;9(10):1462. doi: 10.1001/jamaoncol.2023.3384
- Xiao YT, Cai J, Li GF. Data extraction and handling issues on evidence synthesis of risk of immunoglobulin e-mediated food allergy. JAMA Pediatr. 2023 Sep 1;177(9):983. doi: 10.1001/jamapediatrics.2023.2465
- Wu DN, Guan AJ, Li GF. Data reproducibility issues on evidence synthesis of adverse events associated with HER2-targeted antibody-drug conjugates. EClinicalMedicine. 2023 Apr;58:101904. doi: 10.1016/j.eclinm.2023.101904
- Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Internal Med. 2020 Jun;76:14–20. doi: 10.1016/j.ejim.2020.04.037
- Abenavoli L, Aquila I, Sacco MA, et al. Liver damage and impaired coagulation in COVID-19 patients: a case series. Diseases. 2023 Oct 13;11(4). doi: 10.3390/diseases11040141
- Ramos SG, Rattis B, Ottaviani G, et al. ACE2 down-regulation may act as a transient molecular disease causing RAAS dysregulation and tissue damage in the microcirculatory environment among COVID-19 patients. Am J Pathol. 2021 Jul;191(7):1154–1164. doi: 10.1016/j.ajpath.2021.04.010
- Pan S, Zhang Y, Meng F, et al. Can ursodeoxycholic acid prevent SARS-CoV-2 infection or reduce the COVID-19 severity? Current knowledge and unresolved issues. Infect Immun. 2023;3(3):114–119.
- Li GF, Yu G. Drug-induced liver injury with ritonavir-boosted nirmatrelvir: evidence from coronavirus disease 2019 emergency use authorization adverse event reporting system. Gastroenterology. 2023 Jul;165(1):305–306. doi: 10.1053/j.gastro.2023.02.008