Abstract
Background: Inflammation may contribute to cardiovascular disease (CVD) among antiretrovirally suppressed HIV-infected individuals. We assessed relationships of monocyte, CD8 T-cell activation and plasma biomarkers to changes in carotid artery intima-media thickness (CIMT).
Methods: Longitudinal study of HIV-infected subjects ≥40 years and on stable antiretroviral therapy (ART) ≥3 months. Peripheral blood mononuclear cells were immunophenotyped by multiparameteric flow cytometry to quantify classical (CD14++CD16–), intermediate (CD14++CD16+), non-classical (CD14low/+CD16++) and transitional (CD14+CD16–) monocyte subsets and activated (CD38+HLA-DR+) CD8+ T-cells at baseline. Plasma biomarkers were assessed by multiplex Luminex assay. High-resolution B-mode ultrasounds of right carotid arteries were obtained. Changes in CIMT over two years at the right common carotid artery (CIMTCCA) and right bifurcation (CIMTBIF) were outcome variables.
Results: We studied 50 subjects: 84% male, median age 49 (Q1, Q3; 46, 56) years, median CD4 count 461 (317, 578) cells/mm3, and with HIV RNA ≤ 50 copies/mL in 84%. Change in CIMTBIF correlated with log values of baseline absolute count of non-classical monocytes (r = 0.37, p = 0.020), and with MCP-1 (r = 0.42, p = 0.0024) and TNF-α (r = 0.30, p = 0.036) levels. In multivariable linear regression, only non-classical monocytes and MCP-1 predicted the change in CIMTBIF, independent of Framingham Risk Score and baseline CIMTBIF. No correlation was noted between CD8 T-cell activation and CIMTBIF change. Monocyte subsets, CD8 T-cell activation, and biomarker concentrations were not correlated with changes in CIMTCCA.
Conclusions: Our findings highlight the role of non-classical monocytes and MCP-1 in the progression of CIMTBIF in HIV-infected individuals on stable ART independent of traditional cardio-metabolic risk factors.
Keywords:
Introduction
As human immunodeficiency virus (HIV)-infected individuals are living longer as a result of access to virally suppressive combination antiretroviral therapy (ART) regimens, cardiovascular disease (CVD) has become an important cause of morbidity and mortality in this population.Citation1 While the underlying pathophysiology for this phenomenon has yet to be determined, it is believed that HIV infection leads to chronic inflammation and pro-atherogenic processes.Citation2 Multiple studies have used high-resolution ultrasound to assess carotid artery intima-media thickness (CIMT) as a non-invasive measure of subclinical atherosclerosis. Assessment and progression of CIMT is predictive of future cardiovascular events,Citation3 and has been well-studied amongst HIV-infected patients.Citation4
Although initial studies were mixed regarding the association of HIV-infection with CIMT, the limitations of these results included small sample sizes and inconsistent analyses of carotid artery segments.Citation5 Specifically, a majority of studies showed no differences between controls and HIV patients at the common carotid artery (CIMTCCA),Citation6 whereas recent investigators have emphasized a greater difference in areas of low endothelial shear stress, such as the carotid artery bifurcation. Atherosclerotic lesions are more likely to occur in areas of low endothelial shear stress where factors including downregulation of eNOS, increased uptake of LDL, and promotion of oxidative stress predispose to vascular lesions.Citation7–9 Low flow areas allow for the attachment of inflammatory cells and downstream upregulation of pro-inflammatory cytokines and adhesion molecules.Citation10 Additionally, areas of low shear stress have been linked to the transition of a stable lesion to a vulnerable plaque due to a combination of vessel remodeling and inflammation.Citation2 Given that HIV-uninfected individuals are prone to vascular damage at the carotid artery bifurcation, HIV infection may further augment the inflammatory processes quantified by CIMT at the bifurcation (CIMTBIF). Studies have shown an independent association of HIV infection with more rapid CIMTBIF progression relative to other segments of the carotid artery.Citation11
While studies of HIV-induced immune activation have traditionally focused on the role of CD8 T-cells, there is increasing interest in the role that monocytes may play in non-infectious complications seen among individuals with chronic HIV infection.Citation12–14 Monocytes are a heterogeneous population of cells, and are classified by international consensus into several subsets on the basis of their CD14 and CD16 surface expression: classical monocytes lacking CD16 expression (CD14++CD16–) and those expressing CD16 comprised intermediate (CD14++CD16+) and non-classical (CD14low/+CD16++) monocyte subsets.Citation15 We have recently published data reporting the identification of a fourth “transitional” monocyte subset characterized by reduced but still detectable levels of CD14 (CD14+CD16–) that may represent an immature stage of monocyte development. Within our cohort of HIV-infected individuals on stable ART, expansion of transitional monocytes was found to be associated with increased CIMTCCA at baseline.Citation16,17 Classical monocytes lacking CD16 expression account for roughly 80–90% of circulating monocytes in normal healthy individuals. This population has been reported to increase in acute inflammation and to be rapidly recruited to sites of infection.Citation18–20 The CD16-expressing monocytes, on the other hand, increase with aging and in chronic inflammatory disorders. Compared to classical monocytes, they show higher expression of pro-inflammatory cytokines, higher potency in antigen presentation, and are more permissive for productive HIV infection.Citation21–23 CD16-expressing monocytes have also been shown to expand with HIV infection and acute coronary syndrome.Citation24 An increase in non-classical CD16-expressing monocytes has been reported to correlate with HIV disease progression in ART-naïve subjects.Citation25 Increases in both non-classical and intermediate CD16-expressing monocyte subsets similar in pattern to those in HIV-uninfected subjects with acute coronary syndrome have been reported in HIV-infected individuals with uncontrolled HIV disease.Citation24 In the general population, increases in circulating monocytes have been observed in diabetic subjects compared to non-diabetic controls.Citation26–28 CD16-bearing monocyte subsets have been reported to be increased in patients with type 2 diabetes and in particular in those with diabetic complications such as renal disease.Citation13,26,29 We have reported that increases in insulin resistance are associated with increases in circulating monocytes in HIV-infected subjects Taken together, these data indicate a potentially important role for monocyte populations in the pathogenesis of HIV-associated cardio-metabolic disorders.
This study sought to assess the relationship of monocyte subset phenotypes and T-cell activation with changes in CIMT among HIV-infected individuals on stable ART over time. We also assessed soluble plasma biomarkers of inflammation known to play a substantial role in HIV immune dysregulation.
Materials and methods
Subjects and study design
We analyzed entry data of participants enrolled into the Hawaii Aging with HIV-Cardiovascular Cohort, a five-year longitudinal study investigating the role of immune activation and mitochondrial-specific oxidative stress on the pathogenesis of CVD in HIV-infected patients on ART.Citation30 The study was approved by the Committee on Human Subjects of the University of Hawaii and written informed consents were obtained from all participants.
Entry criteria required subjects to have documented HIV infection, be at least 40 years of age, and be on stable ART for ≥3 months. Blood pressure measurements were obtained in triplicate and averaged. Body mass index (kg/m2) was calculated. CBC, T-cell subsets, plasma HIV RNA assessments, chemistries and metabolic labs (glucose, insulin, total cholesterol, directly measured LDL-C and HDL-C, and triglycerides) were obtained at entry in a fasted state (nothing by mouth except water for 12 h). Subjects were assessed for past and current tobacco use. Diabetes was defined as self-reported history of diabetes, use of diabetic medications, fasting blood glucose ≥126 mg/dL, or a 2-h OGTT glucose level > 200 mg/dL. Participants without concurrent CIMT, monocyte subset and inflammatory measurements were excluded from the analysis.
Framingham Risk Score (FRS ATP III) was calculated based on a model comprising age, gender, total cholesterol, HDL-C, systolic blood pressure, treatment of hypertension, and any cigarette smoking in the past month as previously described.Citation31 FRS was used to categorize subjects into a Framingham risk class (FRC) defined as “low” (<10% 10-year risk of CVD), “intermediate” (10–19% risk of CVD), and “high” risk (>20% risk of CVD). Subjects with diabetes (as a CVD equivalent) or clinical CVD (history of myocardial infarction, angina, coronary disease-related cardiac surgery, or ischemic stroke) were automatically classified under high risk. Clinical CVD events were adjudicated by two physician-researchers.
Carotid artery intima-media thickness
High-resolution B-mode ultrasound images of the right carotid artery were obtained. A single reader measured the CIMT of the far wall of the distal CCA and BIF with automated edge detection. Ultrasound images were acquired at the Queen’s Medical Center in Honolulu and analyzed at the University of Southern California Atherosclerosis Research Unit Core Imaging and Reading Center. Internal landmarks were identified and used at the subsequent visit to ensure that the measurements were performed at the same locations on follow-up visits. Images and measurements were performed at baseline and at 24 months.
Flow cytometric analysis
Cryopreserved PBMC cells were thawed and surface-stained with the following antibodies to identify monocyte sub populations as previously describedCitation32: V500-conjugated anti-CD3, Qdot605-conjugated anti-CD14, Alexa700-conjugated anti-CD16, PE-Cy7-conjugated anti-CD56, PE-Cy7-conjugated anti-CD19, PE-Cy7-conjugated anti-CD20, APC-H7-conjugated HLA-DR monoclonal antibodies (mAbs), All antibodies were from BD Biosciences, except for Q605-conjugated anti-CD14 and yellow Live/Dead (Life Technologies). CD8 T-cell activation was defined using the following antibodies: Alexa700-conjugated anti-CD3, APCH7-conjugated anti-CD8, V450-conjugated anti-CD38, APC-conjugated anti-HLA-DR all mAbs (all from BD Biosciences San Jose, CA). Stained PBMCs were acquired by flow cytometry, using a four-laser custom BD-Fortessa instrument (Becton Dickinson) and analyzed with FlowJo software (Treestar Inc Ashland, OR) as previously described.Citation32
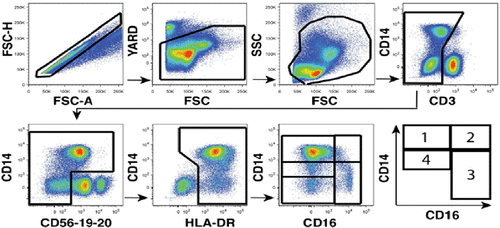
Total monocyte count and CD8 T-cell counts were calculated using white blood cell count (WBC). Monocyte frequencies were obtained on previous flow cytometric evaluations conducted on blood specimens utilized in our prior reportCitation32. We utilized the mean fluorescence intensity of HLA-DR expression to confirm to separate monocyte subpopulations as previously reported.Citation33 See Fig for the multiparametric flow cytometry gating strategy to phenotype four distinct monocyte sub-populations from peripheral blood based on CD16 and CD14 expression.
Figure 1 Multiparametric flow cytometry gating strategy to phenotype four distinct monocyte sub-populations from peripheral blood based on CD16 and CD14 expression: (1) classical monocytes lacking CD16 expression (CD14++CD16-) and those expressing CD16 comprised (2) intermediate (CD14++CD16+) and (3) non-classical (CD14low/+CD16++) monocytes. A fourth MO subset which we have termed (4) “transitional” monocyte subset is characterized by reduced but still detectable levels of CD14 (CD14 + CD16-).
Plasma soluble biomarkers
Testing was conducted using Milliplex Human CVD panels (EMD Millipore, USA) as outlined in the manufacturer’s protocols. Soluble biomarkers assessed included MMP-9, tPAI-1, hsCRP, IL-6, IL-8, IL-10, TNF-α, soluble E-selectin (sE-selectin), soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intercellular cell adhesion molecule-1 (sICAM-1), monocyte chemoattractant protein-1 (MCP-1), and interferon-gamma (IFN-γ).
Statistical analyses
Demographic, clinical, and immunologic information was summarized by median and interquartile (IQR) for continuous variables and frequency (percentage) for categorical variables. Continuous variables (e.g. biomarker levels) were log-10 transformed to improve normality before further analyses were performed if their distributions were skewed. Correlations between variables were performed by Pearson correlation. The outcome variables were the changes in CIMTCCA and CIMTBIF between year 2 and baseline. The relationships between the change in CIMT, baseline CIMT, year 2 CIMT, and various immune variables were assessed by simple and multivariable linear regression analyses, adjusting for immunovirologic and HIV-specific factors. Soluble plasma biomarkers were examined by multivariable analysis if the simple regression analysis produced a p < 0.10. All statistical analyses were conducted in SAS version 9.3 (SAS Institute Inc., Cary, NC). A two-sided p-value < 0.05 was regarded as statistically significant.
Results
Patient characteristics
A total of 50 subjects enrolled into the Hawaii Aging with HIV-Cardiovascular cohort who had available baseline monocyte phenotypic characterization data, concurrent plasma biomarkers previously assessed, and CIMT data two years apart were included in this analysis. The demographic, clinical, and immunologic characteristics of the subjects are summarized in Table . The majority of the subjects were male and of Caucasian ethnicity. Entry criteria for the cohort required subjects to be on ART and 84% of these subjects were virologically suppressed at a plasma HIV RNA level of <50 copies/mL. Subjects aged 40 years or older were recruited and the median age of the selected subjects in this data-set was 49 years (Q1, Q3). The median current CD4 count was relatively high at 461cells/μL (Q1, Q3) while the median nadir CD4 was relatively low at135 cells/μL (Q1, Q3). The monocyte percent and count were 8% and 416 cells/μL, respectively. By subsets, the classical monocytes comprised the majority (78%) of the population, with intermediate (1.4%), non-classical (5%), and transitional (14%) monocytes comprising the rest based on our cellular exclusion/inclusion gating strategy.
Table 1 Baseline characteristics. Values reported as median (Q1, Q3), except for frequency count, n (%)
The majority of subjects had an increase in CIMTCCA and CIMTBIF over the two-year period. Eighty-eight percent of subjects had an increase in CIMTCCA with a median increase of .015 mm and 94% had increased CIMTBIF with a median increase of .016 mm.
Relationship between monocyte subsets, CD8 activation, and CIMT
CIMTCCA at baseline and year 2 correlated with transitional monocytes (CD14+CD16–) but there was no correlation between the transitional monocyte subset and biomarkers with change in CIMTCCA (Tables and ). Classical, non-classical and intermediate monocytes were not correlated with baseline, year 2 or change in CIMTCCA (Table ). No correlation was noted between CD8+ T-cell activation (CD38+HLA-DR+) and CIMTCCA at each endpoint or CIMTCCA change (data not shown).
Table 2 Correlation of monocyte and monocyte subtypes with carotid intima-media thickness, baseline and year 2
Table 3 Correlation between monocyte subtypes and biomarkers
We observed that the frequency (%) of non-classical monocyte (CD14+/lowCD16++) were associated with change in CIMTBIF on simple regression analysis (r = 0.36, p = 0.025) (Table ). Upon adjustment, the proportion of non-classical monocytes predicted change in CIMTBIF (β = 0.13, p = 0.028), independent of Framingham Risk Score (FRS) and baseline CIMTBIF (Table ). (Similar regression coefficients were observed if further adjusted for CD4 count (β = 0.14, p = 0.022, data not shown). Change in CIMTBIF did not correlate with FRS or with baseline CIMTBIF in our study. The frequency of “transitional” monocytes (CD14+CD16–) was marginally correlated with baseline CIMTBIF (r = 0.308, p = 0.053) and year 2 CIMTBIF (r = 0.300, p = 0.060), but not with change in CIMTBIF (Table ). The frequency of classical and intermediate monocytes did not correlate with either baseline, year 2 or change in CIMTBIF. No correlation was noted between CD8+ T-cell activation (CD38+HLA-DR+) and CIMTBIF at each endpoint or CIMTBIF change.
Correlations between soluble plasma biomarkers and change in CIMT
sVCAM-1 was correlated with CIMTCCA at baseline and year 2 but not with change in CIMTCCA Table ). MCP-1 and TNF-α were correlated with change in CIMTBIF but not with CIMTBIF at baseline or year 2. MCP-1 predicted change in CIMTBIF (β = 0.031, p = 0.009), independent of FRS and baseline CIMTBIF (Table ), while TNF-α did not. In multivariable linear regression of change in CIMTBIF, MCP-1 remained significant after adjustment with non-classical monocytes, FRS and baseline CIMTBIF.
Table 4 Multivariable linear regression analysis of the association of non-classical monocyte subset percentage and MCP-1 to the change of CIMTBIF from baseline adjusting for Framingham Risk Score and baseline CIMTBIF
Discussion
The risk for CVD among HIV virally suppressed individuals may be higher than in HIV uninfected individuals.Citation34–37 An appreciation of the role of immune-mediated inflammation and immune activation is gathering tremendous interest as a causal modality in explaining the pathogenesis of this increased risk.Citation38,39 While our previous work found that baseline CIMTCCA was associated with an increase in transitional monocytes, this study assessing change in CIMT found that higher proportions and absolute levels at baseline of the non-classical monocyte subset were associated with increased CIMTBIF, although not with CIMTCCA.Citation17 This association between non-classical monocyte subset and CIMTBIF remained significant even after adjustment for CD4, FRS, and baseline CIMTBIF. Although the majority of study participants were in the low Framingham risk category, the associations between non-classical monocyte subset and CIMTBIF were still seen in the intermediate and high Framingham risk category.
Given the numerous studies demonstrating a relationship between increasing burden of CIMT and future cardiovascular events, one interpretation of our data is that an increase in the non-classical monocyte population may be part of HIV-driven immune dysregulation, which would subsequently drive the increased risk of CVD seen in chronic HIV infection.Citation12 Westhorpe et al. found an increase in CX3CR1 expression on monocytes associated with worse CIMT. Non-classical monocytes have a higher expression of CX3CR1.Citation40
Interestingly, the baseline and longitudinal relationships were not the same. We found a relationship between transitional monocytes and baseline CIMTBIF.Citation17 However, there was no association between transitional monocytes and the change in CIMTBIF. This previously undescribed population of monocytes may serve a unique role as an indicator of CVD shaping a different pathogenic process that is static over time. Further studies to characterize this population are needed.
Our study found an association between change in CIMTBIF and both MCP-1 and TNF-α. In HIV-infected individuals, higher levels of MCP-1 have been associated with increased CIMTCitation41 and with thoracic aorta vessel wall area and vessel wall thickness.Citation42 Monocyte subsets expressing CCR2, the receptor for MCP-1, have been linked to increased HIV associated co-morbidities.Citation43,44 Similarly, higher levels of TNF-α have correlated with IMT specifically for the internal carotid artery.Citation45
In the general population, multiple studies have reported associations between various soluble plasma markers of inflammation and CIMT, particularly at the bifurcation region. A systemic review that more broadly assessed the relationship of various inflammatory markers to CIMT, subsequently concluded that in most studies, the relationship between inflammatory markers and CIMT disappeared after appropriate correction for the presence of traditional risk factors.Citation46 An explanation for this loss of association after adjustment is that inflammatory markers simply function in the causal pathway between risk factors and atherosclerosis, mediating some of the effects of these traditional risk factors.Citation47 It is interesting to note that this was not the case in our study. The association with MCP-1 remained significant when adjusted for traditional CVD risk factors as well as for non-classical monocytes and baseline CIMTBIF, suggesting that the elevation of MCP-1 was not simply a biomarker in the causal pathway between traditional risk factors and atherosclerosis, and had additional predictive factor above and beyond levels of non-classical monocytes. We speculate that this increase in MCP-1 may be secondary to HIV-induced immune dysregulation and may partially mediate the increased risk of CVD in our HIV-infected population.
This study is limited by its relatively small size of the cohort, male predominance, and no HIV sero-negative comparision group. However, the strengths of the study are the careful clinical and metabolic characterization of the subjects in association with detailed phenotypic characterization of monocytes, as well as biomarker assays performed in plasma. Although CIMTBIF also has more variation compared to CIMTCCA especially on repeated measures over time, the study attempted to reduce operator variation by restricting the CIMT measures to a single sonographer and reading center. The study was limited by its ability to assess effects of co-infections such as Hepatitis C and cytomegalovirus (CMV). Host genetics were also not examined in this study. We conclude that chronic HIV in subjects on stable ART is characterized not only by dysregulation and immune activation of CD8 T-cells but also by monocytes. Higher levels of monocytes, specifically non-classical monocytes, may contribute to the increased rates of atherosclerosis in this population and may play a substantial role in the increased risk of cardio-metabolic disease in chronic HIV infection.
Conclusion
The proportion of peripheral non-classical monocytes and MCP-1 predict progression of CIMTBIF in HIV-infected individuals on stable ART independent of traditional cardiometabolic and HIV immuno-virologic factors in our cohort. The role of non-classical monocytes in CVD risk in this vulnerable population requires further study.
Disclaimer statement
Contributors
Dominic C. Chow, MD, PhD, MPH, is a professor of Medicine, John A. Burns School of Medicine, University of Hawaii - Manoa. The author’s research interests include HIV clinical trials and HIV co-morbidities. The author has published 79 journal articles and 5 book chapters.
Jamie M. Kagihara, MD, is a pulmonary critical care fellow at the Los Angeles County + University of Southern California program. His interests include pulmonary hypertension and non-tuberculous mycobacterium in the Asian Pacific Islander population.
Guangxiang Zhang, PhD, is a biostatistician with interests in statistical consulting and biomedical collaborative research. The author has published 20 articles.
Scott A. Souza, PharmD, is an adjunct assistant professor, Department of Medicine, John A. Burns School of Medicine, University of Hawaii. His research interest is in HIV, complications of HIV disease and treatment. He has published 19 articles and 1 chapter.
Howard N. Hodis, MD, is a professor of Medicine, Director, Atherosclerosis Research Unit Harry Bauer & Dorothy Bauer Rawlins Professorship in Cardiology, Keck School of Medicine of University of Southern California. The author is an expert in atherosclerosis, lipid metabolism, and the prevention of cardiovascular disease.
Yanjie Li, MD, RDMS, RDCS, RVT, is the technical director of the Core Imaging and Reading Center at the USC Atherosclerosis Research Unit, Keck School of Medicine of University of Southern California. The author is an expert on cardiovascular disease imaging.
Brooks I. Mitchell, MS, is a PhD student of Tropical Medicine at the University of Hawaii John A. Burns School of Medicine. The author's research interests include innate immunity in chronic HIV infection and HIV co-morbidities. The author has currently published 6 articles.
Beau K. Nakamoto, MD, PhD, MS, is an associate professor of Medicine, University of Hawaii, John A. Burns School of Medicine. The author’s research interest includes HIV-associated neurocognitive disorders. The author has published books/articles: Books: 4 (chapters)/ Articles: 49.
Kalpana J. Kallianpur, PhD, is an assistant researcher in the Hawaii Center for AIDS, John A. Burns School of Medicine. The author's research interests include cognitive dysfunction and brain atrophy in acute and chronic HIV infection; brain resting-state functional connectivity; and normal brain aging. This author has published one book chapter and 32 research articles.
Sheila M. Keating, PhD, MSPH, is the director of the Core Immunology Laboratory at Blood Systems Research Institute. The author’s research interests include HIV, HIV-HCV, HIV co-morbidities, immune responses to parasitic and viral infections, and vaccines. The author has also published books/articles: Books: 1 (chapters)/ Articles: 58.
Philip J. Norris, MD, is a professor of Laboratory Medicine and Medicine at UCSF. The author’s research interests include HIV immunology and cytokine responses in acute viral infections. The author has published 2 book chapters and 110 articles.
Lindsay B. Kohorn, MPH, is a biostatistician/data manager for the Hawaii Center for AIDS. The author's research interests include infectious diseases, clinical trials, and pharmaceuticals. The author has also published three articles.
Lishomwa C. Ndhlovu, MD, PhD, is an associate professor of Tropical Medicine at the University of Hawaii John A. Burns School of Medicine. The author’s research interests include HIV pathogenesis, HIV co-morbidities and HIV reservoirs and the author has also published books/articles: books: 2 (chapters)/ Articles: 58.
Cecilia M. Shikuma, MD, is a professor of Medicine, John A. Burns School of Medicine, University of Hawaii – Manoa. The author’s research interests include HIV clinical trials and HIV co-morbidities. The author has published 158 journal articles.
Funding
National Heart, Lung, and Blood Institute [grant number HL088981]; National Heart, Lung, and Blood Institute [grant number R01HL095135]; National Institute of Neurological Disorders and Stroke [grant number U54NS43049]; National Institute of Neurological Disorders and Stroke [grant numbers N5080656 and R21NS087951]; National Institute on Minority Health and Health Disparities [grant number U19MH081835]; National Institute on Minority Health and Health Disparities [grant number P20RR011091]; National Institute on Minority Health and Health Disparities [grant number U54MD007584]; National Institute on Minority Health and Health Disparities [grant number P20GM103466]; and National Institute on Minority Health and Health Disparities [grant number G12MD007601].
Conflict of interest
None of the authors have any relevant conflicts of interest to disclose.
References
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533.10.1016/S0140-6736(13)61809-7
- Koskinas KC, Chatzizisis YS, Baker AB, et al. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr Opin Cardiol. 2009;24:580–590.
- Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269.10.7326/0003-4819-128-4-199802150-00002
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force. endorsed by the society for vascular medicine. J Am Soc Echocardiogr. 2008;21:93–111; quiz 189-190.
- Chironi G, Escaut L, Gariepy J, et al. Carotid intima-media thickness in heavily pretreated HIV-infected PATIENTS. J Acquir Immune Defic Syndr. 2003;32:490–493.10.1097/00126334-200304150-00004
- Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS. 2007;21:1137–1145.10.1097/QAD.0b013e32811ebf79
- Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. J Am Med Assoc. 1999;282:2035–2042.10.1001/jama.282.21.2035
- Liu Y, Chen BP, Lu M, et al. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol. 2002;22:76–81.10.1161/hq0102.101822
- McNally JS, Davis ME, Giddens DP, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol – Heart Circ Physiol. 2003;285:H2290–H2297.10.1152/ajpheart.00515.2003
- Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393.10.1016/j.jacc.2007.02.059
- Hsue PY, Ordovas K, Lee T, et al. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am J Cardiol. 2012;109:742–747.10.1016/j.amjcard.2011.10.036
- Westhorpe CL, Maisa A, Spelman T, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2014;92:133–138.10.1038/icb.2013.84
- Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: Where are we now? J Am Coll Cardiol. 2013;62:1541–1551.10.1016/j.jacc.2013.07.043
- Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254:102–113.
- Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80.10.1182/blood-2010-02-258558
- Jalbert E, Crawford TQ, D’Antoni ML, et al. IL-1Beta enriched monocytes mount massive IL-6 responses to common inflammatory triggers among chronically HIV-1 infected adults on stable anti-retroviral therapy at risk for cardiovascular disease. PLoS ONE. 2013;8:e75500.10.1371/journal.pone.0075500
- Barbour JD, Jalbert EC, Chow DC, et al. Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis. 2014;232:52–58.10.1016/j.atherosclerosis.2013.10.021
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964.10.1038/nri1733
- Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244–252.10.1189/jlb.0307191
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82.
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592.
- Seidler S, Zimmermann HW, Bartneck M, et al. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30.10.1186/1471-2172-11-30
- Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589.10.4049/jimmunol.178.10.6581
- Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608.10.1182/blood-2012-05-433946
- Han J, Wang B, Han N, et al. CD14highCD16+ rather than CD14lowCD16+ monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr. 2009;52:553–559.10.1097/QAI.0b013e3181c1d4fe
- Min D, Brooks B, Wong J, et al. Alterations in monocyte CD16 in association with diabetes complications. Mediators Inflamm. 2012;2012:649083.
- Corrales JJ, Almeida M, Burgo RM, et al. Decreased production of inflammatory cytokines by circulating monocytes and dendritic cells in type 2 diabetic men with atherosclerotic complications. J Diabetes Complicat. 2007;21:41–49.10.1016/j.jdiacomp.2005.09.006
- Pandzic Jaksic V, Gizdic B, Miletic Z, et al. Association of monocyte CCR2 expression with obesity and insulin resistance in postmenopausal women. Clin Invest Med. 2013;36:E24–31.
- Yang M, Gan H, Shen Q, et al. Proinflammatory CD14+CD16+ monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation. 2012;35:388–396.10.1007/s10753-011-9374-9
- Shikuma CM, Seto T, Liang CY, et al. Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res Hum Retroviruses. 2012;28:793–797.
- Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421.
- Shikuma CM, Chow DC, Gangcuangco LM, et al. Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS ONE. 2014;9:e90330.10.1371/journal.pone.0090330
- Ndhlovu LC, Umaki T, Chew GM, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: Implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20:571–582.10.1007/s13365-014-0279-x
- Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230.10.1097/QAD.0b013e328339192f
- Durand M, Sheehy O, Baril JG, et al. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: A cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–253.10.1097/QAI.0b013e31821d33a5
- Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. J Am Med Assoc Intern Med. 2013;173:614–622.10.1001/jamainternmed.2013.3728
- Womack JA, Chang CC, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035.
- Tawakol A, Lo J, Zanni MV, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66:164–171.
- Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. J Am Med Assoc. 2012;308:379–386.10.1001/jama.2012.6698
- Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1412–1418.
- Coll B, Parra S, Alonso-Villaverde C, et al. HIV-infected patients with lipodystrophy have higher rates of carotid atherosclerosis: The role of monocyte chemoattractant protein-1. Cytokine. 2006;34:51–55.10.1016/j.cyto.2006.03.013
- Floris-Moore M, Fayad ZA, Berman JW, et al. Association of HIV viral load with monocyte chemoattractant protein-1 and atherosclerosis burden measured by magnetic resonance imaging. AIDS. 2009;23:941–949.10.1097/QAD.0b013e328329c76b
- Williams DW, Byrd D, Rubin LH, et al. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflam. 2014;1:e36.
- Ndhlovu LC, D’Antoni ML, Ananworanich J, et al. Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naive HIV-infected Thais. J Neuroimmunol. 2015;288:25–33.10.1016/j.jneuroim.2015.08.020
- Ross AC, Rizk N, O’Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima‐media thickness in HIV‐infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127.10.1086/599190
- Hamirani YS, Pandey S, Rivera JJ, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7.
- Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818.10.1016/j.jacc.2004.07.047
