ABSTRACT
Arctic-alpine vegetation in the eastern United States is unique to northern New England and New York and is disjunct from similar areas in eastern Canada. We present the first study of the non-native flora in the region, specifically focusing on New Hampshire’s White Mountains. By combining literature and herbaria searches, field surveys, and a seventeen-year evaluation at an alpine hut in a hypothesis-driven framework, we document the composition, chronology, and persistence of non-native plant species establishments, regress richness in relation to elevation and disturbed area, and evaluate similarities to nineteen other alpine floras globally. Our results indicate that the White Mountains support one of the most species-rich non-native alpine floras known in the world, with 58 species detected at thirty-one sites since 1874, comprising 19 percent of 300 species documented in New Hampshire’s 22 km2 of alpine tundra. There is a negative relationship between non-native alpine plant richness and elevation along a mountain road on Mt. Washington. Moreover, elevation predicts richness per unit area in proximity to clusters of built structures in alpine areas. The compositions, geographic origins, and dominant ruderal strategies of non-native species are similar to those of Arctic and other alpine non-native floras globally. Sørenson similarity index and nonmetric multidimensional scaling ordination of twenty alpine regions reveals the White Mountains have highest similarity with widely separated mountain regions in Australia, Hawaii, the Rocky Mountains, the Andes, Southern Africa, and Iceland, driven by shared species of Eurasian origin. We report the unexpected discovery of Plagiobothrys hispidulus, a borage native to western North America not previously reported from New Hampshire. These findings have important implications for managers of alpine areas in eastern North America and may facilitate the early detection, monitoring, and control of non-native species, minimizing their establishment and spread.
Introduction
The alpine vegetation of the Presidential Range, Franconia Ridge, and other summits in the White Mountains of New Hampshire has been intensively studied since the early nineteenth century. Botanists soon noted the affinities of New Hampshire’s mountain vegetation to that of treeless, acidic areas in Arctic and alpine regions of eastern Canada (Québec, Newfoundland, Labrador, and Nunavut) and Greenland (Thoreau Citation1906), and their efforts resulted in a remarkably complete understanding of the composition of the White Mountains’ alpine vegetation, summarized most comprehensively by Pease (Citation1916, Citation1924, Citation1964), Fernald (Citation1950), and Harris, Langenheim, and Steele (Citation1964). Storks and Crow (Citation1979) and Crow and Storks (Citation1980) contributed additional treatments of the flora. Bliss (Citation1963) described plant communities of the Presidential Range, and Sperduto and Cogbill (Citation1999) and Sperduto and Nichols (Citation2011) described alpine and subalpine natural communities in New Hampshire’s White Mountains more broadly, including those on the many exposed peaks outside the Presidential Range and Franconia Ridge.
Scientific reporting of non-native plant species has been less deliberate and comprehensive. The earliest collectors, including William Oakes (between 1825–1846) and Henry David Thoreau in 1839 and 1858 (summarized by Pease in 1916; Thoreau Citation1906), did not document any introduced species in their forays to Mt. Washington, just prior to and concurrent with construction of the first road and buildings on the mountain. (Thoreau did document several non-native species from Mt. Monadnock, NH between 1844 and 1860.) The first documented non-native plant species were Agrostis capillaris L., Poa palustris L., and Phleum pratense L., the last noted by Morong on an 1874 specimen label as occurring “from base to summit.” Merrill (Citation1888) noted that “herd grass” (Poa pratensis L., subsp. pratensis) “can be traced far above the limit of trees … along the carriage road” (p. 59). In 1889, John and Alice Northrop catalogued eighty native species above 1,400 m elevation and mentioned only one notable non-native: “On the very summit, 6300 feet [1920 m elevation], a thrifty specimen of that hardy traveler, the dandelion, was in full bloom and apparently as much at home as on the roadsides several thousand feet below” (p. 256). Ultimately, a total of nine non-native species were documented from Mt. Washington between 1874 and 1900, prior to the first ascent by automobile. Many of these collections were from around the summit hotel and a nearby waste pile. Over the course of the next 100 years, reports varied from seven to eighteen species. Antevs (Citation1932) listed eleven non-native species from above tree line in the Presidential Range (based largely on Pease Citation1924). Harris, Langenheim, and Steele (Citation1964) noted seven introduced or questionably native species. Sperduto and Cogbill (Citation1999) tallied 270 vascular plant species from both alpine and subalpine peaks in northern New England and New York, 260 of which occur in the White Mountain alpine areas. These included seventy native taxa restricted primarily to alpine areas and eighteen non-native species. Jones and Willey (Citation2012) noted more than a dozen non-native species known to occur in the Presidential Range’s alpine zone around the turn of the twenty-first century, based on surveys around Lakes of the Clouds Hut between Mt. Washington and Mt. Monroe. In 2014, Bell (Citation2015) discovered thousands of Taraxacum officinale F.H. Wigg in small natural snowbank meadows near the summit of Mt. Washington, sparking renewed attention to the non-native issue.
In 2015, the White Mountain National Forest and partners initiated a rapid-response control program focused on the Taraxacum officinale infestation on Mt. Washington and began documenting novel introduced plant taxa along the Auto Road and at Lakes of the Clouds Hut (United States Department of Agriculture Citation2015, Citation2016, Citation2017). It became evident to us that a comprehensive assessment of introduced plant species in the White Mountains’ alpine tundra was overdue and would provide valuable context for scientists and alpine landowners and managers not only in New Hampshire but elsewhere in northeastern North America.
There has been a documented increase in the presence of non-native plants in Arctic and Antarctic regions, as well as in alpine ecosystems globally. Wasowicz et al. (Citation2020) documented 341 non-native plant species in the Arctic, and Pauchard et al. (Citation2009) noted more than a thousand introduced plant species within mountain regions globally, nearly 200 of which specifically occur in alpine systems (Alexander et al. Citation2016). Many of these introductions are associated with anthropogenic disturbance. Historically, low frequencies of introduced plants in natural alpine ecosystems have been attributed to the barriers imposed by the severe environmental conditions, biotic resistance of natural vegetation, poor adaptation of lowland non-natives to cold, and/or limited propagule availability. However, emerging research and rapidly changing climatic conditions challenge these assumptions. For example, there is evidence that alpine tundra is not particularly resistant to invasion, given enough time for an increase in propagule pressure from cold-adapted non-native taxa or adaptation of lowland non-natives to alpine conditions (Becker et al. Citation2005; Dietz and Edwards Citation2006; Pauchard et al. Citation2009). Essl et al. (Citation2011) referred to this as an accumulating “invasion debt.” Biotic resistance in alpine tundra appears to vary with conditions: some invasions are evidently related to disturbance, amount of bare ground, or type of plant community (Pollnac et al. Citation2012; Milbau et al. Citation2013), whereas others are not related to disturbance or resistance (e.g., Taraxacum officinale in the Andes; Quiroz et al. Citation2009) or are facilitated by native cushion species (T. officinale, Cerastium arvense L., and Rumex acetosella L. in the Andes; Cavieres et al. Citation2007; Cavieres, Quiroz, and Molina-Montenegro Citation2008; Hupp et al. Citation2017). The isolation of high-elevation mountain regions from one another may have limited the opportunities for introduction and establishment of non-native cold-adapted species (Alexander et al. Citation2016). However, numerous documented examples illustrate the capacity for invasiveness once established in new regions, such as the North American Lupinus nootkatensis Donn ex Sims, which was introduced in Iceland and is projected to expand its range in coming decades (Wasowicz, Przedpelska-Wasowicz, and Kristinsson Citation2013; Wasowicz et al. Citation2020). Further, the combination of increasing recreation pressure in mountains, more frequent anthropogenic disturbances, and intensifying climate change (Murray et al. Citation2021) may be accelerating opportunities for invasions in montane and alpine areas globally through increased propagule dispersal, altered ecosystem processes, and relaxed climate filtering.
Climate change, anthropogenic disturbance associated with transportation networks, and recreation activity are the factors most frequently cited that facilitate the establishment (and likely future expansion) of non-native species in alpine and polar systems globally (Jodoin et al. Citation2008; Pauchard et al. Citation2009; Alexander et al. Citation2016; Rew et al. Citation2020). Roads and trails are the primary corridors for the spread of non-native plant species into montane and alpine habitats specifically (Tyser and Worley Citation1992; Pauchard and Alaback Citation2004; Fuentes et al. Citation2010; Meunier and Lavoie Citation2012; Anderson et al. Citation2015; Lembrechts et al. Citation2017), where the spread can be particularly rapid along roads (Vacchiano et al. Citation2013; Kalwij, Robertson, and van Rensburg Citation2015). Becker et al. (Citation2005) linked the spread of non-natives along roads and railways in subalpine and alpine areas in the Alps to abundance and length of establishment in lowlands. Other recreational activities can facilitate their spread away from roads (Pickering and Mount Citation2010; Anderson et al. Citation2015). For example, Morgan and Carnegie (Citation2009) linked the establishment and spread of non-native plants in the Australian Alps to recreation associated with backcountry huts. Invasion may be further facilitated in these contexts by the availability of nutrients, including nitrogen in various forms (Chen and Chen Citation2019). Additionally, climate change is widely expected to contribute to a significant increase of non-native species into montane and alpine habitats (United Nations Environment Programme Citation2009; Diez et al. Citation2012; Kueffer et al. Citation2013; Anderson et al. Citation2015; Rew et al. Citation2020), with at least some of these non-native taxa likely to become invasive. Ultimately, invasions in high-elevation communities facilitated by such human disturbances are governed over time by various interacting ecological processes, including propagule availability, environmental constraints on population growth, evolutionary change, and biotic interactions (Alexander et al. Citation2016).
Our objectives in this study were to (1) present the first comprehensive documentation of non-native vascular plants in an alpine area in eastern North America; (2) evaluate patterns of non-native plant richness, including relationships to area, elevation, and disturbance type; (3) compare the floristic composition of New Hampshire’s alpine non-native flora to other global alpine areas; and (4) determine the extent to which non-native plants have spread and persisted within and beyond human disturbed areas and evaluate their potential for future spread.
Following established ecological theory including species–area relationships and island biogeography (MacArthur and Wilson Citation1967; Simberloff Citation1972; Connor and McCoy Citation1979) and species richness–elevation patterns (Rahbek Citation1995; Grytnes Citation2003), we hypothesize that non-native species richness in New England alpine areas (1) is positively associated with area; (2) decreases with elevation, though the relationship may not be linear; and (3) is higher in disturbed areas with built structures compared to equivalent areas of disturbed roadsides. We also hypothesize that (4) richness of non-native species in alpine areas has increased over time and (5) there is evidence of persistence within and spread beyond sites with a high level of human disturbance. Finally, we hypothesize that (6) New Hampshire’s alpine will consist of many of the same broadly adapted non-native temperate species that occur in other alpine areas in temperate latitudes outside of Eurasia and Africa.
Methods
Geographic area of interest
Our primary focal area is Mt. Washington and the Presidential Range, White Mountains, New Hampshire (), which encompasses ten main summits and the largest extent of alpine tundra in the eastern United States (Bliss Citation1963; Kimball and Weihrauch Citation2000; Jones and Willey Citation2012). The Presidential Range encompasses 19.4 km2 of alpine tundra (Bliss Citation1963) and is densely fragmented by recreational hiking trails and a summit road (Mt. Washington Auto Road) and railway (Cog Railway). There are two recreational huts at the transition to alpine tundra in the Presidential Range, a third near tree line on Franconia Ridge, and seven permanent structures and four parking areas in the immediate vicinity of Mt. Washington’s summit. The Presidential Range is multijurisdictional: the great majority of the Presidential Range is part of the White Mountain National Forest, Mt. Washington State Park occupies 24 ha at the summit of Mt. Washington, the Cog Railway and Auto Road are privately owned corridors, and the Appalachian Mountain Club operates the mountain huts through a Special Use Permit from the U.S. Forest Service and owns part of the footprint of Madison Springs Hut. Two additional huts just below tree line are maintained by the Randolph Mountain Club in the northern Presidential Range but were not surveyed as a part of this study.
Figure 1. Alpine and subalpine peaks in New Hampshire included in the study’s focal area. From Sperduto and Kimball (Citation2011). Courtesy of New Hampshire Natural Heritage Bureau, Concord.
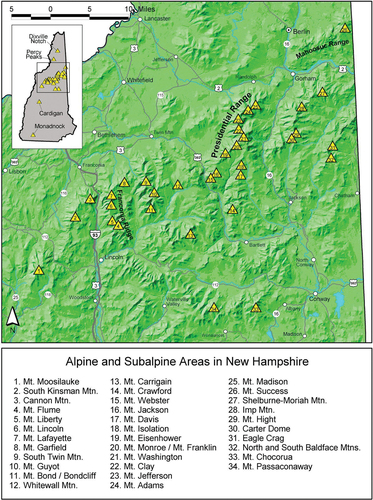
Figure 2. (Top) Alpine tundra (brownish-white background) on Mt. Washington, New Hampshire. Generally, the Auto Road runs from the northeast corner (upper right) to the summit (center) and the Cog Railway from the west edge (left) to the summit; Lakes of the Clouds Hut is near the southwest corner (near lower edge and left of center). Credit: USA Topo Maps, 2013 National Geographic Society. (Bottom) Structures, parking lots, and other disturbed areas on the summit of Mt. Washington, New Hampshire. Credit: 2010–11 Statewide High Resolution Aerial Photography, NH GRANIT, University of New Hampshire, Durham.
Our study also encompasses twenty-seven additional peaks with alpine floristic affinities in the greater White Mountain region of Carroll, Cheshire, Coos, and Grafton Counties, New Hampshire (). This expanded footprint adds approximately 2.6 km2 of alpine habitat (Sperduto and Cogbill Citation1999), for a total of 22 km2 that occur primarily on the White Mountain National Forest and adjacent state parks. Peaks in the larger study area include Franconia Ridge, Mt. Moosilauke, the Bonds, Mahoosuc and Carter Ranges, Cannon Mountain, Mt. Chocorua, and the outlying peaks of Mt. Monadnock and Mt. Cardigan. Some of these are subalpine peaks (below the regional average tree line of 1,495 m elevation) but have open and severe exposures that support otherwise alpine-restricted species.
Human disturbance history of New Hampshire alpine
The White Mountains have been a popular destination for tourists since the early nineteenth century (Crawford Citation1845). The region currently receives more than 4 million visitors per year. The alpine zone is a popular destination year-round and is accessed by tens of thousands of hikers, car drivers, and train riders annually. The chronology of road, railway, and structures (e.g., buildings, storage tanks, towers, other built structures, and adjacent parking lots, collectively referred to as building complexes) in the study area on Mt. Washington includes an original summit house hotel (1852; rebuilt 1872; destroyed by fire 1908), the Tip Top House (1853), the Carriage Road to the summit (1861; first automobile 1901; now called the Auto Road), and the Cog Railway (1869). Other structures in the Presidential Range include Madison Springs Hut (1888) and Lakes of the Clouds Hut (1915), built for overnight accommodations in the alpine zone. Roads and structures above tree line on the western side of the White Mountains include the Mt. Moosilauke summit house (1860), Carriage Road (1870), and Greenleaf Hut (1930). Starting in the early 1800s, horses accessed the summit of Mt. Washington via the Crawford Path (the oldest continuously maintained hiking trail in the United States, completed in 1819) and subsequently to the summits of Mt. Washington and Mt. Moosilauke by Carriage Road and to Mt. Lafayette by a footpath. Between 1930 and 1965, prior to the use of helicopters, the Appalachian Mountain Club used donkeys to bring supplies and construction materials to their expanding backcountry huts operation throughout the White Mountains (). An extensive network of trails has been constructed in the alpine zone, which provides access to all of the mountains and sites in the study area.
Figure 3. (Top left) Donkeys at Madison Springs Hut (right side), Mt. Madison, New Hampshire (circa 1941 or later). (Top right) Burros (donkeys) used to pack materials to Madison Springs Hut in the lowlands of Randolph, New Hampshire. (Bottom left) Donkey and driver in an alpine meadow; drivers let donkeys forage on natural vegetation rather than packing in hay or feed. (Bottom right) Crew and horses at Lakes of the Clouds Hut still under construction in the early twentieth century. Credit: Postcards by Guy Shorey Studio, Gorham, New Hampshire. Courtesy of Appalachian Mountain Club Library & Archives and Gladys Brooks Memorial Library, Mt. Washington Observatory Weather Discovery Center, North Conway, New Hampshire.

To document the diversity, chronology, and extent of introduced plant taxa in the White Mountains, we employed the following methodological approaches.
Documentation of non-native flora
We conducted searches using herbaria portals (Consortium of California Herbaria Citation2021; Consortium of Midwest Herbaria Citation2021; Consortium of Pacific Northwest Herbaria Citation2021; Southeast Regional Network of Expertise and Collections Citation2021; Consortium of Northeastern Herbaria Citation2022). In each of the five portals, searches were made using the names of thirty-seven mountains (and their nomenclatural variations) with alpine vegetation in the study area and by using the scientific names of non-native plant species previously documented in at least one alpine location. Species searches were limited to the four New Hampshire counties with mountains supporting alpine habitat. Determination of native versus non-native infraspecific taxa were not made for new site records for the following two species due to difficulty in making determinations by examining scanned herbaria images: Poa pratensis and Rhinanthus minor L. Specimens and site observations prior to 1990 were considered historical, and those from 1990 onwards were included among the contemporary records. We also documented the rare alpine species that co-occur with non-native species and the natural habitats and communities affected by non-native plants.
Contemporary surveys were conducted on Mt. Washington during the summers of 2016 to 2022, consisting of targeted field surveys on the summit in the vicinity of built structures, along hiking trails that access the summit, and in alpine portions of the Auto Road and Cog Railway. We also conducted surveys at Madison Springs and Lakes of the Clouds Huts in the Presidential Range and at Greenleaf Hut on Mt. Lafayette on Franconia Ridge. These non-native species-focused surveys in disturbed environments amounted to ca. 100 person hours. In addition, approximately 219 hours were spent at other alpine locations from 2015 to 2022 conducting dandelion removal work, surveying trail corridors, and conducting vegetation monitoring off trail. Voucher specimens for new site records will be deposited at the Hodgdon Herbarium at the University of New Hampshire and Massachusetts State Herbarium at the University of Massachusetts. The building complex footprint at the summit of Mt. Washington was considered as a single location, as was a zone around a set of storage tanks ~200 m from the summit and zones around the three alpine huts. Individual species observations were recorded by Global Positioning System (GPS) along the 5.9-km alpine portion of the Mt. Washington Auto Road and tallied within 50-m elevational segments between tree line from 1,200 to 1,900 m, just below the summit building complex.
Vascular plant taxonomy, nomenclature, and nativity status follow Haines (Citation2019). Authorities for taxa not listed in the online appendix are otherwise given after the taxon’s first mention. Abbreviations for authors’ names follow the International Plant Names Index (Citation2022).
Richness
The Mt. Washington Auto Road presented the best opportunity to compare richness at different elevations with minimal confounding effects of existing building complexes. We tallied species richness within each of fourteen segments along the Auto Road from 1,200 to 1,900 m elevation, each segment spanning 50-m elevation. We regressed the number of non-native species per road segment with the midpoint elevation of the segment. At sites with structures (building complexes on the summit of Mt. Washington and around the three alpine huts), we calculated the disturbed footprint area (excluding impervious surface area) and elevation in Google Earth Pro (Citation2022). We divided the richness (S) of non-native species by the disturbed area of the site (excluding impervious surface area) to obtain a standardized richness per hectare of disturbed area and regressed the standardized richness against elevation using the package “car” in the statistical program R (Fox and Weisberg Citation2019).
Floristic composition and comparison to global alpine areas
Alexander et al. (Citation2016, online supplemental material) assembled non-native floras in alpine regions from fifteen different mountain ranges around the world. We used these data to generate a species by site matrix and added non-native lists from five other alpine regions in North and Central America: New Hampshire, the Cascade Mountains and two additional regions in the Rocky Mountains (Parks et al. Citation2005), and the Trans-Mexican Volcanic Belt, Mexico (Steinmann et al. Citation2021). We used the Sørensen similarity index to compare New Hampshire’s non-native alpine flora to each of the other nineteen lists. The Sørensen index is based on the ratio of twice the number of species shared between two sites and the total number of species in the two floras combined. It is calculated with the equation: 2C/(N1 + N2), where C is the number of non-native species in common between two floras, and N1 and N2 are the total number of non-native species in each flora. Index values can range from zero (no overlap) to one (complete overlap). To further interpret similarities of the White Mountain non-native floras to these other regions, we ran a nonmetric multidimensional scaling (NMS) ordination of the twenty regions and 260 species and present an ordered table of most frequent species globally. The NMS ordination was run using PC-ORD software (McCune and Mefford Citation2018) using a medium level of iterations, three dimensions, and Sørensen (Bray-Curtis) distance measure settings. We also considered the phytogeographic origins of New Hampshire’s alpine non-natives that contribute to degree of similarity with other regions.
Immigration, persistence, and evidence of spread
Immigration, persistence, and evidence of spread were examined at two scales: across the entire study area and at Lakes of the Clouds Hut. At the broader scale, immigration rate was calculated by plotting cumulative richness against time of first observation on Mt. Washington. Persistence was evaluated by tallying the number of species observed prior to 1990 that remain extant on Mt. Washington. Although newly observed species, those first documented between 2016 and 2022, do not have a lengthy establishment record, we note the identity of those species that are entrenched at known locations (species that are likely to persist based on their current abundance and degree of establishment). Evidence of spread is evaluated by recording locations of non-native species beyond the perimeters of human disturbed sites in natural alpine settings across the broader study area.
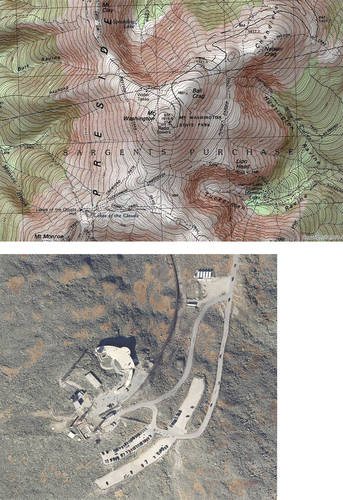
At the local scale, we conducted surveys across a two-decade time interval at Lakes of the Clouds Hut, a backcountry lodging facility constructed in 1915 by the Appalachian Mountain Club above tree line on Mt. Washington. From 1999 to 2001, we gathered preliminary lists of non-native plant species in the vicinity of the hut. From mid-July to mid-August 2003, we initiated a survey at the hut by mapping the distribution of five widespread non-native forbs and by noting the distribution of six additional non-native plant species, which at the time were narrowly confined to one or two areas of occurrence. Our search area encompassed twelve zones delineated post hoc within the disturbed areas immediately surrounding the hut, extending to 75 m from the center of the hut (). We conducted a comparable resurvey of the area during this study (2020) to document apparent changes in the distribution and abundance of the eleven non-native species observed and characterized in 2003.
Figure 4. Schematic map of Lakes of the Clouds Hut and associated outbuildings (as they were in 2003) at 1,539 m elevation between Mt. Washington and Mt. Monroe, New Hampshire. Five widespread non-native forbs were visually mapped onto a schematic of the hut and its outbuildings in 2003 and were resurveyed with GPS in 2020. Visible aboveground structures are shown as black boxes; the perimeter of each was searched. To evaluate distributional and compositional change, twelve zones were designated corresponding to 25-m bands from the geometric center of the main hut.
Results
Documentation of non-native flora
Searches in the Consortium of Northeastern Herbaria portal (2022) generated approximately seventy-five new collection records; other herbaria consortium portals produced fourteen new records. Review of data in the New Hampshire Natural Heritage Bureau (Citation2021) database revealed two new records. In combination with Harris, Langenheim, and Steele (Citation1964), Pease (Citation1924), and Sperduto and Cogbill (Citation1999), there were seventy-two historical collection records (prior to 1990), totaling 26 non-native species. Contemporary surveys (1990 onwards) contributed 194 non-native species records (153 from this study), which added 32 species to the documented flora of alpine and subalpine peaks in the White Mountains. In all, historical plus contemporary surveys total 266 records at thirty-one sites (Appendix). We documented 44 extant non-native species during our 2014 to 2022 surveys on Mt. Washington, as well as forty-three records of 23 species outside of Mt. Washington.
In total, fifty-eight non-native species were documented from alpine or exposed subalpine sites near tree line in New Hampshire (). The full chronology of each species and site documentation is provided in the Appendix. There were two main periods of collections and observations: ~1890–1903 and 1990–present (). Nineteen species (33 percent) were first documented more than one hundred years ago, consisting of common non-native pasture and roadside species apparently brought in by people, horses, carriages, animal feed, and other supplies. Fourteen of the eighteen most frequent species—those at three or more sites—were originally documented more than one hundred years ago, mostly from Mt. Washington (). Forty additional species were documented at one or two sites. The most frequent species on Mt. Washington were Taraxacum officinale (thirty-eight local stations); Pilosella aurantiaca (twelve stations); Tanacetum vulgare L. (seven stations); Rumex acetosella subsp. pyrenaica, Tussilago farfara, and Vicia cracca subsp. cracca (six stations each); and Trifolium repens L. (five stations). Thirteen additional species were newly documented along the Auto Road at one or two sites, mostly along the narrow ~2- to 3-m-wide dirt shoulders. The immediate Cog Railway corridor above tree line appears to have few non-natives, other than the summit cone area. The Cog is on the more wind-exposed western side of Mt. Washington. Only three of the fifty-eight species were not observed during contemporary surveys from the Presidential Range: Anthoxanthum hirtum (Schrank) Y. Schouten & Veldkamp, Fallopia convolvulus (L.) Á. Löve, and Sinapis arvensis O.F.Müll., all not recorded since the summit building on Mt. Washington burned down in 1908. Ranunculus bulbosus L. from Mt. Monadnock summit was also not documented during contemporary surveys.
Figure 5. Cumulative species richness over time on Mt. Washington, New Hampshire. There were two primary periods of documentation, from 1874 to 1903 and from ~1990 to present.
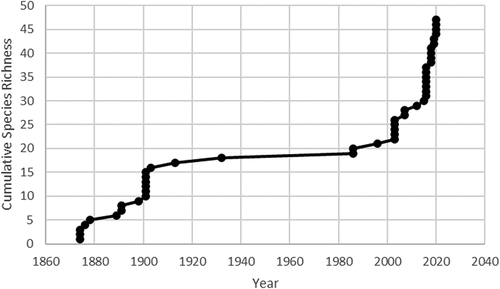
Table 1. Non-native vascular plant species composition of alpine and exposed subalpine areas in New Hampshire, USA.
We report a surprising disjunct population of Plagiobothrys hispidulus [syn. Plagiobothrys scouleri I.M. Johnst. var. hispidulus (Greene) Dorn], a North American native of the Sierra Nevada, Cascade, and northern Rocky Mountain regions. Plagiobothrys scouleri sensu lato has been introduced to a few widely scattered locations in eastern North America, as well as in Scotland and Northern Europe (Gimingham et al. Citation2002), where it is abundant in certain settings, especially areas that are seasonally flooded in winter. Plagiobothrys hispidulus appears to have persisted for at least twenty-two years (1999–2021) on a disturbed, wet and icy terrace on the north side of the Lakes of the Clouds Hut, but it does not appear to have changed its general distribution. Plagiobothrys hispidulus was misidentified tentatively in 2003 as Buglossoides arvensis (L.) I.M. Johnst.
Non-native plants grow in the immediate proximity of sixteen regionally rare alpine plants that are on the federal Sensitive Species List (United States Department of Agriculture Citation2023) and/or New Hampshire state rare plant list (New Hampshire Natural Heritage Bureau Citation2021). These include Epilobium hornemannii Rchb., Festuca prolifera Fernald, Geum peckii Pursh, Luzula spicata (L.) DC., Nabalus boottii DC., Omalotheca supina (L.) DC., Oxyria digyna Hill, Phleum alpinum L., Phyllodoce caerulea (L.) Bab., Poa laxa subsp. fernaldiana (Nannf.) Hyl., P. pratensis subsp. alpigena (Lindm.) Hiitonen, Rhinanthus minor subsp. groenlandicus (Chabert) Neuman, Salix planifolia Pursh, Saxifraga rivularis L., Solidago leiocarpa DC., and Viola palustris L. Three of these species are regional endemics (G. peckii, N. boottii, and S. leiocarpa), restricted to northeastern U.S. alpine, with two occurrences of G. peckii in Nova Scotia, Canada. Luzula spicata, N. boottii, both Poa species, R. minor subsp. groenlandicus, S. rivularis, and S. leiocarpa apparently tolerate lightly disturbed natural alpine habitats as well as certain human disturbed contexts within building complexes or Auto Road margins. Phyllodoce caerulea occurs in relatively well drained snowbank settings. Omalotheca supina occurs on an eroding scree slope in a ravine. The remaining species occur along rills and other wet, seepy cirque ravine snowbank habitats on soils vulnerable to erosion from trampling. Primary sites for the co-occurrence of these rare species include the building complex and surrounding natural sedge meadows and snowbank habitats on the cone and shoulders of Mt. Washington, Lakes of the Clouds Hut, Madison Springs Hut, the Great Gulf, and other cirque ravines. Invaded natural communities include sedge meadows dominated by Carex bigelowii Torr. ex Schwein and several types of herbaceous, heath, and mixed snowbank and rill communities (Sperduto and Nichols Citation2011).
At least four species native to lowland areas of New Hampshire have expanded into the alpine zone. These species might be considered examples of native range-expanders, in that they have extended their geographic distribution and apparent climatic range within New Hampshire. Potentilla norvegica L. was documented from three alpine sites prior to 1964 (Pease Citation1964) and currently known from at least eight alpine sites in ravines, disturbed summits, and areas around huts. Contemporary observations of Euthamia graminifolia Elliott, Hordeum jubatum L. subsp. jubatum, and Iris versicolor L. were made at single sites, though these species lack historical records in New Hampshire’s alpine. Euthamia graminifolia is not known to have spread from its current location over a twenty-year period. Iris versicolor, previously only known well below tree line in New Hampshire, was documented at 1,222 m near the transition to tree line on Cannon Mt.
Richness
Total species richness of non-native plants was summed for each primary study location, including the Mt. Washington Auto Road (fourteen segments spaced at 50-m elevational increments, including “The Horn”) and five building complexes on the Presidential and Franconia Ranges (three at mountain huts, a storage tank area, and the summit of Mt. Washington). These complexes include transportation corridors, communications towers, and state park buildings), and the summit of Mt. Moosilauke (; ).
Figure 6. Species richness of non-native plants along the margins of the alpine portion of the Mt. Washington Auto Road (teal line) in fourteen segments spanning 50-m elevation each (New Hampshire). Richness of each segment is indicated in circles along a color gradient. Building complexes and corresponding richness are indicated for the summit of Mt. Washington and the Lakes of the Clouds Hut (star). Trails are denoted by dashed tan lines.
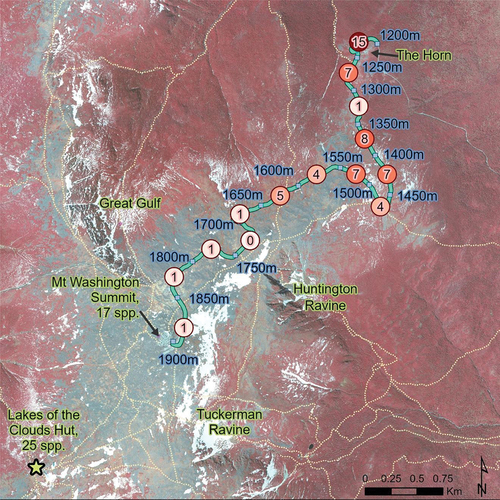
Table 2. Composition and richness of non-native species along the Mt. Washington Auto Road (New Hampshire), sorted by frequency.
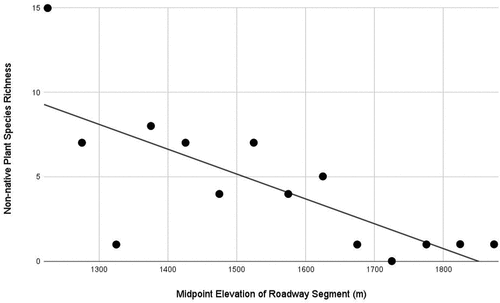
Fifty-meter elevational roadway segments of the Mt. Washington Auto Road ranged in length from 330 to 578 m (mean = 424 m; median = 433 m). Midpoint elevation of 50-m elevational roadway segments predicted non-native plant species richness (S) along the Mt. Washington Auto Road from 1,200 to 1,900 m. The regression equation was significant, F(1,12) = 14.59, p < .001, with an R2 of 0.55 ().
Figure 7. Linear regression of midpoint elevation of fourteen 50-m elevation roadway segments from 1,200 to 1,900 m on Mount Washington, New Hampshire, against extant (2020–2021) non-native species richness.
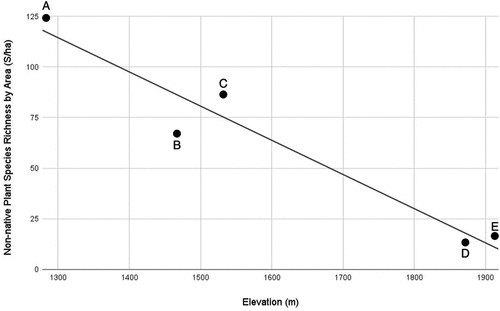
Estimates of total area of impervious surfaces (existing structures and parking lots) in 2021 at five building complexes ranged from 0.0389 ha at Greenleaf Hut on Mt. Lafayette to 0.2733 ha at Mt. Washington summit. These areas were subtracted from the total disturbed area footprints to produce an estimate of area potentially available for plant growth. Resulting net areas used in regressions were as follows: Mt. Washington summit, 1.4875 ha; Mt. Washington storage tanks, 0.1967 ha; Lakes of the Clouds Hut, 0.3617 ha; Madison Springs Hut, 0.2376 ha; and Greenleaf Hut, 0.1917 ha. Elevation predicted the richness by area (S/ha) of non-native plant species associated with building complexes. The regression equation was also significant, F(1,3) = 51.14, p = .003, with an R2 of 0.94 ().
Figure 8. Linear regression of site elevation (m) against non-native species richness (S) divided by the total area of disturbed nonimpervious habitat (ha) for five building complexes above or near tree line in the White Mountains, New Hampshire: (A) Greenleaf Hut, (B) Madison Springs Hut, (C) Lakes of the Clouds Hut, (D) Mount Washington Storage Tanks, and (E) Mount Washington Summit Complex.
Composition and comparison to global alpine areas
More than half of the fifty-eight New Hampshire alpine non-native plants are either grasses (20 species, 34 percent) or composites (13 species, 22 percent), members of the Poaceae and Asteraceae families. Twelve other families are represented, five of which comprise 28 percent of the remaining species: four Fabaceae and three species each from the Brassicaceae, Caryophyllaceae, Plantaginaceae, and Ranunculaceae. This adds 40 species to the previously documented flora of 260 species (Sperduto and Cogbill Citation1999; included 18 non-natives) on New Hampshire alpine and subalpine peaks. Thus, the 58 non-natives comprise 19 percent of the 300-species flora. In comparison, native Arctic-alpine restricted plants comprise 23 percent of the flora (70 species). Nearly all of the non-native species are ruderals or competitors, as opposed to stress tolerators adapted to extreme environments (Grime Citation1977; Alexander et al. Citation2016).
The combined list of non-native species from twenty alpine regions (Alexander et al. Citation2016) plus the five additional regions included here consists of 260 species. Sørensen similarity index values of New Hampshire non-native species compared to nineteen other alpine areas around the world () indicate that New Hampshire’s non-native flora was most similar to the floras of the Australian Alps, Hawaii, Northern and Central Andes, Drakensberg Mountains, Mexican stratovolcanoes, and Iceland (values from 0.14 to 0.27, 5–11 species in common). The most dissimilar floras were from Europe, Africa, Asia, and southwestern North America (La Reunion; East African Mountains; Sierra Nevada, USA; Qinghai, Tibet; Tenerife; and the Swiss Alps; values range from 0 to 0.09, 0–3 species in common). The remaining five ranges had intermediate values (0.09 to 0.13 and 3–6 species in common with New Hampshire).
Table 3. Sørensen similarity index values (Index) comparing non-native species composition of the White Mountains, New Hampshire, to nineteen other global alpine areas.
To examine the underlying structure of the relationships between global alpine floras, we employed NMS ordination (). The ordination plot displayed clear separation among the sampled sites, indicating potentially distinct ecological patterns. New Hampshire’s non-native alpine flora (NA_App) is near the middle of the ordination space and closest to the other sites with relatively high Sørensen index values, which are also roughly in the middle of the graph. The more dissimilar sites occupy the marginal areas of the graph, with north temperate and subarctic sites on the upper third and remaining African sites and Tibet on the lower portion and extreme left. The species that drive the higher similarity values and proximities in the NMS are the most frequent non-native species in global alpine areas, documented from five or more sites (), here listed in order of frequency: Taraxacum officinale, Rumex acetosella, Poa annua L., Trifolium repens, Poa pratensis subsp. pratensis, Stellaria media, Capsella bursa-pastoris, and Phleum pratense. These species, all native to Western Europe or Eurasia, are present in many of the most similar sites and infrequent in the most dissimilar sites. The ordination included three dimensions and produced a final stress value of 15.530. The stress values, a measure of how well the data are represented in the multidimensional space, for the first two dimensions portrayed in for the 100 runs of the real data were as follows: Axis 1 minimum = 42.850, mean = 51.870, maximum = 55.364; Axis 2 minimum = 23.168, mean = 26.811, maximum = 37.755. The proportion of 250 randomized data Monte Carlo runs where stress values were less than or equal to the real data stress for Axes 1, 2, and 3 was 0.008, 0.004, and 0.004, respectively.
Figure 9. NMS ordination of twenty global alpine sites in PC-ORD software (McCune and Mefford Citation2018) using a medium level of iterations and Sørensen (Bray-Curtis) distance measure settings. For region abbreviations, see .
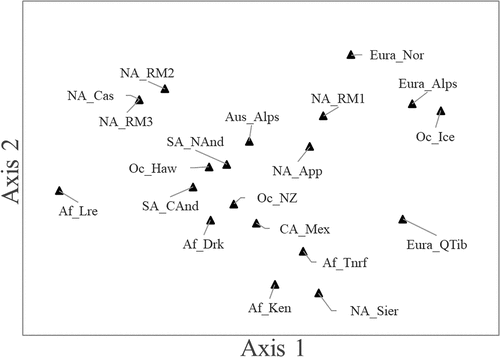
Table 4. Non-native species documented from three or more global alpine regions.
Immigration, persistence, and evidence of spread
Evidence of change in the number of species, their persistence, and their ability to spread comes primarily from the flora chronology, limited observations of distributional change over time at the site level, and evidence of spread beyond sites with a high level of human disturbance. Although documentation of non-native species has been sporadic over time, on Mt. Washington, there has been an average interval of 2.55 years for each new species establishment over the entire 148-year time period since the first non-native species observation in 1874. Of the fifty-six species documented from the primary White Mountain alpine areas (excluding the disjunct Mt. Monadnock), fifty-three species are extant. This indicates a high degree of persistence among non-natives that become established in alpine tundra of the White Mountains.
Thirty-three records of fourteen species at twenty-two sites are documented from the Presidential Range and Franconia Ridge away from the highly disturbed settings of roads, building complexes, or tree line settings of a ski area (Appendix). This includes eleven sites for Taraxacum officinale in natural alpine communities, such as snowbank meadows high on the slopes of Mt. Washington, in cirque ravines, and on ridgelines. The Great Gulf site consists of numerous individual stations recorded by Jones, Peters, and Sperduto on separate trips (Appendix). Taraxacum officinale is established at two stations outside of the disturbed footprint around the Lakes of the Clouds Hut. Pilosella aurantiaca occurs at four sites away from highly disturbed settings, including in a ravine on Mt. Washington, where it grows with the last population of Omalotheca supina in New Hampshire, a rare native alpine plant. In addition to these four Pilosella aurantiaca sites, this species is spreading far downstream from Madison Springs Hut and Lakes of the Clouds Hut along subalpine streambanks, as well as into heath–krummholz openings more than 15 m from the immediate managed edge of the Auto Road. Rumex acetosella subsp. pyrenaica was observed in a natural snowbank setting on Mt. Franklin, consisting of hundreds of well-established plants mixing with native species. It is unknown whether this is the same or a derivative population of a 1916 observation on Mt. Franklin by Pease (Appendix) or whether it is novel. Poa pratensis subsp. pratensis is documented from four sites away from buildings or along the Auto Road, including a new 2022 location in a meadow where thousands of dandelion plants have been removed. The overall composition and condition of the meadow has been largely restored based on detailed annual monitoring of composition since 2015 (U.S. Forest Service, unpublished data); the appearance of Poa pratensis subsp. pratensis is apparently novel and was likely introduced by people working in the meadow. Agrostis stolonifera L. and Taraxacum officinale were newly documented on Franconia Ridge between 2021 and 2022, on the summit of Little Haystack. These species had not been documented during intensive surveys of the ridgeline conducted over the previous forty-five-year period (Cogbill Citation2016). From these records, it is clear that non-native species have and will continue to spread beyond highly human-disturbed settings.
Two species exhibit evidence of spread within and adjacent to mountain huts or the Auto Road. Euphrasia stricta has apparently expanded its range at Madison Springs Hut, where it was not observed by C. Cogbill in 1996 during his documentation of flora around the hut (unpublished data). By 2020, it was very broadly established around Madison Springs Hut, including ~50 m away along a trail above the hut and along an alpine rill below the hut. At a third location near the hut, E. stricta invaded a reclaimed bootleg trail bed between 2015 and 2020 (D. Sperduto, pers. obs.). Scree was placed in entrenched trail areas in 2015, sediments subsequently accumulated among the scree, and by 2020, E. stricta had established in the reclaimed trail. Vicia cracca subsp. cracca is well established and spreading in disturbed and natural alpine turfs at several sites. At The Horn, a location at tree line adjacent to the Auto Road, it is spreading from human-disturbed areas into adjacent natural and relatively undisturbed heath shrub communities and occurs at four other stations along the Auto Road, where it is well established in krummholz openings beyond the immediate road margin. Evidence of spread for both of these species is discussed in the next section as well.
Several other species are frequent and exhibit local entrenchment, although evidence of local spread is indeterminate. Poa annua was documented in the study area only recently (2016), although it may have been present for many years given its degree of entrenchment around the structures and along heavily traveled pathways at all three alpine huts (Greenleaf, Lakes of the Clouds, and Madison Springs) and on the summit of Mt. Washington. It grows with several rare species including Nabalus boottii, P. pratensis subsp. alpigena, and P. laxa subsp. fernaldiana at Lakes of the Clouds Hut and with Luzula spicata and Saxifraga rivularis around infrastructure on Mt. Washington’s summit. In addition, P. annua has been observed in disturbed sites along trails and at campsites at high elevations below tree line in Great Gulf, Oakes Gulf, Mt. Pierce, and Shelburne–Moriah Mountain. Tanacetum vulgare is frequent on Mt. Washington along the Auto Road, occurring at seven stations above 1,500 m in elevation, and at the summit next to the Cog Railway. Some stems of T. vulgare were 1 m in height and have persisted despite several years of casual manual removal. One plant was observed growing from a root crown through a layer of fresh macadam along the Auto Road at 1,525 m elevation.
Longitudinal survey at Lakes of the Clouds Hut
We recorded the distributions of eleven non-native plant species in the immediate vicinity (to ~75 m from the hut perimeter) of Lakes of the Clouds Hut in 2003 and resurveyed the same area with handheld GPS units in 2020. Five widespread forb species were visually mapped onto a site locus map in 2003 and resurveyed in 2020: Matricaria discoidea, Pilosella aurantiaca, Ranunculus acris, Stellaria media, and Taraxacum officinale (). The most widely distributed non-native species in 2003 were Stellaria media, Taraxacum officinale, and Matricaria discoidea, which were observed in eight, eight, and seven zones, respectively. The most widely distributed non-native species in 2020 were Taraxacum officinale, Ranunculus acris, and Stellaria media, which were found in nine, seven, and six zones, respectively. Six additional non-native forb species were recorded in 2003 because of their apparent rarity, because they were noted in only one or two zones: Capsella bursa-pastoris, Euphrasia stricta, Plagiobothrys hispidulus, Rumex acetosella subsp. pyrenaica, Tussilago farfara, and Vicia cracca subsp. cracca. Plagiobothrys hispidulus was misidentified tentatively in 2003 as Buglossoides arvensis (L.) I.M. Johnst.
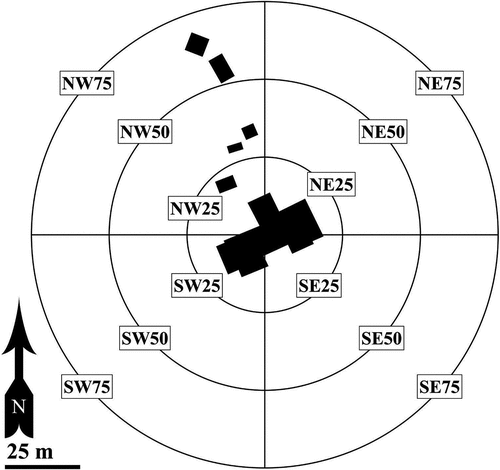
Figure 10. Distribution of five non-native species at Lakes of the Clouds Hut (New Hampshire), visually mapped in 2003 and resurveyed with GPS in 2020: Pilosella aurantiaca, Matricaria discoidea, Ranunculus acris, Stellaria media, and Taraxacum officinale. The bands are 25 m apart and the outermost boundary is 75 m from the geometric center of the main hut. In both years the greatest species richness of non-native species occurred in the northwest quadrant in association with the septic outflow, food compost, and propane storage areas. Taraxacum officinale and R. acris also occur immediately outside the 75-m band to the northwest, and T. officinale is established near the upper lake of the Lakes of the Clouds, 175 m E/NE of the hut.
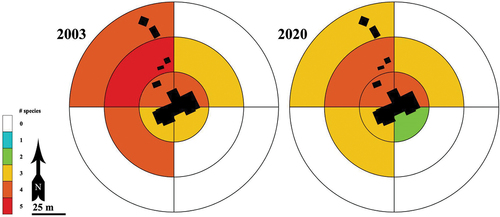
Nine of the eleven species initially recorded at Lakes of the Clouds Hut in 2003 were observed upon resurvey in 2020 (); the two species not detected were Capsella bursa-pastoris and Rumex acetosella subsp. pyrenaica. Two species, Matricaria discoidea and Stellaria media, may have declined in overall extent. Three species had similar distributional footprints: Pilosella aurantiaca, Plagiobothrys hispidulus, and Tussilago farfara (the latter two of which remain narrowly distributed to a single localized occurrence). In June 2021, P. hispidulus occurred along an arcing path roughly 1.5 × 11 m immediately north of the hut’s northeast corner, in the same general area it was documented in 2003. Taraxacum officinale also appears to exhibit a similar distribution in 2020 as in 2003 and is now also known to occur in a disturbed bootleg campsite ~175 m from the hut near the upper lake of the Lakes of the Clouds, and in an undisturbed snowbank 10 m below and north of the lowest septic component. Species that appeared to have increased in overall distributional extent were Euphrasia stricta, Ranunculus acris, and Vicia cracca subsp. cracca. Euphrasia stricta was first observed as a localized occurrence immediately west of the hut in 2003 but in 2020 was found abundantly on the north, west, and south sides of the hut.
Table 5. Generalized distribution and apparent net distributional change of eleven non-native forb species recorded in 2003 and 2020 at Lakes of the Clouds Hut, located between Mt. Washington and Mt. Monroe, New Hampshire.
In addition to the eleven forb species evaluated above, several non-native taxa were observed at the hut between 2016 and 2020: Gnaphalium uliginosum L. (2016), Phalaris arundinacea L. (2018), Plantago major L. (2016), and Poa annua (2018). Within the immediate vicinity of the hut, the only major new ground disturbance between 2003 and 2020 was a septic line reconstruction that occurred in 2017 (United States Department of Agriculture Citation2017). These species were well established when observed and likely to have predated the 2017 septic rebuild.
Discussion
Floristic composition
A group of common, non-native pasture and roadside species arrived early on Mt. Washington and at other disturbed alpine locations, whereas others are more novel. Most of the first introductions apparently arrived via horse and donkey manure, hay, carriages, or passengers and thrived in disturbed environments along road and railway corridors and around summit buildings and mountain huts and have persisted since their first appearance. Three early arrivals on the summit of Mt. Washington (Anthoxanthum hirtum, Fallopia convolvulus, and Sinapis arvensis) may have perished as a result of a hotel fire in 1908, owing to other subsequent disturbance and building replacements around the summit, or potentially from exposure to the extreme climate. Nineteen species first documented more than one hundred years ago (33 percent of fifty-eight total species) account for 102 (59 percent) of the 173 species–location observations (; Appendix), indicative of their invasive capacity. The frequency of the more novel recorded species may not accurately represent their invasive capacity.
Building complexes and the Auto Road are concentration areas for non-natives plants and potential source areas for spread within the alpine zone. The occurrence of fourteen non-native species at twenty-two alpine sites in the White Mountains—away from these highly disturbed footprints—clearly indicates that non-native species can, and have, spread to less disturbed and more natural habitats. Many of these are in locations with late-melting snowbanks or lee positions of topographic features. More exposed surveyed locations have few records of non-natives, despite disturbance in some areas. Relatively few species appear to have established along exposed alpine trails, some of which were popular routes for both horse and donkey ascents. The limited number of non-natives along the Cog Railway corridor (only locations are close to tree line and near the summit) may be due to a combination of heavy disturbance along most of the corridor’s length and slow revegetation overall of the disturbed area (Capers and Taylor Citation2014) and the extreme exposure to prevailing winds from the west and northwest. Similarly, deliberate introductions in the late 1980s of non-native grasses (Poa pratensis subsp. pratensis and Festuca rubra L. subsp. rubra) in disturbed alpine on Franconia Ridge to facilitate revegetation (Cogbill Citation2016) ultimately failed, and these species did not persist on the very exposed ridgeline.
Several factors likely contribute to the high number of non-native species reported from the alpine tundra of the White Mountains, including the relatively small area (~22 km2) that has a long history of botanical study and the concentration of anthropogenically disturbed areas associated with roads, trails, and built structures. In addition, vascular plant collections in major herbaria have been recently digitized across the United States and Canada. This allowed us to research collections made in our study area via herbaria portals, a tool that may not yet be similarly available to researchers studying alpine regions elsewhere around the globe. Finally, thorough targeted contemporary surveys focused in anthropogenically disturbed alpine areas helped yield a more complete list of non-native plant species than previously documented.
Richness
Our findings support our initial hypotheses that non-native plant species richness decreases with elevation, is positively associated with disturbed area, and is higher in disturbed areas with built structures. Specifically, the results of our Auto Road surveys found that midpoint roadway elevation predicted non-native plant species richness, and our analysis of disturbed habitats associated with building complexes found that elevation predicted the richness of non-native plant species per unit area.
Though our study was not designed to compare roadway segments to sites with building complexes, our findings suggest that non-native species richness may be higher in disturbed areas with built structures compared to equivalent areas of disturbed roadsides, which could reflect increased microclimatic heterogeneity around built structures, increased nutrient enrichment, or both. However, our sample size was small, and the relationship between elevation and richness may not be linear. In fact, our results suggest that non-native plant species richness may be higher in tree line, krummholz, and similar late-melting snowbank settings than in exposed tundra areas, indicating a potentially disproportionate risk (and impact) in these ecologically important zones.
Our preliminary findings would be strengthened by a broader regional approach—inclusion of areas in Vermont, Maine, New York, and Québec—to provide a more comprehensive evaluation of the potential effects of elevation, proximity to tree line or krummholz, and disturbed area.
Global comparisons
The composition, geographic origins, and ecological profiles of New Hampshire’s non-native alpine species are similar to those of other alpine regions. Globally, 69 percent of alpine non-natives examined by Alexander et al. (Citation2016) are members of just five families, all well represented in New Hampshire (listed in order of species richness): Poaceae, Asteraceae, Caryophyllaceae, Fabaceae, and Brassicaceae (Alexander et al. Citation2016). A similar pattern is seen in the non-native flora of Arctic subregions: Poaceae, Asteraceae, and Brassiceae contribute 144 species (42 percent of the non-native flora); species-rich genera include Poa, Ranunculus, Rumex, Trifolium, and Vicia. The geographic origins of most alpine non-natives globally—and all of those in New Hampshire’s alpine—are from the Northern Hemisphere. The dominant ecological affinity of the non-native alpine species analyzed by Alexander et al. (Citation2016) and Wasowicz (Citation2016) is shared by New Hampshire’s species: the majority are common and widespread in temperate region lowlands and are tolerant of broad temperature ranges, and very few are strictly adapted to Arctic-alpine or high-mountain conditions. Alexander et al. (Citation2016) reported that all of the non-native alpine species for which data were available were ruderals or competitors (Grime Citation1977), a pattern that holds for New Hampshire’s alpine non-natives as well (none are considered stress tolerators). Presumably stress-tolerant alpine non-natives from other alpine regions of the world might do well in local alpine settings if they could get there, but the large intervening lowland distances between regional alpine areas make these migrations and establishments rare events (Pauchard et al. Citation2009). The expansion of globalization, recreation, and transportation networks may be diminishing these barriers. In New Hampshire, one possible example of an unintentional introduction from a different region may be Plagiobothrys hispidulus at Lakes of the Clouds Hut, whose native range includes high mountain regions of the Western United States. The North American alpine native Lupinus nootkatensis was introduced to Iceland, where it is now considered the most problematic invasive species in highland areas (Wasowicz Citation2016) and expected to continue to expand rapidly (Wasowicz, Przedpelska-Wasowicz, and Kristinsson Citation2013).
The similarity index comparison, NMS, and ordered frequency table (; ) of non-native species in the White Mountains compared to nineteen other alpine areas globally highlight similarities with the widely separated mountain systems including the Australian Alps, Rocky Mountains, Hawaii, Drakensberg, the northern and central Andes, Iceland, and Mexico. Most of the Western European or more broadly distributed Eurasian species that drive the similarities between these regions are infrequent in other African and Eurasian alpine regions and the Sierra Nevada in North America. Several sites occur at tropical or subtropical latitudes (0°–23°; ), in contrast to our prediction of higher similarities among temperate latitudes. This may reflect the fact that the Eurasian species that drive similarities among these alpine regions are broadly adapted generalists (primarily from cool to warm temperate climates; Alexander et al. Citation2016) and can establish readily in lowland areas beyond their native range and subsequently in disturbed and often slightly protected areas in adjacent colder alpine environments. The similarity index calculations are lower overall for regions that have reports of only a few non-native species, which limits the number of potential species in common and resulting index value. Some of this variation in richness among regions may be due to differences between individual study areas and completeness of non-native floristic surveys (many derive from studies only along mountain roads). In addition, 139 of the 260 species occur at only a single site, contributing to dissimilarity and smaller index values. Nonetheless, the general pattern of common floristics among many regions is apparent. In the case of Switzerland, detailed studies by Becker et al. (Citation2005) documented only three non-native species that are common to both the Swiss Alps and the White Mountains (Becker et al. Citation2005): Bromus inermis Leyss. and Matricaria discoidea (up to 2,300 m in the Alps) and Lupinus polyphyllus Lindl. (up to 2,100 m in Europe), with the latter two being native to western North America.
The New Hampshire non-native alpine flora is also similar to floras in some Arctic regions. Eleven of the sixteen species found in three or more Arctic subregions are also found in New Hampshire alpine (Wasowicz et al. Citation2020; Bartlett et al. Citation2021). Five of nine invasive species in Denali National Park (National Park Service Citation2014) targeted for control efforts also occur in New Hampshire alpine.
This study documents 58 non-native plant species from the alpine tundra in the White Mountains of New Hampshire, among the highest species richness for any alpine region (; Alexander et al. Citation2016). The composite richness of all twenty regions compared is 260 species. The non-native flora of the Arctic is higher, totaling 341 documented non-natives for the entire region (i.e., species considered non-native in at least one Arctic subregion): 153 of these plants are considered “casual” establishments, 188 are considered “naturalized” to the Arctic, and 11 of the naturalized species are considered invasive (Wasowicz et al. Citation2020). Subregions within the Arctic vary greatly in terms of total non-native plant species richness. They ranged from none in some subregions to 47 in Arctic Alaska (Alaska Exotic Plants Information Clearinghouse Citation2020); 98 in Svalbard, Norway (Bartlett et al. Citation2021); and 206 species in Kanin-Pechora, Russia, with an average of 40 non-native species in each subregion and a median of 19 species (Wasowicz et al. Citation2020).
Immigration, persistence, and evidence of spread
The immigration of fifty-eight species into White Mountain alpine over the past 148 years (one species every 2.55 years on average), the high degree of persistence, the high frequency of species that have been here the longest, and the evidence of spread beyond highly disturbed contexts suggest that non-native plants will continue to immigrate and increase without management intervention. Furthermore, the heavily disturbed areas of building complexes and the Auto Road are the most likely source areas for the fourteen species that have spread beyond these human contexts. It is unclear the extent to which the warming climate documented on Mt. Washington in recent decades (Murray et al. Citation2021) has reduced climate filtering of non-native species and contributed to the large number of contemporary observations. However, higher richness at lower elevations along the Auto Road suggests that climate filtering may be occurring on Mt. Washington, and therefore as the climate continues to warm, the richness and upper elevational limits of non-native species may shift as well, similar to upward shift of species in the European Alps (Becker et al. Citation2005) and other movements in alpine areas and the Arctic summarized by Rew et al. (Citation2020). The species of highest concern are identified below based on evidence of spread, abundance, and several other factors.
Species of concern
Eight species are identified as higher concern in the White Mountains’ alpine tundra based on the combination of persistence, frequency, and evidence of spread; degree of entrenchment at the site level; and behavior in polar regions and alpine areas globally. The abundance and frequency of Taraxacum officinale is most striking, occurring at thirty-eight stations, eleven of which are in mostly natural alpine settings away from roads or buildings, with some locations consisting of thousands of plants. Its high frequency and local abundance may be indicative of its adaptation to disturbed alpine conditions, having had a long period of time to build capacity to spread, or both. Becker et al. (Citation2005) suggested that the extended altitudinal range of many introduced alpine plants over time in the Swiss Alps may be the result of progressive local adaptation. Through a common garden experiment comparison of T. officinale from the French Alps and invasive alpine T. officinale stock from the Andes, Quiroz et al. (Citation2009) demonstrated that the Andean plants were more drought tolerant than French plants and had a nitrogen-demanding/potassium-avoiding adaptation that differed from the French plants (i.e., the French plants showed no nitrogen or potassium preference). Lipowsky et al. (Citation2012) suggested that rapid genetic differentiation (via differential selection of clones) among T. officinale populations resulted after five years of differential selective pressures imposed on plants from a common origin (experiment conducted in lowland Germany). Adaptation of T. officinale or other non-native species in New Hampshire’s alpine environment has not been documented, but these studies suggest that adaptation in alpine areas is plausible if not likely and worthy of further study.
Cold-adapted Taraxacum officinale have also invaded Arctic and alpine regions in North, Central, and South America; Eurasia; Australia; and Japan (Johnson and Pickering Citation2001; McDougall et al. Citation2005; Carlson et al. Citation2008; Quiroz et al. Citation2009; Alexander et al. Citation2016; Steinmann et al. Citation2021). In the Chilean Andes, plots dominated by T. officinale had reduced cover of several native herbaceous species (Cavieres et al. Citation2005), and Muñoz and Cavieres (Citation2008) found a density-dependent preference of pollinators on T. officinale, resulting in reduced pollination success of competing native plants. In Svalbard, Norway, Bartlett et al. (Citation2021) detected a rapid expansion of Taraxacum section Ruderalia over the last several decades. Taraxacum officinale also grows directly with fifteen of the sixteen rare plants noted in the results as co-occurring with non-natives in the White Mountains (does not occur with Omalotheca supina). Recognition of these threats and documented effects of T. officinale in alpine regions from around the world in combination with the forb’s apparent success in establishing large populations in natural alpine settings on Mt. Washington prompted the initiation of an eradication and monitoring program in 2015 by the United States Department of Agriculture Forest Service and multiple partners. Initial results indicate effective control by repeated hand-digging (D. Sperduto, R. Capers, and N. Slack, unpublished data; United States Department of Agriculture Citation2015, Citation2016).
Seven species other than Taraxacum officinale are of high concern based on evidence of local expansion, abundance, coincidence with rare plants and vulnerable habitats, and/or invasive tendencies in other alpine or polar regions. These include Euphrasia stricta, Pilosella aurantiaca, Poa annua, Ranunculus acris, Rumex acetosella subsp. pyrenaica, Tanacetum vulgare, and Vicia cracca subsp. cracca (all discussed in more detail below). A preliminary survey of non-natives in the alpine zone on Mt. Mansfield, Vermont, in 2022 by the authors documented the first four species (in addition to eight others), indicating that non-natives species of concern are not limited to the White Mountains and underscoring the need for additional study within the region.
Four species of high concern are documented from several of the nineteen global alpine comparison sites () and exhibit evidence of local spread or entrenchment in White Mountain alpine and/or invasiveness in alpine or polar regions. Pilosella aurantiaca is the second most frequent non-native in New Hampshire alpine, where it has spread to both human and naturally disturbed settings and grows with the last remaining Omalotheca supina population. Pilosella aurantiaca occurs at three other alpine sites globally () and is rated as highly invasive in Alaska, where it occurs in southeast and boreal zones (Carlson et al. Citation2008). Although not yet known from Arctic Alaska, it is known from Arctic Iceland (Wasowicz et al. Citation2020) and is a major focus of invasive control efforts in high subalpine areas of Australia. Pilosella officinarum L. (recently discovered on the Mt. Washington Auto Road) is also the subject of control efforts in the Australian Alps (Kueffer et al. Citation2013). Poa annua occurs at all of the alpine huts and around buildings on the summit of Mt. Washington, where it is locally abundant and co-occurs with many rare plants. It also has been documented in the alpine tundra of six other alpine regions (compiled in ), as well as in montane and alpine settings in Gaspésie, Québec, where it has high reproductive capacity at all elevations regardless of the elevation origin of parental strains (Deschenes, Caubel, and Sirois Citation2019). Poa annua was also observed at the summit of Mt. Mansfield, Vermont, by the authors. It is one of the five most widely distributed species documented in the world, occupying closely cropped and trampled turf environments (Wódkiewicz et al. Citation2013). It is cold-adapted and spreading and persistent on King George’s Island, Antarctica (Wódkiewicz et al. Citation2013; Galera et al. Citation2021). Here it can maintain a functional seed bank of more than 5,000 seeds per square meter and is evidently competing with the only two species of native vascular plants in Antarctica (Molina-Montenegro et al. Citation2019). Rumex acetosella occurs in a natural snowbank community in the White Mountains and several other sites and is the second most frequent non-native species among global alpine areas (), documented in seven other regions. Hupp et al. (Citation2017) found that the cushion species Azorella juliana facilitated establishment of R. acetosella in alpine areas of the Andes, and it is common in the Australian Alps, where it has been established for more than one hundred years (Mallen Citation1986; McDougall et al. Citation2005). Tanacetum vulgare is persistent along the Mt. Washington Auto Road, despite initial manual control efforts. This species is considered invasive in the cold-climate regions of the U.S. Mountain West and northern lake states (Jacobs Citation2008; Clasen et al. Citation2011) and considered invasive in Arctic-alpine systems in Alaska (Carlson et al. Citation2008). It is native to Siberia in subalpine montane river valleys (Jacobs Citation2008).
Three of the seven species were not reported from the other nineteen global alpine areas but remain as high concern species in the study area based on evidence of local spread, adaptation to alpine or boreal climates in Eurasia, and/or invasiveness in polar regions. Euphrasia stricta and Ranunculus acris appear to be abundant and spreading within and beyond disturbed building complex sites in White Mountain alpine areas and are present in disturbed alpine settings on Mt. Mansfield, Vermont, as well. As an annual species, E. stricta may have considerable interannual variability, and with a relatively late flowering season, it may have lower detectability during some summers. Ranunculus acris is native to Europe including alpine areas (Totland Citation1996) and thus may be well adapted to northeastern alpine, particularly snowbank settings. Vicia cracca subsp. cracca is spreading at some White Mountain alpine sites and exhibits invasive tendencies in the Arctic, including in northern and western Alaska (Wasowicz et al. Citation2020), where it is considered highly invasive (Carlson et al. Citation2008).
Many of the non-native species could remain confined to small areas for years or decades and may never spread extensively into natural alpine habitats, but those that can or will spread more widely could be ecologically disruptive. In fact, we observed multiple trajectories for species at Lakes of the Clouds Hut, where some species apparently expanded their cover, others exhibited little change, some were no longer detected, and two species have spread beyond the immediate disturbed footprint of the hut. This may reflect that some species have been on-site for many years or decades prior to our initial evaluation from 1999 to 2003 and have possibly reached distributional equilibriums within the site, whereas others continue to build capacity and spread. Notably, the greatest number of non-native species near the backcountry hut were observed along the disturbed corridor of septic system components, emphasizing the possible significance of augmented nutrients to the persistence of non-native species in the otherwise nutrient-poor edaphic context of alpine tundra.
Snowbank communities in the alpine tundra of the White Mountains, with their unique and diverse mix of rare alpine and montane species, may be particularly at risk of invasion from non-native plant species. The late-melting snowpacks create a moderated alpine environment, allowing plants some natural protection from wind exposure and avoidance of extreme temperatures beneath the snow from fall through early summer. Ranunculus acris, Rumex acetosella subsp. pyrenaica, and Taraxacum officinale have all been documented as spreading within human-disturbed alpine areas, and the latter two also occur within natural snowbank settings with no apparent human disturbance. Additional surveys are warranted in alpine ravines, other snowbank settings, and naturally disturbed habitats including avalanche and landslide paths, scree slopes, streambanks, mineral soils subject to cryogenic (freeze-thaw) action, and meadows occupied by mammals such as woodchucks (Marmota monax L.).
Most of the other documented non-native species () appear to be confined to one or two sites, and invasive capacity is uncertain based solely on frequency. However, invasive tendencies in the Arctic or other alpine regions may provide context for some of these infrequent species. For example, of the eleven species considered invasive in the Arctic (Wasowicz et al. Citation2020), five occur in our alpine tundra zone, and four of those are currently documented from a single site: Bromus inermis, Cirsium arvense (L.) Scop., Hordeum jubatum subsp. jubatum, and Leucanthemum vulgare Lam. Three other Arctic invaders occur in the White Mountain lowlands within miles of alpine tundra areas: Anthriscus sylvestris (L.) Hoffm., Linaria vulgaris Mill., and Melilotus albus Medik. (Carlson et al. Citation2008; Wasowicz et al. Citation2020). Given their invasive capacity in the Arctic, all of these species warrant careful monitoring and potentially early detection and rapid response removal should they become established in the alpine tundra.
Other than possibly Potentilla norvegica, there is no evidence that native range expanders (such as Euthamia graminifolia, Hordeum jubatum subsp. jubatum, and Iris versicolor) are spreading in White Mountain alpine areas, but they bear monitoring. Though technically a native species in New Hampshire, Hordeum jubatum subsp. jubatum is considered anomalous and non-native to the alpine zone in the context of this article, where it occurs at Madison Springs Hut 1,300 m above the nearest lowland record. Hordeum jubatum subsp. jubatum is invasive in Arctic Alaska and Yukon (Carlson et al. Citation2008; Wasowicz et al. Citation2020) and has been targeted for management in Denali National Park, where more than 680 kg was collected from 2014 to 2015 (National Park Service Citation2014). Native range expanders have had substantial impacts in several alpine areas globally including Sasa kurilensis Makino & Shibata in Japan (Kudo et al. Citation2011) and Pinus mugo Turra in the Austrian Alps (Dullinger, Dirnböck, and Grabherr Citation2003) and Czech Republic (Kašák et al. Citation2015). Plagiobothrys hispidulus is a cross-continental range expander into a habitat consistent with its source area. How it became established at Lakes of the Clouds Hut is unknown. One plausible pathway is via the footwear of a hiker who had recently been in the U.S. Mountain West, given the noted efficacy of footwear in transporting non-native plant species to Svalbard, Norway, from elsewhere in the world (Ware et al. Citation2012). Another possibility is inadvertent introduction through contaminated seed mix. Gimingham et al. (Citation2002) concluded that Plagiobothrys scouleri in northeast Scotland likely established from contaminated seed mix sourced from the northwestern United States. Although it is apparently restricted to a single site, its extreme diminutive stature may mean that it is easily overlooked. It may yet be discovered on other seasonally flooded, anthropogenically disturbed alpine sites in the region.
Invasive pathways, future prospects, and management considerations
Multiple invasive pathways have likely contributed to the non-native alpine flora in New Hampshire. Although it is difficult to establish which of the present-day pathways contribute most to newer establishments, the likely pathways include motor vehicles; materials associated with Auto Road, Cog Railway, and recreational facility maintenance activities (including mowing equipment and gravel additions for road resurfacing); and via seeds on clothing, boots, and packs from tourists, hikers, and staff of private and public entities working in huts and summit buildings. Building complexes harbor forty-one of the fifty-eight species documented in the White Mountains and are thus primary source areas for potential spread, similar to the high richness at mountain huts in the Australian Alps (Morgan and Carnegie Citation2009). In the Arctic, escapes from cultivation, transport stowaways, and transport contamination were the major pathways of introduction of non-natives, with the first two being the likely source of 85 percent of Arctic invasive plant species (Wasowicz et al. Citation2020). On the island of Svalbard, Ware et al. (Citation2012) recorded 1,019 seeds from the footwear of 259 travelers (~4 seeds per traveler); 26 percent of the seed germinated in local conditions, revealing fifty-three species from seventeen families, the majority of which were non-native species. The number of seeds was highest from travelers whose footwear had been in forested and alpine areas within the previous three months. Chown et al. (Citation2012) obtained similar results from visitors to Antarctica, showing an average of 9.5 seeds/visitor, with a higher number of seeds for scientists than tourists. Casterline (Citation2020) found that non-native invasive plants had become established at thirteen of thirty-five (37 percent) lowland sites on the White Mountain National Forest where gravel sediments had been sourced from private gravel pits in the local vicinity. These studies quantify the efficacy of unintentional introductions via just two of the sources mentioned (footwear and gravel).
Management efforts globally in Arctic, Antarctic, and alpine tundra areas vary greatly. The perceived threat is still considered low in many regions because of the isolation of extensive tundra from the limited establishment locations concentrated around human disturbances, including in the European Alps (Kueffer et al. Citation2013) and the North American Cascade and Rocky Mountain Ranges (Schreiner Citation1982; Parks et al. Citation2005). In contrast, invasive control efforts have been initiated in other tundra regions, including for several Pilosella taxa and other species in the Australian Alps (Kueffer et al. Citation2013), Poa annua in Antarctica (Galera et al. Citation2021), Taraxacum officinale in Denali National Park (National Park Service Citation2014), and Anthriscus sylvestris in Svalbard, Norway (Bartlett et al. Citation2021). The scale of the infestations and/or the biology of the species in these areas have made these eradication efforts difficult and ongoing, with the exception of an early detection and rapid response to the A. sylvestris infestation in Svalbard, which was successful. The T. officinale eradication effort on the summit of Mt. Washington in New Hampshire adds to this growing list.
Without monitoring and remedial control efforts by landowners and managers targeting the most concerning taxa, as well as addressing likely pathways for colonization (such as extensive or frequent ground disturbance), a future increase in the distribution and abundance of non-native plant species is probable in the White Mountain alpine zone, as well as in other northeastern North American alpine areas. This conclusion is supported by the observed expansion of Taraxacum officinale and other species in White Mountain alpine over time (representing a growing “invasion debt”), the expansion of these same species elsewhere globally in tundra ecosystems, increasing recreation pressure and disturbance, the suite of invadable human-disturbed and natural habitats present, and reduced climate filtering from climate change documented in the alpine and montane climate of the White Mountains of New Hampshire (Murray et al. Citation2021).
The Mountain Invasion Research Network (Citation2014) is a model collaboration that seeks to create global information links, awareness, and capacity among managers from different mountain regions. Similar collaborations stem from the polar regions (Rew et al. Citation2020), including from the Arctic Council and the Antarctic Treaty. The Arctic Council, an intergovernmental cooperative among all eight Arctic nations, spurred the development of the Arctic Invasive Alien Species Strategy and Action Plan (Conservation of Arctic Flora and Fauna Citation2022; Protection of the Arctic Marine Environment Citation2022). A 1991 protocol to the Antarctic Treaty prohibits the introduction of non-native species to Antarctica, and Hughes et al. (Citation2020) identified species most likely to invade the Antarctic Peninsula and the areas most at risk for invasion. Bartlett et al. (Citation2021) developed and implemented a monitoring protocol on Svalbard to detect change among non-native species, techniques that may have applicability to the White Mountains. We recommend that landowners and managers of northeastern North American alpine areas utilize these resources to document non-native species and develop preventative practices, monitoring protocols, and control plans (including early detection and rapid response for high-risk plants). Ultimately, collaboration among principal parties at local, regional, and global scales is needed to ensure that non-native invasive plant species do not adversely affect these distinctive globally rare habitats and the numerous state, regional, and global rare native plant species they support.
Supplemental Material
Download Zip (42.4 KB)Acknowledgments
The Mt. Washington Auto Road provided access to the summit during our surveys. Thanks to Erica Duda for assistance with mapmaking and to the anonymous reviewers and editors for their critical review and comments. The authors thank Doug Weihrauch, Charlie Cogbill, Matt Peters, and Brett Engstrom for information and field assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15230430.2023.2243704
Additional information
Funding
References
- Alaska Exotic Plants Information Clearinghouse (AKEPIC). 2020. Alaska Center for Conservation Science, University of Alaska. http://aknhp.uaa.alaska.edu/apps/akepic/ ( accessed June, 2020).
- Alexander, J. M., J. J. Lembrechts, L. A. Cavieres, C. Daehler, S. Haider, C. Kueffer, G. Liu, et al. 2016. Plant invasions into mountains and alpine ecosystems: Current status and future challenges. Alpine Botany 126, no. 2: 89–32. doi:10.1007/s00035-016-0172-8.
- Anderson, L. G., S. Rocliffe, N. R. Haddaway, and A. M. Dunn. 2015. The role of tourism and recreation in the spread of non-native species: A systematic review and meta-analysis. PLoS One 10, no. 10: e0140833. doi:10.1371/journal.pone.0140833.
- Antevs, E. 1932. Alpine zone of Mt. Washington Range. Auburn, ME: Merrill & Webber Company.
- Bartlett, J. C., K. B. Westergaard, I. M. G. Paulsen, R. E. M. Wedegärtner, F. Wilken, and V. Ravolainen. 2021. Moving out of town? The status of alien plants in high-Arctic Svalbard, and a method for monitoring of alien flora in high-risk, polar environments. Ecological Solutions and Evidence 2, no. 1. doi: 10.1002/2688-8319.12056.
- Becker, T., H. Dietz, R. Billeter, H. Buschmann, and P. J. Edwards. 2005. Altitudinal distribution of alien plant species in the Swiss Alps. Perspectives in Plant Ecology, Evolution and Systematics 7: 173–83. doi:10.1016/j.ppees.2005.09.006.
- Bell, A. W. 2015. Dandelions – Lowland nuisance, alpine menace. Randolph Mountain Club News. June issue, p. 9.
- Bliss, L. C. 1963. Alpine plant communities of the Presidential Range, New Hampshire. Ecology 44, no. 4: 678–97. doi:10.2307/1933014.
- Capers, R. S., and D. W. Taylor. 2014. Slow recovery in a Mount Washington, New Hampshire, alpine plant community four years after disturbance. Rhodora 116, no. 965: 1–24. doi:10.3119/13-01.
- Carlson, M. L., I. V. Lapina, M. Shephard, J. S. Conn, R. Densmore, P. Spencer, J. Heys, J. Riley, and J. Nielsen. 2008. Invasiveness ranking system for non-native plants of Alaska. USDA Forest Service. Region 10-TP-143.
- Casterline, J. 2020. A survey of non-native plants associated with gravel sediment projects in the White Mountain National Forest. Master’s Thesis. Plymouth State University, Plymouth, NH.
- Cavieres, L. A., E. I. Badano, A. Sierra-Almeida, and M. A. Molina-Montenegro. 2007. Microclimatic modifications of cushion plants and their consequences for seedling survival of native and non-native herbaceous species in the high Andes of central Chile. Arctic, Antarctic, and Alpine Research 39, no. 2: 229–36. doi:10.1657/1523-0430(2007)39[229:MMOCPA]2.0.CO;2.
- Cavieres, L. A., C. L. Quiroz, and M. A. Molina-Montenegro. 2008. Facilitation of the non-native Taraxacum officinale by native nurse cushion species in the high Andes of central Chile: Are there differences between nurses? Functional Ecology 22: 148–56.
- Cavieres, L. A., C. L. Quiroz, M. A. Molina-Montenegro, A. A. Muñoz, and A. Pauchard. 2005. Nurse effect of the native cushion plant Azorella monantha on the invasive non-native Taraxacum officinale in the high-Andes of central Chile. Perspectives in Plant Ecology, Evolution and Systematics 7, no. 3: 217–26. doi:10.1016/j.ppees.2005.09.002.
- Chen, W. B., and B. M. Chen. 2019. Considering the preference for nitrogen forms by invasive plants: A case study from a hydroponic culture experiment. Weed Research 59: 49–57.
- Chown, S. L., A. H. L. Huiskes, N. J. M. Gremmen, J. E. Lee, A. Terauds, K. Crosbie, Y. Frenot, et al. 2012. Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proceedings of the National Academy of Sciences of the United States of America 109, no. 13: 4938–43.
- Clasen, B. M., N. G. Moss, M. A. Chandler, and A. G. Smith. 2011. A preliminary genetic structure study of the non-native weed, common tansy (Tanacetum vulgare). Canadian Journal of Plant Science 91, no. 4: 717–23. doi:10.4141/cjps10203.
- Cogbill, C. V. 2016. Vegetation of Franconia Ridge, New Hampshire: Evaluation of 42 years of trail management and vegetation change. Beyond Ktaadn for USDA Forest Service. Campton, NH: White Mountain National Forest.
- Connor, E. F., and E. D. McCoy. 1979. The statistics and biology of the species-area relationship. The American Naturalist 113, no. 6: 791–833. doi:10.1086/283438.
- Conservation of Arctic Flora and Fauna (CAFF). 2022. Arctic Council Secretariat. https://caff.is ( accessed March, 2022).
- Consortium of California Herbaria (CCH). 2021. https://www.cch2.org/portal/collections/index.php ( accessed January, 2021).
- Consortium of Midwest Herbaria (CMH). 2021. https://midwestherbaria.org/portal/collections/index.php ( accessed January, 2021).
- Consortium of Northeastern Herbaria (CNH). 2022. http://www.neherbaria.org/portal/collections/index.php ( accessed January, 2021).
- Consortium of Pacific Northwest Herbaria (CPNWH). 2021. https://www.pnwherbaria.org/data/search.php ( accessed January, 2021).
- Crawford, L. 1845. The history of the White Mountains, from the first settlement of Upper Coos and Pequaket. Portland, ME: B. Thurston and Company.
- Crow, G. E., and I. M. Storks. 1980. Rare and endangered plants of New Hampshire: A phytogeographic viewpoint. Rhodora 82, no. 829: 173–89.
- Deschenes, E., E. Caubel, and L. Sirois. 2019. Parental plant elevation does not affect nonnative Poa annua’s seed germination and propagation potential. Natural Areas Journal 39, no. 2: 333–8. doi:10.3375/043.039.0305.
- Dietz, H., and P. J. Edwards. 2006. Recognition of changing processes during plant invasions may help reconcile conflicting evidence of the causes. Ecology 87, no. 6: 1359–67. doi:10.1890/0012-9658(2006)87[1359:RTCPCD]2.0.CO;2.
- Diez, J. M., C. M. D’Antonio, J. S. Dukes, E. D. Grosholz, J. Olden, C. J. B. Sorte, D. M. Blumenthal, et al. 2012. Will extreme climatic events facilitate biological invasions? Frontiers in Ecology and the Environment 10, no. 5: 249–57. doi:10.1890/110137.
- Dullinger, S., T. Dirnböck, and G. Grabherr. 2003. Patterns of shrub invasion into high mountain grasslands of the Northern Calcareous Alps, Austria. Arctic, Antarctic, and Alpine Research 35, no. 4: 434–41. doi:10.1657/1523-0430(2003)035[0434:POSIIH]2.0.CO;2.
- Essl, F., S. Dullinger, W. Rabitsch, P. E. Hulme, K. Hülber, V. Jarošík, I. Kleinbauer, et al. 2011. Socioeconomic legacy yields an invasion debt. Proceedings of the National Academy of Sciences of the United States of America 108, no. 1: 203–7.
- Fernald, M. L. 1950. Gray’s Manual of Botany. 18th ed. New York, NY: Van Nostrand Reinhold Company.
- Fox, J., and S. Weisberg. 2019. An R companion to applied regression, Third Edition. https://socialsciences.mcmaster.ca/jfox/Books/Companion/ ( accessed April, 2023).
- Fuentes, N., E. Ugarte, I. Kühn, and S. Klotz. 2010. Alien plants in Southern South America. A framework for evaluation and management of mutual risk of invasion between Chile and Argentina. Biological Invasions 12, no. 9: 3227–36. doi:10.1007/s10530-010-9716-9.
- Galera, H., A. Znój, K. J. Chwedorzewska, and M. Wódkiewicz. 2021. Evaluation of factors influencing the eradication of annual bluegrass (Poa annua L.) from Point Thomas Oasis, King George Island, Maritime Antarctica. Polar Biology 44, no. 12: 2255–68. doi:10.1007/s00300-021-02941-1.
- Gimingham, C. H., D. Welch, E. J. Clement, and P. Lane. 2002. A large population of Plagiobothrys scouleri (Boraginaceae) in north-east Scotland, and notes on occurrences elsewhere in Britain. Watsonia 24: 159–69.
- Google Earth Pro 7.3. 2022. Presidential Range (44.27°, -71.30°) and Franconia Ridge (44.16°, -71.64°), NH, USA. Available at: google.com/earth/versions (accessed 24 March 2023).
- Grime, J. P. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111, no. 982: 1169–94. doi:10.1086/283244.
- Grytnes, J. A. 2003. Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography 26, no. 3: 291–300.
- Haines, A. 2019. Tracheophyte checklist of New England (4 February 2019). http://www.arthurhaines.com/tracheophyte-checklist/ ( accessed March, 2022).
- Harris, S. K., J. H. Langenheim, and F. L. Steele. 1964. Appalachian Mountain Club field guide to the mountain flowers of New England. Boston, MA: Appalachian Mountain Club.
- Hughes, K. A., O. L. Pescott, J. Peyton, T. Adriaens, E. J. Cottier-Cook, G. Key, W. Rabitsch, et al. 2020. Invasive non-native species likely to threaten biodiversity and ecosystems in the Antarctic Peninsula region. Global Change Biology 13, no. 26: 2702–16. doi:10.1111/gcb.14938.
- Hupp, N., L. D. Llambi, L. Ramirez, and R. Callaway. 2017. Alpine cushion plants have species-specific effects on microhabitat and community structure in the tropical Andes. Journal of Vegetation Science 28, no. 5: 1–11. doi:10.1111/jvs.12553.
- International Plant Names Index (IPNI). 2022. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries, and Australian National Botanic Gardens. http://www.ipni.org ( accessed April, 2022).
- Jacobs, J. 2008. Ecology and management of common tansy (Tanacetum vulgare L.). United States Department of Agriculture, Natural Resources Conservation Service, Invasive Species Technical Note No. MT-18.
- Jodoin, Y., C. Lavoie, P. Villeneuve, M. Theriault, J. Beaulieu, and F. Belzile. 2008. Highways as corridors and habitats for the invasive common reed Phragmites australis in Quebec, Canada. Journal of Applied Ecology 45, no. 2: 459–66. doi:10.1111/j.1365-2664.2007.01362.x.
- Johnson, F. M., and C. Pickering. 2001. Alien plants in the Australian Alps. Mountain Research and Development 21, no. 3: 284–91. doi:10.1659/0276-4741(2001)021[0284:APITAA]2.0.CO;2.
- Jones, M. T., and L. L. Willey. 2012. Eastern alpine guide: Natural history and conservation of mountain tundra east of the Rockies. New Salem, MA: Beyond Ktaadn.
- Kalwij, J. M., M. P. Robertson, and B. J. van Rensburg. 2015. Annual monitoring reveals rapid upward movement of exotic plants in a montane ecosystem. Biological Invasions 17, no. 12: 3517–29. doi:10.1007/s10530-015-0975-3.
- Kašák, J., M. Mazalová, J. Šipoš, and T. Kuras. 2015. Dwarf pine: Invasive plant threatens biodiversity of alpine beetles. Biodiversity and Conservation 24, no. 10: 2399–415. doi:10.1007/s10531-015-0929-1.
- Kimball, K. D., and D. M. Weihrauch. 2000. Alpine vegetation communities and the alpine-treeline ecotone boundary in New England as biomonitors for climate change. In S. F. McCool, D. N. Cole, W. T. Borrie, and J. O’Loughlin, eds., Wilderness Science in a Time of Change. 2000. USDA Forest Service Proceedings RMRS-P-15-VOL-3, Missoula, MT, pp. 93–101.
- Kudo, G., Y. Amagai, B. Hoshino, and M. Kaneko. 2011. Invasion of dwarf bamboo into alpine snow-meadows in northern Japan: Pattern of expansion and impact on species diversity. Ecology and Evolution 1, no. 1: 85–96. doi:10.1002/ece3.9.
- Kueffer, C., K. McDougall, J. M. Alexander, C. Daehler, P. J. Edwards, S. Haider, A. Milbau, et al. 2013. Plant invasions into mountain protected areas: Assessment, prevention and control at multiple spatial scales. In Plant invasions in protected areas: Patterns, problems and challenges, ed. L. C. Foxcroft, P. Pyšek, D. M. Richardson, and P. Genovesi, 89–116. Dordrecht, NL: Springer.
- Lembrechts, J. J., J. M. Alexander, L. A. Cavieres, S. Haider, J. Lenoir, C. Kueffer, K. McDougall, et al. 2017. Mountain roads shift native and non-native plant species’ ranges. Ecography 40, no. 3: 353–64. doi:10.1111/ecog.02200.
- Lipowsky, A., C. Roscher, J. Schumacher, and B. Schmid. 2012. Density-independent mortality and increasing plant diversity are associated with differentiation of Taraxacum officinale into r- and K-strategists. PLoS ONE 7, no. 1: e28121. doi:10.1371/journal.pone.0028121.
- MacArthur, R., and E. O. Wilson. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press.
- Mallen, J. 1986. Introduced vascular plants in the high altitude and high latitude areas of Australasia, with particular reference to the Kosciuszko alpine area, New South Wales. In Flora and fauna of the alpine Australasia: Ages and origins, ed. B. A. Barlow, 249–58. Melbourne, AU: CSIRO.
- McCune, B., and M. J. Mefford. 2018. PC-ORD. Multivariate analysis of ecological data. Version 7.10.
- McDougall, K. L., J. W. Morgan, N. G. Walsh, and R. J. Williams. 2005. Plant invasions in treeless vegetation of the Australian Alps. Perspectives in Plant Ecology, Evolution and Systematics 7, no. 3: 159–71. doi:10.1016/j.ppees.2005.09.001.
- Merrill, G. D. 1888. History of Coos County. Syracuse, NY: W. A. Fergusson & Co.
- Meunier, G., and C. Lavoie. 2012. Roads as corridors for invasive plant species: New evidence from smooth bedstraw (Galium mollugo). Invasive Plant Science and Management 5, no. 1: 92–100. doi:10.1614/IPSM-D-11-00049.1.
- Milbau, A., A. Shevtsova, N. Osler, M. Mooshammer, and B. J. Graae. 2013. Plant community type and small-scale disturbances, but not altitude, influence the invasibility in subarctic ecosystems. New Phytologist 197, no. 3: 1002–11. doi:10.1111/nph.12054.
- Molina-Montenegro, M. A., D. M. Bergstrom, K. J. Chwedorzewska, and S. L. Chown. 2019. Increasing impacts by Antarctica’s most widespread invasive plant species as a result of direct competition with native vascular plants. NeoBiota 51: 19–40. doi:10.3897/neobiota.51.37250.
- Morgan, J. W., and V. Carnegie. 2009. Backcountry huts as introduction points for invasion by non-native species into subalpine vegetation. Arctic, Antarctic, and Alpine Research 41, no. 2: 238–45. doi:10.1657/1938-4246-41.2.238.
- Mountain Invasion Research Network (MIREN). 2014. Linking local and global scales for addressing an ecological consequence of global change. GAIA 23, no. 3: 263–5. doi:10.14512/gaia.23.3.11.
- Muñoz, A. A., and L. A. Cavieres. 2008. The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. Journal of Ecology 96, no. 3: 459–67. doi:10.1111/j.1365-2745.2008.01361.x.
- Murray, G. L. D., A. M. Colgan, S. J. Nelson, E. P. Kelsey, and K. D. Kimball. 2021. Climate trends on the highest peak of the Northeast: Mount Washington, NH. Northeastern Naturalist 28, no. 11: 64–82. doi:10.1656/045.028.s1105.
- National Park Service. 2014. Invasive and exotic plant management in Denali National Park and Preserve. U.S. Department of the Interior, National Park Service, Natural Resource Stewardship and Science, Fort Collins, Colorado. NPS/DENA/NRDS—2014/739.
- New Hampshire Natural Heritage Bureau. 2021. Biotics database. Division of Forests and Lands. Concord, NH: Department of Natural and Cultural Resources.
- Northrop, J. I., and A. R. Northrop. 1889. Notes on the Plant Distribution of Mt. Washington, N.H. In A naturalist in the Bahamas: Life and writings of John I. Northrop. 1910, ed. H. F. Osborn, 255–8. New York, NY: Columbia University Press.
- Parks, C. G., S. R. Radosevich, B. A. Endress, B. J. Naylor, D. Anzinger, L. J. Rew, B. D. Maxwell, and K. A. Dwire. 2005. Natural and land-use history of the Northwest mountain ecoregions (USA) in relation to patterns of plant invasions. Perspectives in Plant Ecology, Evolution and Systematics 7, no. 3: 137–58. doi:10.1016/j.ppees.2005.09.007.
- Pauchard, A., and P. B. Alaback. 2004. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of south-central Chile. Conservation Biology 18, no. 1: 238–48. doi:10.1111/j.1523-1739.2004.00300.x.
- Pauchard, A., C. Kueffer, H. Dietz, C. Daehler, J. Alexander, P. Edwards, J. Arévalo, et al. 2009. Ain’t no mountain high enough: Plant invasions reaching new elevations. Frontiers in Ecology and the Environment 7, no. 9: 479–86. doi:10.1890/080072.
- Pease, A. S. 1924. Vascular flora of Coos County, New Hampshire. Proceedings of the Boston Society of Natural History 37: 339–88.
- Pease, A. S. 1964. A flora of northern New Hampshire. Cambridge, MA: New England Botanical Club, Inc.
- Pease, A. S. 1916. Notes on the botanical explorations of the White Mountains. In Appalachia, Vol. XIV. 1916–1919, and C. E. Fay, 157–78. Boston, MA: The Appalachian Mountain Club.
- Pickering, C., and A. Mount. 2010. Do tourists disperse weed seed? A global review of unintentional human-mediated terrestrial seed dispersal on clothing, vehicles, and horses. Journal of Sustainable Tourism 18, no. 2: 239–56. doi:10.1080/09669580903406613.
- Pollnac, F., T. Seipel, C. Repath, and L. J. Rew. 2012. Plant invasion at landscape and local scales along roadways in the mountainous region of the Greater Yellowstone Ecosystem. Biological Invasions 14, no. 8: 1753–63. doi:10.1007/s10530-012-0188-y.
- Protection of the Arctic Marine Environment (PAME). 2022. Arctic Council Secretariat. www.arctic-council.org ( accessed March, 2022).
- Quiroz, C. L., P. Choler, F. Baptist, M. González-Teuber, M. A. Molina-Montenegro, and L. A. Cavieres. 2009. Alpine dandelions originated in the native and introduced range differ in their responses to environmental constraints. Ecological Research 24, no. 1: 175–83. doi:10.1007/s11284-008-0498-9.
- Rahbek, C. 1995. The elevational gradient of species richness: A uniform pattern? Ecography 18, no. 2: 200–5. doi:10.1111/j.1600-0587.1995.tb00341.x.
- Rew, L. J., K. L. McDougall, J. M. Alexander, C. C. Daehler, F. Essl, S. Haider, C. Kueffer, et al. 2020. Moving up and over: Redistribution of plants in alpine, Arctic, and Antarctic ecosystems under global change. Arctic, Antarctic, and Alpine Research 52, no. 1: 651–65. doi:10.1080/15230430.2020.1845919.
- Schreiner, E. G. 1982. The role of non-indigenous species in plant succession following human disturbance in an alpine area of Olympic National Park, Washington. Ph.D. Thesis. University of Washington, Seattle.
- Simberloff, D. S. 1972. Models in biogeography. In Models in paleobiology, ed. T. J. M. Schopf, 160–91. San Francisco, CA: Freeman, Cooper, and Co.
- Southeast Regional Network of Expertise and Collections (SERNEC). 2021. https://sernecportal.org/portal/collections/index.php ( accessed January 2021).
- Sperduto, D., and C. Cogbill. 1999. Alpine and subalpine vegetation of the White Mountains, New Hampshire. New Hampshire Natural Heritage Inventory, Department of Resources and Economic Development. Concord, NH.
- Sperduto, D., and B. Kimball. 2011. The nature of New Hampshire: Natural communities of the Granite State. Hanover and London: University of New Hampshire Press, Durham, NH and University Press of New England. p. 40.
- Sperduto, D., and W. Nichols. 2011. Natural communities of New Hampshire. 2nd ed. Durham: New Hampshire Natural Heritage Bureau, Concord, NH. Pub. UNH Cooperative Extension.
- Steinmann, V. W., L. Arredondo-Amezcua, R. A. Hernández-Cárdenas, and Y. Ramírez-Amezcua. 2021. Diversity and origin of the central Mexican alpine flora. Diversity 13, no. 31: 1–25.
- Storks, I. M., and G. E. Crow. 1979. Endangered, threatened and rare plants of the White Mountain National Forest, New Hampshire. Report prepared for the White Mountain National Forest, USFS in cooperation with the New Hampshire Agricultural Experiment Station, Durham, NH.
- Thoreau, H. D. 1906. The writings of Henry David Thoreau. Journal. XI. 7/2/1858–2/28/1859. Houghton Mifflin and Company, Boston and New York.
- Totland, O. 1996. Flower heliotropism in an alpine population of Ranunculus acris (Ranunculaceae): Effect on flower temperature, insect visitation, and seed production. American Journal of Botany 83, no. 4: 452–8. doi:10.1002/j.1537-2197.1996.tb12726.x.
- Tyser, R., and C. Worley. 1992. Alien flora in grasslands adjacent to road and trail corridors in Glacier National Park, Montana (U.S.A.). Conservation Biology 6, no. 2: 253–62. doi:10.1046/j.1523-1739.1992.620253.x.
- United Nations Environment Programme (UNEP), 2009. Climate Change Science Compendium 2009. Nairobi, KE: EarthPrint.
- United States Department of Agriculture (USDA). 2015. Mount Washington dandelion removal project. Campton, NH: Supervisor’s Office, White Mountain National Forest.
- United States Department of Agriculture (USDA). 2016. Invasive dandelion removal in the alpine zone. Campton, NH: Supervisor’s Office, White Mountain National Forest.
- United States Department of Agriculture (USDA). 2017. Lakes of the Clouds septic system replacement project. Campton, NH: Pemigewasset Ranger District, White Mountain National Forest.
- United States Department of Agriculture (USDA). 2023. National Threatened, Endangered and Sensitive Species (TES) Program. (threatened, endangered & sensitive species | US Forest Service) www.usda.gov ( accessed May, 2023).
- Vacchiano, G., E. Barni, M. Lonati, D. Masante, A. Curtaz, S. Tutino, and C. Siniscalco. 2013. Monitoring and modeling the invasion of the fast spreading alien Senecio inaequidens DC. in an alpine region. Plant Biosystems 147, no. 4: 1139–47. doi:10.1080/11263504.2013.861535.
- Ware, C., D. Berge, E. Muller, and I. G. Alsos. 2012. Humans introduce viable seeds to the Arctic on footwear. Biological Invasions 14, no. 3: 567–77. doi:10.1007/s10530-011-0098-4.
- Wasowicz, P. 2016. Non-native species in the vascular flora of highlands and mountains of Iceland. PeerJournal 4: e1559. doi:10.7717/peerj.1559.
- Wasowicz, P., E. M. Przedpelska-Wasowicz, and H. Kristinsson. 2013. Alien vascular plants in Iceland: Diversity, spatial patterns, temporal trends, and the impact of climate change. Flora - Morphology, Distribution, Functional Ecology of Plants 208, no. 10–12: 648–73. doi:10.1016/j.flora.2013.09.009.
- Wasowicz, P., A. N. Sennikov, K. B. Westergaard, K. Spellman, M. Carlson, L. J. Gillespie, J. M. Saarela, et al. 2020. Non-native vascular flora of the Arctic: Taxonomic richness, distribution and pathways. Ambio 49, no. 3: 693–703. doi:10.1007/s13280-019-01296-6.
- Wódkiewicz, M., H. Galera, K. J. Chwedorzewska, I. Giełwanowska, and M. Olech. 2013. Diaspores of the introduced species Poa annua L. in soil samples from King George Island (South Shetlands, Antarctica). Arctic, Antarctic, and Alpine Research 45, no. 3: 415–9. doi:10.1657/1938-4246-45.3.415.
