ABSTRACT
Background: This cohort study investigated the role of the active matrix metalloproteinase-8 (aMMP-8) and interleukin-6 (IL-6) as oral fluid biomarkers for monitoring the periodontal degeneration occurring in head and neck cancer (HNC) patients treated by radiotherapy.
Research design and methods: Eleven patients, aged 28–74, diagnosed with HNC were included in the study. Complete periodontal and oral examinations were performed pre-radiotherapy and 1 month after radiotherapy. Mouthrinse samples (pre-radiotherapy, after 6 weeks of radiotherapy and 1 month after radiotherapy) were assayed by aMMP-8 point-of-care-kit (PerioSafe®/ORALyzer®) for aMMP-8 and ELISA for IL-6.
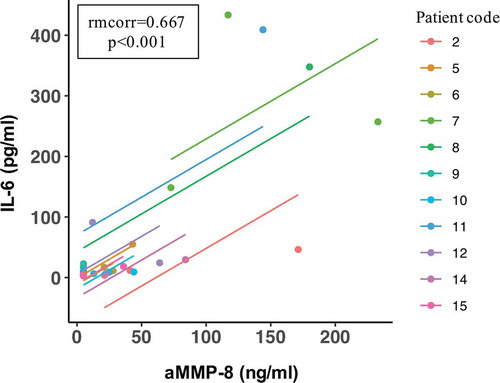
Results: HNC radiotherapy had a deteriorating impact on the periodontium and a significant impact on periodontal biomarkers aMMP-8 and IL-6 and increased their levels in mouthrinse. Clinical-attachment-loss (CAL) (site of greatest loss: mean = 1.7 mm, range = 1–3 mm) corresponding to rapid progression of periodontitis. There was a positive repeated measures correlation (rmcorr = 0.667) between the aMMP-8 and IL-6 levels.
Conclusions: Elevated aMMP-8 levels were observed 1 month after radiotherapy among some HNC patients suggesting a prolonged increased susceptibility to further periodontal tissue destruction. Currently available aMMP-8 point-of-care testing could be useful to monitor and assess quantitatively online and real-time the risk of deterioration of periodontal health during HNC radiotherapy.
1. Introduction
Head and neck cancers (HNC) constitute a heterogenous group of different malignant tumors that arise in several anatomical locations, including the oral cavity, larynx, pharynx, oropharynx, nasopharynx, and sinonasal. In 2018, HNC was the seventh most common cancer worldwide with 890,000 new cases and 450,000 deaths were reported [Citation1]. HNC represents nearly 4% of all cancers (65,630 new cases) and 2.4% of all cancer deaths (14,500 deaths) in the United States [Citation2]. One of the major reasons leading to the high HNC mortality is the late diagnosis, as symptoms usually appear at a late stage of the disease. Thus, early detection and early treatment are of utmost importance to improve the cure rate of these malignancies and to avoid massive, mutilating operations.
Radiotherapy is the standard treatment modality for most HNC patients either alone or in combination with other treatment options to eliminate the tumor cells [Citation3]. In addition to a direct cytotoxic effect on the tumor cells, radiotherapy can also substantially affect the tumor-surrounding immune microenvironment, i.e. the amount and configuration of immune cells infiltrating into the tumor [Citation3]. HNC radiotherapy carries several side effects, including a deteriorating effect on the oral immune fitness and periodontium making it more susceptible to periodontitis and progression of attachment loss [Citation4–8]. Furthermore, periodontitis may exert a negative effect on the quality of life of the patients including emotional, social, functional and esthetic aspects, which are amplified by the severity of periodontitis [Citation9]. Thus, management of the oral health of head and neck cancer patients going through radiotherapy is important for both medical and oral health professionals [Citation5].
The above described supports the imperative need for sensitive biomarkers to improve the early detection of tissue destruction of the periodontium as a side effect of the radiotherapy. In this regard, measurement of biomarkers for periodontitis from mouthrinse by an adjunctive point-of-care (PoC) method could offer an inexpensive, easy, and noninvasive means of detecting this destructive process in HNC patients [Citation10,Citation11]. Biomarkers for diagnosing periodontitis and its progressive phases, such as active matrix metalloproteinase-8 (aMMP-8) and interleukin-6 (IL-6), have been studied extensively [Citation10,Citation11]. Matrix metalloproteinase (MMP)-8 is particularly related to inflammatory conditions, systemic diseases, and cancer, and it is a major destructive collagenase in periodontitis as well [Citation10,Citation12]. The progression of periodontitis has been repeatedly associated with pathologically excessive elevation of active MMP-8 (aMMP-8), which is reflected in oral fluids, i.e. gingival crevicular fluid (GCF), peri-implant sulcular fluid (PISF), mouthrinse, and saliva [Citation10,Citation12–14]. MMP-8, also known as collagenase-2 or neutrophil collagenase, is expressed mainly by neutrophils but also by several other cells, such as macrophages, plasma cells, T-cells, fibroblasts, chondrocytes, epithelial cells, and oral squamous carcinoma cells [Citation12]. MMP-8 is secreted as an inactive latent pro-enzyme and activation can be initiated by extracellular proteinases such as tumor-associated trypsin-2 or by other MMPs, as well as by microbial proteases from periodontopathogens and Candida [Citation12,Citation15]. Tissue inhibitor of metalloproteinases 1 (TIMP-1) is the major endogenous inhibitor of MMP-8 [Citation10,Citation12].
IL-6 is a pro-inflammatory cytokine secreted by several cells, including immune cells, fibroblasts, keratinocytes, adipose tissue, and muscles [Citation16]. IL-6 is involved in inflammation and infection responses, regulates host-response to bacterial infections and is part of the pathogenesis of periodontitis by its link to molecular mediators of active periodontal degeneration and progression of periodontitis (APD) [Citation16,Citation17]. IL-6 can induce pro-inflammatory and tissue destructive cascades and potentiate the amplitude and severity of host response and activation cascades involving other pro-inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α [Citation16–18]. Furthermore, IL-6 modulates the bone homeostasis and turnover by anti-osteogenic and pro-osteoclastic effects resulting in inhibition of bone formation [Citation18].
At the moment, there is a gap in knowledge on the utility of oral fluid biomarkers for prediction of and online monitoring of the side effects of HNC radiotherapy. Modern rapid noninvasive PoC oral fluid chairside technologies, which are developed for monitoring the progression of periodontal tissue degradation and periodontitis, could be particularly useful in HNC patients treated by radiotherapy. Currently, such technologies are already commercially available for aMMP-8 and we used one in this study. Thus, the aim of this study was to investigate the possible utility of two periodontal tissue degeneration biomarkers, aMMP-8 and IL-6, in identifying and predicting the periodontal side effects of the HNC radiotherapy.
2. Patients and methods
2.1. Study design and participants
Ten males and one female HNC patients (aged 28–74 years) from the Bakırköy Eğitim Araştırma Hastanesi (BEAH) hospital, Istanbul, Turkey, were included in this cohort study. Tumor types were based on the routine pathology reports and confirmed by the BEAH oncologists. The dose of radiotherapy administered to the patients was planned by experienced radiation oncologists according to the stage of the disease and based on The National Comprehensive Cancer Network® (NCCN®) guidelines. Complete periodontal and oral examinations were carried out pre-radiotherapy and 1 month after radiotherapy. Oral fluid samples for periodontitis biomarkers aMMP-8 and IL-6 were collected pre-radiotherapy, during radiotherapy, and 1 month after radiotherapy. Current systemic diseases, medications, and smoking habits were reported by the participants. All study participants signed an informed consent and the study was approved by the BEAH ethical committee (Protocol Number: 2018/453), Istanbul, Turkey. The study was conducted according to the principles of the Declaration of Helsinki.
The inclusion criteria to this study were the following: patients at least 21 years old; histological diagnosis of an oropharyngeal/neck cancer; presence of at least ten teeth; and no periodontal treatment during the previous 1 year. The exclusion criteria were as follows: patients who could not receive radiotherapy treatment; patients whose radiotherapy treatment was interrupted; patients with Eastern Cooperative Oncology Group (ECOG) performance 3 and higher; patients with immune associated disorders (i.e. chronic inflammatory diseases such as lupus erythematosus, rheumatoid arthritis, multiple sclerosis, Crohn’s disease); and patients with the human immunodeficiency virus (HIV) positivity.
2.2. Procedures
2.2.1. Radiotherapy procedure
Patients were immobilized in the supine position from the vertex to shoulders with thermoplastic masks. The planning CT datasets at 3 mm slice thickness were generated with Toshiba Aquilian Computed Tomography simulation (Toshiba, Japan). All CT images were imported to the Monaco treatment planning system (CMS Inc, Version 5.1, St. Louis, MO) to outline all target volumes and critical structures located in the head and neck region. Treatment plans were calculated using the Monaco treatment planning system. For definitive cases, the primary targets were prescribed to a dose of 70–72 Gy while the regional lymph nodes were prescribed to a dose of 54–58 Gy. For post‐operative cases, the prescription doses to the primary tumor beds were 60–66 Gy. 6 MV photon beams generated by Elekta Synergy linear Accelerator (Elekta Oncology, UK) was used to design IMRT plans and patients were treated based on these plans.
2.2.2. Dental examination
Periodontal examinations were performed pre-radiotherapy (baseline) and 1 month after the end of radiotherapy by a single experienced periodontist (MK). The following clinical parameters were assessed: probing pocket depth (PPD), bleeding on probing (BOP), clinical attachment loss (CAL), plaque index (PI) and mobility index [Citation19]. Clinical parameters were evaluated for all teeth present (including the third molars). PPD and CAL were recorded from six sites, BOP and PI from four sites of each tooth. CAL was measured from the cementoenamel junction to the base of the periodontal pocket. BOP was recorded based on the presence or absence of bleeding 10 seconds after probing (0 or 1, respectively). PI was scored as 0 to 3 [Citation20]. All probing measurements were performed using a manual millimeter periodontal probe (Williams Periodontal Probe PW; Hu-Friedy®, Chicago, IL, USA). Patients were diagnosed according to the new classification system of periodontitis described by Tonetti et al. (2018) [Citation21].
2.2.3. Mouthrinse collection/analysis of samples
Mouthrinse sampling was performed before the radiation therapy; 1, 3, and 6 weeks after radiotherapy had started; and 1 month after radiotherapy had ended. The study participants were instructed to avoid tooth brushing and eating 1 hour prior to the visit. First, each participant rinsed and gargled the mouth with water for 30 seconds and then spit out. After 60 seconds of waiting, they re-rinsed the mouth with 5 ml of rinsing fluid (sterile purified water) for 30 seconds and then poured the liquid in their mouth into a measuring cap. The sample was taken from the measuring cup with a syringe, filtered and transferred to the aMMP-8 PoC kit (Periosafe®) for the aMMP-8 PoC analysis. The active form of matrix metalloproteinase-8 (aMMP-8) was analyzed quantitatively by the digital reader (ORALyzer®) according to the manufacturer’s instructions as described earlier [Citation22]. The detection limit for aMMP-8 was 10 ng/ml. The remaining mouthrinse samples were transferred into eppendorf tubes and stored in −70°C for further analysis. The concentrations of IL-6 from mouthrinse samples were determined by commercially available enzyme-linked immunosorbent (ELISA) kit according to the protocol of the manufacturer (Human IL-6 Quantikine® ELISA kit, R&D Systems, Minneapolis, MN, USA). The detection limit was 0.70 pg/ml.
2.3. Statistical analysis
The periodontal parameters furcation involvement, mobility, clinical attachment loss, probing depth, bleeding on probing and plaque index were examined pre-radiotherapy and post-radiotherapy (1 month after the radiotherapy). The mean was calculated for each periodontal parameter (all teeth and categorized to maxilla and mandible). The differences in measurements pre-radiotherapy and post-radiotherapy indicated changes in the parameter. The significance between these two time-points was calculated by the paired t-test for each parameter (for all teeth, maxilla, and mandible). Missing values in periodontal parameters were caused by extracted teeth and edentulous maxilla and were deleted pairwise. In addition, aMMP-8 and IL-6 levels were measured pre-radiotherapy, after 6 weeks of radiotherapy and post-radiotherapy (1 month after the radiotherapy). The difference in the mean levels of aMMP-8 and IL-6 in the patient sample between the three time-points were assessed by the repeated measures ANOVA analysis. Pairwise comparisons from the repeated measures ANOVA analysis were adjusted for multiple comparisons by the Bonferroni post hoc test. The repeated sampling of participants was controlled by calculating the repeated measures correlation (rmcorr) between aMMP-8 and IL-6 [Citation23]. There were no missing values regarding aMMP-8 and IL-6 levels. A two‐tailed P-value below 0.05 was considered statistically significant. Statistical analyses were made using the rmcorr package (version 0.4.1) in R statistical software version 3.6.3, and the SPSS version 25.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA). Analyses were done without an a priori power calculation, because of the lack of information about the effect of radiotherapy on repeated measures of oral fluid biomarkers aMMP-8 and IL-6.
3. Results
3.1. Demographic of the study population
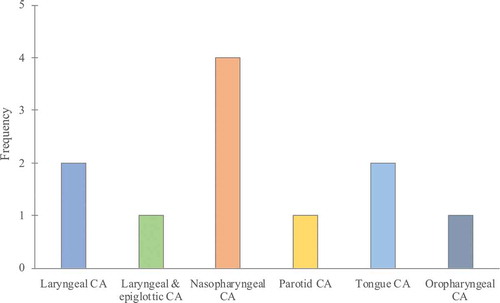
Patient demographic and oncological characteristics are presented in and . Briefly, location of the HNCs were the following in this study population: two laryngeal tumors (squamous cell carcinoma, SCC), one laryngeal and epiglottic (SCC), four nasopharyngeal (three nonkeratinizing and one keratinizing carcinoma), one parotid cancer (myoepithelial carcinoma), two tongue cancer (SCC) and one oropharyngeal cancer (SCC). The mean age of patients was 53.1 years (range 28–74 years); 10 (90.9%) were male; all patients had a history of smoking (≥10 cigarettes a day, more than 5 years). Six (54.5%) patients were systemically healthy, two (18.2%) patients had Type II diabetes, two (18.2%) patients had chronic obstructive pulmonary disease (COPD), one (9.1%) patient hypertension, one (9.1%) patient had cardiovascular disease and one (9.1%) patient had hypothyroidism. The mean number of teeth was 21.6 (range 14–28; maxilla & mandible), 9.5 (range 0–14; maxilla) and 12.1 (range 5–16; mandible). One (9.1%) of the patients had an edentulous maxilla. Five (45.4%) patients were diagnosed with stage II periodontitis, two (18.2%) patients with stage III periodontitis and four (36.4%) patients with stage IV periodontitis. The mean of total radiotherapy dose was 6686.4 (cGy) (range 5800–7000 cGy). Six of the patients (54.5%) were treated by chemotherapy in adjunct with radiotherapy.
Table 1. Patient characteristics (n = 11)
3.2. The difference in clinical periodontal parameters before and after the radiotherapy
The impact of radiotherapy was deterioration of the periodontium (). Firstly, results showed that the change in CAL between pre- and post-radiotherapy (site of greatest loss) was on average 1.7 mm (range 1–3 mm), which corresponds to grade C (= rapid progression) in the classification system of periodontitis [Citation21]. All patients had at least a 1 mm CAL loss (site of greatest loss) during the follow-up time, a much shorter than 5 years. Secondly, there was a small increase in the mean of CAL, probing depth, mobility, and furcation involvement after radiotherapy in all teeth, and when categorized for maxilla and mandible. However, the difference between these two time-points was significant only for CAL in all teeth (p < 0.001), maxilla (p = 0.001) and mandible (p = 0.001) and for probing depth in all teeth (p = 0.043) and mandible (p = 0.015). Similarly, bleeding on probing increased significantly in all teeth (p = 0.002), maxilla (p = 0.047) and mandible (p < 0.001) after radiotherapy. The difference in plaque index was not significant between the two time-points.
Table 2. Periodontal parameters when all teeth, maxilla, and mandible were examined pre- and post-radiotherapy (1 month after the radiotherapy). The significance between these two time-points calculated by paired t-test for each parameter
3.3. Estimated marginal means of aMMP-8 and IL-6 levels between pre-and postradiotherapy
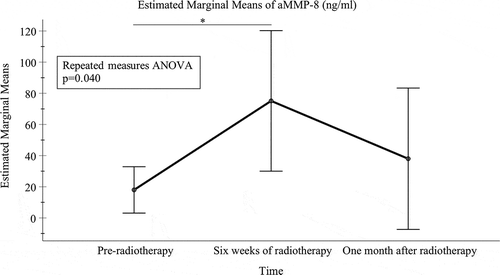
Regarding the impact of radiotherapy on aMMP-8 levels in mouthrinse, there was a significant difference in the mean levels of aMMP-8 between the three time-points (pre-radiotherapy: 17.99 ng/ml [95% confidence interval (CI) = 3.10–32.87 ng/ml]; 6 weeks of radiotherapy: 75.12 ng/ml [30.01–120.23 ng/ml]; and 1 month after radiotherapy: 38.00 ng/ml [−7.37–83.37 ng/ml]) (p = 0.040) (). Pairwise comparisons revealed that the difference was specifically significant for the mean levels of aMMP-8 between the pre-radiotherapy and 6 weeks of radiotherapy (p = 0.049) (). Other pairwise comparisons were not significant (between pre-radiotherapy and 1 month after radiotherapy, p = 0.713; and between 6 weeks of radiotherapy and 1 month after radiotherapy, p = 0.551).
Figure 2. Mean levels of aMMP-8 (ng/ml) with 95% confidence interval bars for time-points of pre-radiotherapy, at the end of 6 weeks of radiotherapy and one month after radiotherapy. All significant (* p < 0.05) pairwise comparisons from repeated measures ANOVA analysis adjusted for multiple comparisons (the Bonferroni post hoc test) are marked in the figure
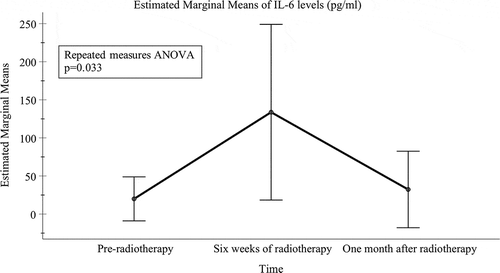
Likewise, there was a significant difference in the mean levels of IL-6 in the mouthrinse between the three time-points (pre-radiotherapy: 19.94 pg/ml [95% CI = −8.94–48.83 pg/ml]; after 6 weeks of radiotherapy: 133.73 pg/ml [18.40–249.06 pg/ml]; and 1 month after radiotherapy: 32.27 pg/ml [−17.95–82.49 pg/ml]) (p = 0.033) (). However, none of the pairwise comparisons adjusted for multiple comparisons were significant (between pre-radiotherapy and 6 weeks of radiotherapy, p = 0.095; between pre-radiotherapy and 1 month after radiotherapy, p = 0.729; or between 6 weeks of radiotherapy and 1 month after radiotherapy, p = 0.117).
Figure 3. Mean levels of IL-6 (pg/ml) with 95% confidence interval bars for time-points of pre-radiotherapy, at the end of 6 weeks of radiotherapy and one month after radiotherapy. No significant pairwise comparisons were found from repeated measures ANOVA analysis adjusted for multiple comparisons (the Bonferroni post hoc test)
Finally, there was a significant positive correlation between the aMMP-8 and IL-6 levels measured pre-radiotherapy, after 6 weeks of radiotherapy and 1 month after the end of radiotherapy (rmcorr = 0.667 [95% CI = 0.331–0.853], p < 0.001) ().
4. Discussion
Currently, there are no studies on the use of oral fluid biomarkers for the detection of deteriorating side effects of HNC radiotherapy on the periodontium. With this background, we studied here the usefulness and ability of mouthrinse aMMP-8 point-of-care/chairside lateral flow immunotest and IL-6 levels (ELISA) to diagnose and characterize the potential deterioration of oral and periodontal tissues induced by the HNC radiotherapy. Our main finding in this study was that radiotherapy was associated with both deterioration of the periodontal health as well as elevated aMMP-8 and IL-6 levels in HNC patients. The most prominent changes in clinical periodontal measures were observed in CAL, which corresponded to a rapid progression (from grade A to grade C) of periodontitis according to the new classification system of periodontitis [Citation21]. At the same time, radiotherapy had a significant impact on the mean levels of aMMP-8 and IL-6 in the mouthrinse. The aMMP-8 levels increased significantly during the 6 weeks of radiotherapy and decreased after the radiotherapy, although not significantly when measured 1 month after the end of radiotherapy. In this regard, it seems that for some patients it may take longer than 1 month before the aMMP-8 levels and active collagenolysis diminish to their original, pre-radiotherapy level. This suggests a prolonged increase in susceptibility to further progression of periodontitis after radiotherapy among these patients. There were significant differences in the mean levels of IL-6 in mouthrinse between the before, during and after the radiotherapy time-points, as well. IL-6 levels followed the same trend as the aMMP-8 levels by increasing during radiotherapy and decreasing after radiotherapy. However, the pairwise differences in IL-6 levels between the three time-points were not significant.
Our results support and further extend the previous studies that have demonstrated a significant damage to the periodontium and loss of periodontal attachment due to radiotherapy [Citation4–8]. This deteriorating effect of radiotherapy on the periodontium is significantly reflected in elevated aMMP-8 levels measured and detectable by the aMMP-8 point-of-care mouthrinse test utilized in this study. Furthermore, we observed a moderate to strong positive correlation between the aMMP-8 and IL-6 levels measured in the mouthrinses before, during and after the HNC radiotherapy. To our knowledge, this is the first time this kind of an association between these two periodontitis biomarkers, aMMP-8 and IL-6, is reported in oral fluid in vivo. Although a correlation does not necessarily imply causality, it has been previously shown that MMP-8 activity can modulate the production of proinflammatory IL-6 and IL-8 in inflammation [Citation24]. In this regard, it should be noted that elevated IL-6 levels may cause excessive osteoclastic activity and osteolysis [Citation18]. Furthermore, previous studies have reported that IL-6 plays an important role mainly in the initiation and acute phase of periodontitis [Citation17]. Thus, MMP-8 is not only a proteinase but has also inflammatory regulatory and immunomodulatory roles, as has been previously shown, as well [Citation10]. Nevertheless, activation of MMP-8 is a complex process [Citation10]. Further research is still needed to better understand the initiation of the periodontal pathogenesis in HNC radiotherapy and the role of different molecules and cascades in it, such as TIMP-1, the major endogenous inhibitor of MMP-8 activation, and, for example, if its downregulation occurs in effect of HNC radiotherapy.
Our results support the clinical value of the aMMP-8 test that is performed in 5 minutes. It has the ability to detect and highlight active periodontal and dental peri-implant diseases as well as periodontal and peri-implant tissue degeneration before clinical or X-ray manifestations. Previous studies have shown that the increased collagenolytic activity in the periodontium and peri-implant tissues is reflected in the pathological elevation of aMMP-8 levels in oral fluids, but not in total or latent MMP-8 levels [Citation10,Citation12–14,Citation25]. Pathological elevation of active collagenolysis is a key characteristic of active periodontal disease resulting in attachment loss and progression of periodontitis and dental peri-implantitis [Citation10,Citation12–14,Citation25]. Conventional periodontal and dental peri-implant diagnostic procedures, i.e. clinical measurements of gingival pocket depth, clinical attachment loss and bleeding on probing, together with a radiological examination, can assess only the past tissue destruction but they do not provide any exact information about the disease status (active or inactive) or its future progression [Citation26]. The quantitative point-of-care oral fluid aMMP-8 immunotest has been repeatedly and independently validated in Finland, Germany, Italy, Nigeria, Turkey, Netherlands and United States to successfully screen susceptible sites and patients, differentiate active and inactive periodontal and dental peri-implant sites and periodontitis and dental peri-implantitis [Citation25,Citation27], predict the future disease progression and monitor treatment response and maintenance therapy [Citation25,Citation27]. Furthermore, previous studies have shown that the aMMP-8 test could be potentially useful related to diabetes and related diseases in the medical settings [Citation28–30].
Previous research has not so far, to the best of our knowledge, contributed to the development of the diagnostic and eventually preventive methodology for the adjunctive periodontal diagnostics and monitoring of the periodontal side effects of radiotherapy in HNC patients. Overall, we were able – for the first time – to demonstrate the potential usefulness of oral fluid, i.e. mouthrinse aMMP-8 lateral flow immunotest as a quantitative online and real-time chairside/PoC diagnostic tool to monitor the development and consequences of the degenerative weakening and deteriorating oral immune fitness induced by HNC radiotherapy. The deteriorating side effects reflecting weakening and worsening of the oral immune fitness/health could possibly be reflected by another pro-inflammatory biomarker, IL-6, as well. However, to our knowledge, a rapid quantitative point-of-care/chairside mouthrinse test technology for periodontal disease monitoring is currently available only for aMMP-8, but not for IL-6 and other potential biomarkers. Naturally, the results of this study should be considered with respect to the small sample size. Further research is still needed and warranted in larger cohorts to confirm the results of this pilot study.
5. Conclusions
This pilot study extends our knowledge on the periodontal side effects of radiotherapy in HNC patients and the results could help in developing diagnostic and eventually preventive methods for these severe side effects of radiotherapy. The analysis confirmed that HNC radiotherapy had a significant impact on periodontal biomarkers, aMMP-8 and IL-6, and their levels in mouthrinse in addition to deteriorating impact on the periodontium. Furthermore, there was a moderate to strong positive correlation between the aMMP-8 and IL-6 levels in mouthrinse. To our knowledge, the results are the first to present the potential usefulness of oral fluid biomarkers and an aMMP-8 point-of-care/chairside mouthrinse test as an adjunctive diagnostic methods in HNC radiotherapy patients, as well as, the association between the two periodontitis biomarkers, aMMP-8 and IL-6, reflected in oral fluids in vivo.
The results of this study support the potential usefulness of oral fluid, i.e. a mouthrinse aMMP-8 lateral flow point-of-care immunotest as a quantitative online and real-time chairside/PoC diagnostic tool to monitor the development and consequences of the degenerative weakening and deteriorating oral immune fitness induced by HNC radiotherapy. Elevated mouthrinse aMMP-8 levels observed among HNC patients after radiotherapy suggest an increased susceptibility to further periodontal degeneration and a need for targeted periodontal prevention and treatment. Further research is needed in larger cohorts to confirm these preliminary results.
Article highlights
Head and neck cancer (HNC) radiotherapy had a deteriorating impact on the periodontium.
Elevated aMMP-8 levels were observed 1 month after radiotherapy among some HNC patients suggesting a prolonged increased susceptibility to further periodontal tissue destruction.
There was a moderate to strong positive correlation between the aMMP-8 and IL-6 levels in mouthrinse during HNC radiotherapy, indicating an association between the two periodontitis biomarkers that was reflected in oral fluids in vivo for the first time.
Periodontal biomarkers, especially aMMP-8, may be useful for identifying increased susceptibility to further periodontal degeneration during HNC radiotherapy.
Point-of-care/chairside oral fluid biomarker diagnostics could offer an online and real-time tool to monitor and assess quantitatively the risk of deterioration of periodontal health during HNC radiotherapy.
Contributors
M. Keskin, T. Sorsa, A.M. Şenışık, D. Karaçetin, A. Yentek Balkanay, U.K. Gursoy, J. Hagström, J. Rautava, C. Haglund, and T. Tervahartiala were involved in study conception or design, or in data acquisition, data collection and data management. I.T. Räisänen, M. Keskin, N Rathnayake, H. Lähteenmäki, P. Pärnänen, P. Heikkilä, A. Silbereisen, N. Bostanci, and T. Sorsa were involved in data analysis and interpretation. M. Keskin, T. Sorsa, I.T. Räisänen, and T. Tervahartiala verified the underlying data. All authors were involved in manuscript writing, provided important intellectual content and critically reviewed and approved the paper. Authors agree to be accountable for all aspects of the work.
Declaration of interest
T. Sorsa is the inventor of US-patents 5652223, 5736341, 5866432, 6143476, 20170023571A1 (granted 6.6.2019), WO2018/060553A1 (granted 31.5.2018), 10488415B2, and a Japanese patent 2016-554676. P. Pärnänen is the inventor and holder of the patent EP2585087B. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download PDF (864.9 KB)Acknowledgments
The authors would like thank all the patients and research staff for their contributions and commitment to the present study.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
- Karam SD, Raben D. Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol. 2019;20(8):e404–e416.
- Schuurhuis JM, Stokman MA, Witjes MJH, et al. Patients with advanced periodontal disease before intensity-modulated radiation therapy are prone to develop bone healing problems: a 2-year prospective follow-up study. Support Care Cancer. 2018;26(4):1133–1142.
- Irie MS, Mendes EM, Borges JS, et al. Periodontal therapy for patients before and after radiotherapy: A review of the literature and topics of interest for clinicians. Med Oral Patol Oral Cir Bucal. 2018;23(5):e524–e530.
- Sroussi HY, Epstein JB, Bensadoun RJ, et al., Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6(12):2918–2931.
- Marques MA, Dib LL. Periodontal changes in patients undergoing radiotherapy. J Periodontol. 2004;75(9):1178–1187.
- Ammajan RR, Joseph R, Rajeev R, et al. Assessment of periodontal changes in patients undergoing radiotherapy for head and neck malignancy: a hospital-based study. J Cancer Res Ther. 2013;9(4):630–637.
- Ferreira MC, Dias-Pereira AC, Branco-de-Almeida LS, et al. Impact of periodontal disease on quality of life: a systematic review. J Periodontal Res. 2017;52(4):651–665.
- Sorsa T, Gursoy UK, Nwhator S, et al., Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000. 2016;70(1):142–163.
- Ghallab NA. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: review of the current evidence. Arch Oral Biol. 2018;87:115–124.
- Sorsa T, Tjäderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38(5):306–321.
- Lee W, Aitken S, Sodek J, et al. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30(1):23–33.
- Romanelli R, Mancini S, Laschinger C, et al. Activation of neutrophil collagenase in periodontitis. Infect Immun. 1999;67(5):2319–2326.
- Pärnänen P, Sorsa T, Tervahartiala T, et al. Isolation, characterization and regulation of moonlighting proteases from Candida glabrata cell wall. Microb Pathog. 2020;149(104547). DOI:10.1016/j.micpath.2020.104547
- Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888.
- Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11(3):30.
- Harmer D, Falank C, Reagan MR. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front Endocrinol (Lausanne). 2019;9:788.
- Miller PD Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):8–13.
- Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135.
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159–S172.
- Sorsa T, Alassiri S, Grigoriadis A, et al. Active MMP-8 (aMMP-8) as a grading and staging biomarker in the periodontitis classification. Diagnostics (Basel). 2020;10(2):61.
- Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456.
- Thirkettle S, Decock J, Arnold H, et al. Matrix metalloproteinase 8 (collagenase 2) induces the expression of interleukins 6 and 8 in breast cancer cells. J Biol Chem. 2013;288(3):16282–16294.
- Al-Majid A, Alassiri S, Rathnayake N, et al. Matrix metalloproteinase-8 as an inflammatory and prevention biomarker in periodontal and peri-implant diseases. Int J Dent. 2018;2018:7891323.
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038.
- Alassiri S, Parnanen P, Rathnayake N, et al. The ability of quantitative, specific, and sensitive point-of-care/chair-side oral fluid immunotests for aMMP-8 to detect periodontal and peri-implant diseases. Dis Markers. 2018;2018:1306396.
- Grigoriadis A, Sorsa T, Räisänen I, et al. Prediabetes/diabetes can be screened at the dental office by a low-cost and fast chair-side/point-of-care aMMP-8 immunotest. Diagnostics (Basel). 2019;9(4):151.
- Chaparro A, Realini O, Hernández M, et al. Early pregnancy levels of gingival crevicular fluid matrix metalloproteinases-8 and −9 are associated with the severity of periodontitis and the development of gestational diabetes mellitus. J Periodontol. 2020. DOI:10.1002/JPER.19-0743.
- Räisänen IT, Umeizudike A, Pärnänen P, et al. Periodontal disease and targeted prevention using aMMP-8 point-of-care oral fluid analytics in the COVID-19 era. Med Hypotheses. 2020;144:110276.