Abstract
Background: Although HIV therapy is delivered to millions globally, treatment default (especially soon after entering care) remains a challenge. Community health workers (CHWs) can provide many services for people with HIV, including in rural and resource-limited settings.
Objectives: We designed and implemented a 32 site community randomized trial throughout southern Ethiopia to assess an intervention using CHWs to improve retention in HIV care.
Methods: Sixteen district hospital and 16 local health center HIV clinics were randomized 1:1 to be intervention or control sites. From each site, we enrolled adults newly entering HIV care. Participants at intervention sites were assigned a CHW who provided: HIV and health education; counseling and social support; and facilitated communication with HIV clinics. All participants are followed through three years with annual health surveys, plus HIV clinic record abstraction including clinic visit dates. CHWs record operational data about their client contacts.
Results: 1799 HIV patients meeting inclusion criteria were enrolled and randomized: 59% were female, median age = 32 years, median CD4 + count = 263 cells/mm3, and 41% were WHO Stage III or IV. A major enrollment challenge was fewer new HIV patients initiating care at participating sites due to shortage of HIV test kits. At intervention sites, 71 CHWs were hired, trained and assigned to clients. In meeting with clients, CHWs needed to accommodate to various challenges, including HIV stigma, distance, and clients lacking cell phones.
Conclusions: This randomized community HIV trial using CHWs in a resource-limited setting was successfully launched, but required flexibility to adapt to unforeseen challenges.
Community health workers (CHWs) can play a critical role in the delivery of prevention and treatment services, especially in resource-poor and hard to reach settings, including in sub-Saharan Africa.Citation1–4 In many areas, CHWs have a critical function in allowing health systems to deliver health care in a cost-effective manner.Citation5 Task-shifting of certain health care tasks to CHWs has been employed in a variety of health programs, such those for tuberculosis and malaria.Citation6–8
CHWs have provided a wide range of services for people living with HIV (PLWH), including patient counseling, social support, adherence checks, and monitoring for symptoms of illness or adverse HIV drug reactions.Citation9–19 Such assistance may be particularly beneficial in rural areas, where HIV clinics with limited numbers of skilled health personnel are facing a growing volume of patients as a result of decentralization of HIV care to peripheral health facilities, as well as increasing numbers of PLWH due to improved life expectancy from ART and other treatment advances.Citation20–24
Although antiretroviral therapy (ART) has been delivered to over 20 million PLWH globally,Citation24 discontinuity of care remains a major reason why those on ART are not virally suppressed.Citation23 Those who default from care may die without treatment or return only after they have developed severe immune deficiency disease.Citation25,26 PLWH not virally suppressed are at increased risk of transmitting HIV to their sexual partners, increasing HIV transmission in their community.Citation27
The greatest likelihood of default from care is within the first year after treatment initiation. One meta-analysis of African clinics reported that at 6 and 12 months after ART initiation, only 82 and 76%, respectively, were retained in care.Citation28 Data from 2016 indicate that after 12 months, only 72% of PLWH from western and central Africa and 80% from eastern and southern Africa were retained on treatment after initiation.Citation23
Trained CHWs can play a valuable role in supporting PLWH to remain engaged in care.Citation14,15,17–19,23 In 2010, the estimated 12 month retention in ART rate in Ethiopia was 86% with considerable site-specific variation.Citation29 We initiated at that time a pilot study in rural Ethiopia, in which 142 PLWH new to care were assigned to a CHW who was also HIV-positive and from the same geographic area.Citation30 CHWs provided education, social support and facilitated communication with the HIV clinic. After one year, 7 clients had died and 3 transferred to other clinics; of the remaining 132, 131 (99%) were documented as remaining in care, based on having their last documented clinic encounter either at or after the 12-month follow-up date, or within 90 days prior to the 12-month follow-up date.Citation30
Although these results were encouraging, because there was no control group, it was unclear whether these findings resulted from our CHW intervention or other factors, including those at clinic facility level. However, this pilot did provide “proof of concept,” supporting a larger randomized study of CHWs carried out in multiple and more geographically dispersed locations. Our primary hypothesis was that recipients of this intervention (compared to controls) would have higher rates of retention in care that persisted through three years of follow-up. Secondary objectives were to measure differences between groups over time in HIV knowledge, attitudes towards being HIV-positive, feelings of social support, and clinical status. This report describes how we designed and implemented a cluster randomized trial at multiple clinical sites throughout southern Ethiopia, challenges encountered, and how they were addressed.
Methods
Cluster identification, recruitment, and randomization
In Ethiopia where 80% of the population is rural,Citation31 primary care is provided through a network of geographically dispersed health centers typically staffed by clinical officers and nurses; secondary and tertiary care is provided at district and regional referral hospitals typically staffed by physicians. HIV care in Ethiopia, initially rolled out in hospital HIV clinics, is also decentralized to local health centers as part of the national HIV plan.Citation32
This study was conducted in the Southern Nations Nationalities and Peoples Region (SNNPR) of Ethiopia, one of the most rural of the major regions. In SNNPR, all district hospitals and local health centers are in the public sector and under direction of the SNNPR Regional Health Bureau. Before initiating this project we met with and secured approval of the Regional Health Bureau, which encouraged their health facilities to support this study.
Although we initially suggested enrolling from 8 hospitals and 32 health centers, after discussions with local co-investigators, we chose to enroll from 16 district hospitals and 16 local health centers. To select participating sites, we obtained from the SNNPR Health Bureau a list of all district hospitals that provided HIV treatment, and selected the 16 hospitals with the greatest numbers of PLWH enrolled in care; this list was further subdivided into two strata: eight hospitals with the largest numbers of HIV patients (called “larger hospitals”), and the next eight hospitals with the second largest numbers of HIV patients (called “smaller hospitals”) (Figure ). Within each stratum, we then randomized four larger hospitals and four smaller hospitals to the intervention arm, and an equal number to the control arm.
Figure 1 Randomization of 32 District Hospitals and Local Health Centers with HIV Clinics to Intervention (Community Health Worker) or Control Sites, Southern Nations Nationalities and Peoples Region of Ethiopia, October 2015–April 2017.
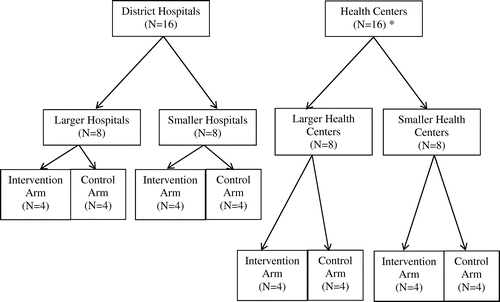
We then listed all local health centers that provided HIV treatment by numbers of PLWH enrolled in care. We first eliminated from this list any health center that was in the same town or in a village near to one of the previously selected 16 hospitals, in order to avoid possible intervention contamination by geographic proximity. From the remaining list, we selected 16 health centers with the greatest numbers of PLWH enrolled in care; this list was further subdivided into eight health centers with the largest numbers of HIV patients (called “larger health centers”), and the eight with the second largest numbers of HIV patients (called “smaller health centers”) (Figure ). Within each stratum, we then again randomized to intervention or control arms. Both intervention and control arms therefore had 16 sites: four larger hospitals, four smaller hospitals, four larger health centers, and four smaller health centers. All hospitals and health centers that were identified as potential study sites signed formal memoranda of agreement, outlining roles and responsibilities. Before beginning the study, we also held a “launch meeting” attended by senior staff from participating facilities, to again explain the study and expectations.
Participant recruitment and enrollment
Because loss to follow-up (LTFU) is greatest in the first six months after ART initiation,Citation28 our inclusion criteria were new enrollment in HIV care at one of the 32 participating hospitals or health centers within the previous 3 months, and age ≥18 years. Those unable to give informed consent were excluded.
Recruitment was done sequentially from among all patients attending each clinic who met inclusion criteria. The initial approach to participation in this study was made by the HIV clinic nurse at the end of a patient’s medical visit. The nurse provided a brief description of the project and asked the patient if they were interested in learning more about the study. Those who indicated potential interest were then referred to a study coordinator responsible for that facility who explained the study in more detail and obtained informed consent. In order to minimize potential enrollment bias, potential participants were told during consent that they might be randomized to either intervention or control arms; they were not to be informed about which arm they would be assigned to until after consent was obtained.
Based upon previous data for the number of new patients initiating HIV care at each site, we set as an enrollment goal 168 PLWH from each of the larger hospitals, 112 from each of the smaller hospitals, and 42 from local health centers. In order to allow for representation and participation from slower enrolling sites, recruitment at a specific site that more rapidly enrolled participants ended when this goal was reached. In total, we projected enrolling 2640 PLWH over 18 months from the 32 participating sites.
CHW intervention
All participants at sites randomized to the intervention arm were assigned to a CHW. This intervention was based on the theoretical framework that PLWH face multiple stressors, including insufficient information and social isolation, which can be “buffered” by support provided by a CHW.Citation33–35 CHWs had three major responsibilities. First, they provided HIV and health education on topics including ART drugs and side effects, importance of drug adherence and attending medical appointments, and nutrition. Second, they provided personal counseling and social support, including on issues such as disclosure of HIV status, feelings of hopelessness, and internalized stigma. Third, CHWs had cell phones and contact information for HIV clinic nurses, in case clients had medical questions or developed new symptoms. CHWs were also aware of local support organizations (such as food supplementation programs) they could refer clients to if needed.
The study protocol was for CHWs to initially meet with clients weekly for the first three months, then twice a month for three months, and then (as clients became more stable) monthly. CHWs had the option, at their discretion, to visit clients more frequently if needed, such as if the client had an acute problem or was in an unstable situation. Meetings could take place in various locations, wherever the client felt most comfortable.
Participants in the control sites received standard of care, as specified in Ethiopian guidelines for HIV treatment.Citation36 These guidelines, which also applied to participants at intervention sites, included any support counseling at the facility level to help promote retention in care. The CHW intervention in this study therefore represented community-based supplementation, rather than replacement, of existing facility-based counseling and support services.
Data collection
All participants are to be followed for 36 months, or until such time as they die, transfer to another HIV clinic, or are classified as LTFU. At enrollment and every 12 months thereafter, the following data are abstracted by data clerks from the participants’ HIV clinic record: CD4 + count, body mass index (BMI), World Health Organization (WHO) HIV clinical stage, and (as applicable) ART start date. Every twelve months, we also record dates of all HIV clinic visits in the previous year. If the patient has died or transferred to another clinic, this information (with dates) is recorded. At certain sites (primarily at larger hospitals) a separate HIV pharmacy exists. For these sites during the annual follow-up data abstraction, the dates of all ART refills in the last 12 months are to be recorded by the pharmacist.
At enrollment and every 12 months thereafter, participants complete a health survey. The survey was prepared as a scan-able bubble survey form using Amharic font (to allow for scanned in data entry), and is verbally administered by a study coordinator. In developing the survey, items were translated into Amharic, back-translated, and pilot tested. Items include: (a) demographics; (b) distance from home to clinic; (c) knowledge about HIV and HIV treatment (e.g. benefits of ART, importance of adherence); (d) physical symptoms (e.g. chronic fever or pain); (e) feelings of social support and companionship; (f) internalized HIV stigma (e.g. feelings of self-blame or shame); (g) mental health symptoms (e.g. depression, hopelessness); and (h) disclosure of HIV status to others. Health survey items for social support, mental health, and internalized stigma were adapted and drawn from different validated measurement tools.Citation37–39 During follow-up surveys, participants are also asked to self-rate their ART adherence during the previous three days. All follow-up clients receive 100 birr (~$5 US) as transportation reimbursement.
In the study protocol, the primary outcome for this study, based upon review of HIV clinic dates, is LTFU, defined as >90 days from last mised appointment (a missed clinical or drug pickup appointment without any follow-up contacts).Citation40 Major secondary outcomes were changes in: knowledge about HIV care and treatment, feelings of social support and companionship, attitudes of internalized HIV stigma, and symptoms of depression. Clinical health status was assessed by responses on the survey (such as presence of chronic symptoms), and data recorded in the HIV clinic record, such as CD4 + count and BMI. All primary and secondary outcomes will be compared through 36 months between participants in the intervention and the control arms.
Ethics
All participants provided informed consent. The consent form was translated into Amharic and verbally explained to all participants, including those who could not read. After explaining the consent form, enrollees were asked a series of questions to assess comprehension of key provisions, such as the ability to refuse participation or withdraw at a later date.
Participants were asked to provide their cell phone number and (if they were willing) the cell phone for a close contact. This information was used to contact participants for the annual follow-up surveys. All data collection forms included only the subject identification number; no personal identifiers were recorded. Approval was obtained from the University of Minnesota’s Institutional Review Board and the Ethiopian Ministry of Science and Technology National Research Ethics Committee. The study was registered with ClinicalTrials.gov, NCT02448394.
A Data Safety and Monitoring Board committee was established for this study, and will received annual interim and final reports by intervention arm for participant characteristics and the primary endpoint. The DSMB will communicate recommendations and any concerns regarding the progress of the study, patient safety, or intervention effectiveness to the study investigators. Full results for all primary and secondary outcomes by treatment arm will be made available to all investigators at the conclusion of study.
Results
Recruitment and enrollment
Although we projected recruiting 2640 PLWH from participating sites, after our study started in October 2015, we found that the monthly rate of enrolling new participants from many sites was less than projected. Follow-up investigations at these sites revealed that compared with previous years, fewer PLWH were newly entering care, leading to a smaller pool of patients from which to recruit for this study. The reason for this decline was because less HIV testing was being done at both the community and facility level, resulting in fewer new HIV diagnoses, and therefore fewer persons newly initiating HIV care. We learned that shortly after the study began, the Ethiopian government changed the brand of HIV test kits being used. The old test kits were used up, but because of delays in distributing new test kits to clinics, voluntary counseling and testing services were discontinued at many locations, and provider initiated counseling and testing services were significantly curtailed.
Based upon enrollment rates at participating sites after the study started, we recalculated that we would be able to enroll at least 1700 participants during our recruitment period. Using an estimated LTFU rate at 36 months of 23%, an estimated intraclass correlation (ICC) of 0.01, and a correction for the variation in cluster size (coefficient of variation = 0.84), revised calculations confirmed that with a sample size of 1700 from 32 sites, we had sufficient power (0.80) to detect a difference of 7.6% between intervention and control arms as significant (p = 0.05). If the ICC were 0.05, with the same other assumptions and numbers of participants, we would be able to detect a difference of 12% between the two arms as significant. These revised calculations confirmed that we still had enough statistical power to meet study objectives in detecting meaningful differences in LTFU rates between intervention and control groups.
By the end of the recruitment period in April 2017, 1799 PLWH enrolled in the study, including 819 from intervention sites and 980 from control sites. According to records kept by HIV clinic nurses, information about the study was given to 1952 HIV patients who met inclusion criteria, of whom 1901 (97%) expressed interest in learning more about the study. Contact information was obtained and follow-up meetings with study staff were scheduled for 1815 persons; the reminder included those who could not be reached because they lacked cell phones or other contact information to schedule a meeting, with many of these persons living a great distance from the clinic. Of the 1815 persons to whom the study was further explained, 1799 (99%) agreed to enroll. Therefore, of 1952 HIV patients who potentially met inclusion criteria, 92% ultimately enrolled. Participation rates among potentially eligible HIV patients were 93% from intervention sites and 92% from control sites.
Of those who enrolled in this study, 733(41%) were male and 1066 (59%) female; the median age was 32 years (interquartile range [IQR] = 26,40). In terms of education, 64% either had no schooling (26%) or had attended less than eight years of primary school (37%). Many participants already had evidence of HIV disease progression. The median CD4 + count at enrollment was 263 cells/mm3 (IQR = 123,450); 39% had < 200 cells/mm3. Forty-one percent were WHO Stage III (35%) or IV (6%). Sixty-seven percent reported weight loss for more than one month; the median BMI was 19.4 kg/m2 (IQR = 17.5, 21.7).
CHW intervention
In total, 71 CHWs were hired for this project; 49 were assigned to clients from one of the intervention hospitals, and 22 to clients from a health center. All CHWs were HIV-positive themselves, and from the same geographic area as their assigned health facility. To select CHWs, we collaborated with local PLWH associations to identify potential candidates, who were then interviewed and provisionally accepted. Final approval was made after observing applicant performance during the initial training. Selection criteria included demonstrated understanding of training content, maturity, good communication skills, and expressed commitment toward supporting PLWH. Training included educational presentations about HIV, ART, and health-promoting behaviors. CHWs also participated in group exercises on counseling clients in supportive and empathic ways, based on principles of motivational interviewing.Citation41
CHWs were supplied with a cell phone to contact clients and HIV clinics, monthly phone cards, and a monthly allowance of 700 birr (~$35 US) for transportation and other expenses. CHWs from each specific intervention site met every month as a group with their supervising study coordinator assigned to that site, to discuss problems encountered in working with clients, potential strategies to meet these challenges, and lessons learned. After each client contact, CHWs filled out a client contact form that included date and location of meeting, time spent with client, and topics discussed; these forms were also used to help monitor CHW performance and supervise them.
CHWs faced certain challenges in setting up meetings with their assigned clients, especially with arranging the first visit. Because CHWs were recognized within the community as working with PLWH, some participants were initially reluctant to meet with CHWs in their home locations. CHWs adopted various strategies to minimize such concerns, including meeting at neutral locations such as coffee shops or on the grounds of the hospital or clinic. Although our protocol called for CHWs initially visiting with clients on a weekly basis, in some situations clients expressed a preference for meeting their CHW at the clinic during monthly medical visits. Often after clients became more trusting and comfortable with CHWs, ongoing meetings were easier to establish. Other challenges faced by CHWs were distance (with some clients coming from far away locations), clients who lacked cell phones (making regular contact and scheduling meetings more challenging), and mobile clients (such as seasonal migrant workers) who were away from their homes for several months at a time. In all cases, CHWs tried to work with clients to meet as often and regularly as possible, at whatever location was feasible and most comfortable for the client. This information is documented in client contact forms.
Discussion
This article describes a cluster randomized trial of HIV CHWs with 1799 participants enrolled from 32 hospitals and health centers throughout southern Ethiopia, with those at intervention sites being assigned to one of 71 CHWs. Cluster randomization minimizes potential intervention contamination that could occur if subjects were randomized to intervention or control arms within a given facility. A community randomized trial design was also selected because if this intervention were successful, it would be implemented at a facility level.
In group randomized trials, two factors that have a major role in determining statistical power are the ICC and the number of groups per condition.Citation42 Although we ended up enrolling a smaller number of participants than originally projected, with the number of sites remaining at 32, we still had sufficient power to meet our statistical objectives. In addition, when we designed our protocol, we conservatively proposed enrolling a larger number of participants than the minimum number needed, in order to account for unforeseen circumstances; this proved a very useful decision.
Our intervention was grounded in the theory that CHWs can help “buffer” stressors faced by PLWH by providing informational support (facts or advice to help patients understand their situation and solve problems), emotional support (empathy and acceptance to let PLWH know they are cared about and valued), instrumental support (provision of tangible aid, such as facilitating communication with the HIV clinic), and social companionship (reducing feelings of isolation and fulfilling needs for social contact).Citation33–35 As demonstrated in our pilot, the fact that CHWs were also HIV-positive and members of the same communities as their clients was especially valuable in facilitating client relationships and acceptance.Citation30 Social support as coping assistance may be strengthened when it comes from those who have effectively managed similar stressors and life circumstances.Citation35
As others have noted, proper recruitment and selection, initial and continued training, regular supportive supervision, and adequate incentives/financial support are all important components of a CHW program,Citation1,2,11,43 and failure to attend to each of these can result in poor CHW performance or increased attrition from a support program. We emphasized that CHWs should be treated as respected members of the team, with appreciation for their contributions. CHWs were allowed a degree of flexibility and adaptability in dealing with specific client circumstances and challenges, such as in arranging meetings with clients who did not want visits in their home communities. The client contact forms filled out by CHWs after each visit will allow us to describe operational and implementation characteristics of this intervention, and to analyze relationships between intensity of the intervention (e.g. frequency and duration of visits) and client outcomes.
HIV-related stigma, with fear by PLWH of negative judgments and discriminatory practices by others, was a significant issue faced in this study. Our previous research in one town in which this study was implemented confirmed the presence of such attitudes in community members.Citation44 Although participation in this study was over 90%, during recruitment for this trial some HIV clinic patients indicated that they did not want to join because they were afraid (despite our assurances of confidentiality) that others outside the clinic might learn they had HIV. Some potential participants feared that if they were randomized to the intervention arm and were seen with a CHW, others in the community would learn their status. As noted above, some clients who joined the study did not want to meet with CHWs in their home communities for similar reasons. These examples reinforce that HIV stigma and fear of discrimination can be significant barriers to successful HIV prevention and care programs,Citation23,45,46 and emphasize the need for stigma reduction efforts. It is also important to counsel PLWH about their own feelings of internalized stigma (blame or shame) because of their HIV status.Citation39
In summary, this trial conducted in rural Ethiopia among PLWH newly enrolled in care, was designed to assess the impact on retention in care of an intervention using CHWs to provide educational counseling, social support, and facilitated linkage to HIV clinics. Although we had established protocols and training of project staff, we also needed to be flexible to adapt to unforeseen challenges. Our ability to meet these challenges was due to close communication and coordination with local co-investigators who were intimately involved in all phases of designing and implementing this project, and who best understood the clinical and community settings and local circumstances in which this research is being conducted.
Clinical trials registry
ClinicalTrials.gov number: NCT02448394.
Conflict of interest
No potential conflict of interest was reported by the authors.
Funding
Funding for this project was provided by the National Institute of Mental Health, National Institutes of Health [grant number 5R01MH105290].
Geolocation
This study was conducted in the Southern Nations Nationalities and Peoples Region (SNNPR) of Ethiopia.
Notes on contributors
Alan Lifson is a Professor, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota.
Sale Workneh is a Community Care and Support Advisor, Ethiopian Office, National Alliance of State and Territorial AIDS Directors.
Abera Hailemichael is Technical Officer for Research, Ethiopian Office, National Alliance of State and Territorial AIDS Directors.
Richard MacLehose is an Associate Professor, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota.
Keith Horvath is an Associate Professor, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota.
Rose Hilk is a Systems Database Administrator, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota.
Lindsey Fabian is a Research Project Specialist, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota.
Anne Sites is Senior Manager, Global Program, National Alliance of State and Territorial AIDS Directors.
Tibebe Shenie is Country Director, Ethiopian Office, National Alliance of State and Territorial AIDS Directors.
Acknowledgements
We wish to acknowledge the Ethiopian study coordinators who are working on this study: Anteneh Mengistu, Behailu Dagne, Engidaw Ayele, Hiwot Tekle, Simret Girma, Signe Tefera, Tesfaye Gemechu, Tsedey Ayele, Tewabe Tamiru, and Yayush Tesfaye. We also wish to thank: Lucy Slater; Madelyn Tillemans; the Southern Nations Nationalities and Peoples Regional Health Bureau; the National Alliance for State and Territorial AIDS Directors Global Program; clinical staff, data clerks, pharmacists and hospital managers at all participating hospital and health center HIV clinics; and all of the PLWH who are participating in this study.
References
- Lehmann U, Sanders D. Community health workers: what do we know about them? The state of the evidence on programmes, activities, costs and impact on health outcomes of using community health workers. WHO; 2007. www.who.int/hrh/documents/community_health_workers.pdf. Accessed July 24, 2017.
- Global Health Workforce Alliance, World Health Organization. Global experience of community health workers for delivery of health related Millennium Development Goals: a systematic review, country case studies, and recommendations for integration into national health systems. WHO; 2010. www.who.int/workforcealliance/knowledge/resources/chwreport/en. Accessed July 24, 2017.
- Singh P, Sachs JD. 1 million community health workers in sub-Saharan Africa by 2015. Lancet. 2013;382(9889):363–365.10.1016/S0140-6736(12)62002-9
- Dahn B, Woldemariam AT, Perry H, et al. Strengthening primary health care through community health workers: investment case and financing recommendations. WHO; 2015. www.who.int/hrh/news/2015/CHW-Financing-FINAL-July-15-2015.pdf. Accessed July 24, 2017.
- McCord GC, Liu A, Singh P. Deployment of community health workers across rural sub-Saharan Africa: financial considerations and operational assumptions. Bull World Health Organ. 2012;91:244–253B.10.2471/BLT.12.109660
- Ong’ang’o JR, Mwachari C, Kipruto H, Karanja S. The effects on tuberculosis treatment adherence from utilising community health workers: a comparison of selected rural and urban settings in Kenya. PLoS ONE. 2014;9(2):e88937.10.1371/journal.pone.0088937
- Uwimana J, Zarowsky C, Hausler H, Swanevelder S, Tabana H, Jackson D. Community-based intervention to enhance provision of integrated TB-HIV and PMTCT services in South Africa. Int J Tuberc Lung Dis. 2013;17(10):S48–S55.
- Siribié M, Ajayi IO, Nsungwa-Sabiiti J, et al. Training community health workers to manage uncomplicated and severe malaria: experience from 3 rural malaria-endemic areas in sub-Saharan Africa. Clin Infect Dis. 2016;63(suppl 5):S264–S269.
- Wools-Kaloustian KK, Sidle JE, Selke HM, et al. A model for extending antiretroviral care beyond the rural health centre. J Int AIDS Soc. 2009;12:22.10.1186/1758-2652-12-22
- Selke HM, Kimaiyo S, Sidle JE, et al. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic Syndr. 2010;55(4):483–490.10.1097/QAI.0b013e3181eb5edb
- Celletti F, Wright A, Palen J, et al. Can the deployment of community health workers for the delivery of HIV services represent an effective and sustainable response to health workforce shortages?Results of a multicountry study AIDS. 2010;24(Suppl 1):S45–57.
- Kabore I, Bloem J, Etheredge G, et al. The effect of community-based support services on clinical efficacy and health-related quality of life in HIV/AIDS patients in resource-limited settings in sub-Saharan Africa. AIDS Patient Care STDs. 2010;24(9):581–594.10.1089/apc.2009.0307
- Chang LW, Kagaayi J, Nakigozi G, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS One. 2010;5(6):e10923.10.1371/journal.pone.0010923
- Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012;61(4):e50–e58.10.1097/QAI.0b013e31826a6aee
- Wouters E, Van Damme W, van Rensburg D, Masquillier C, Meulemans H. Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Serv Res. 2012;12:194.
- Kipp W, Konde-Lule J, Saunders LD, et al. Antiretroviral treatment for HIV in rural Uganda: two-year treatment outcomes of a prospective health centre/community-based and hospital-based cohort. PLoS One. 2012;7(7):e40902.10.1371/journal.pone.0040902
- Franke MF, Kaigamba F, Socci AR, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clin Infect Dis. 2013;56(9):1319–1326.10.1093/cid/cis1193
- Mwai GW, Mburu G, Torpey K, Frost P, Ford N, Seeley J. Role and outcomes of community health workers in HIV care in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18586.10.7448/IAS.16.1.18586
- Lubega M, Tumwesigye NM, Kadobera D, et al. Effect of community support agents on retention of people living with HIV in pre-antiretroviral care: a randomized controlled trial in eastern Uganda. J Acquir Immune Defic Syndr. 2015;70(2):e36–e43.10.1097/QAI.0000000000000723
- Nkhata MJ, Muzambi M, Ford D, et al. Shifting human resources for health in the context of ART provision: qualitative and quantitative findings from the Lablite baseline study. BMC Health Serv Res. 2016;16(1):660.
- Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–965.10.1126/science.1230413
- Reidy WJ, Sheriff M, Wang C, et al. Decentralization of HIV care and treatment services in Central Province, Kenya. J Acquir Immune Defic Syndr. 2014;67(1):e34–e40.
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Ending AIDS: Progress Towards the 90-90-90 Targets. UNAIDS 2017. www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. Accessed October 28, 2017.
- UNAIDS. Fact Sheet–World AIDS Day, 2017. Global HIV Statistics. UNAIDS; 2017. www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed March 20, 2018.
- Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790.10.1371/journal.pone.0005790
- Ndiaye B, Ould-Kaci K, Salleron J, et al. Characteristics of and outcomes in HIV-infected patients who return to care after loss to follow-up. AIDS. 2009;23(13):1786–1789.10.1097/QAD.0b013e32832e3469
- Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–1404.10.1097/QAD.0b013e32832b7dca
- Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69(1):98–108.10.1097/QAI.0000000000000553
- Assefa Y, Gilks CF, Lynen L, et al. Performance of the antiretroviral treatment program in Ethiopia, 2005-2015: strengths and weaknesses toward ending AIDS. Int J Infect Dis. 2017;60:70–76.10.1016/j.ijid.2017.05.012
- Lifson AR, Workneh S, Hailemichael A, Demisse W, Slater L, Shenie T. Implementation of a Peer HIV community support worker program in rural Ethiopia to promote retention in care. J Int Assoc Provid AIDS Care. 2017;16(1):75–80.10.1177/2325957415614648
- Federal Democratic Republic of Ethiopia, Central Statistical Agency. Population projection of Ethiopia for all regions at Wereda level from 2014–2017. Central Statistical Agency; 2013. www.csa.gov.et/ehioinfo-internal?download=724:population-projection-of-ethiopia-for-all-regions-at-wereda-level-from-2014-2017. Accessed March 21, 2018.
- Federal HIV/AIDS Prevention and Control Office. Strategic Plan II for Intensifying Multisectoral HIV and AIDS Response in Ethiopia, 2010/11–2014/15. Federal Ministry of Health; 2010. www.nationalplanningcycles.org/sites/default/files/country_docs/Ethiopia/aids_hiv_strategic_plan_2010-2015.pdf. Accessed March 21, 2018.
- Cohen S, Wills TA. Stress, social support and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357.10.1037/0033-2909.98.2.310
- Langford CP, Bowsher J, Maloney JP, Lillis PP. Social support: a conceptual analysis. J Adv Nurs. 1997;25(1):95–100.10.1046/j.1365-2648.1997.1997025095.x
- Thoits PA. Social support as coping assistance. J Consult Clin Psychol. 1986;54(4):416–423.10.1037/0022-006X.54.4.416
- Federal Democratic Republic of Ethiopia, Ministry of Health. National Guidelines for Comprehensive HIV Prevention, Care and Treatment. Ministry of Health; 2014 https://aidsfree.usaid.gov/sites/default/files/ethiopia_natl_gl_2014.pdf. Accessed October 31, 2017.
- Sherbourne CD, Stewart AL. The MOS Social Support Survey. Social Sci Med. 1991;32(6):705–714.
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.10.1177/014662167700100306
- Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-related Stigma Scale. AIDS Care. 2009;21(1):87–93.
- World Health Organization. Operations manual for delivery of HIV prevention, care and treatment at primary health centres in high-prevalence, resource-constrained settings: Edition 1 for field-testing. World Health Organization; 2008. www.who.int/hiv/pub/imai/operations_manual/en. Accessed March 25, 2018.
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York,NY: Guilford Press; 2013.
- Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: a review of recent methodological developments. Am J Public Health. 2004;94(3):423–432.10.2105/AJPH.94.3.423
- Hermann K, Van Damme W, Pariyo GW, et al. Community health workers for ART in sub-Saharan Africa: learning from experience–capitalizing on new opportunities. Human Resour Health. 2009;7:31.
- Lifson AR, Demissie W, Tadesse A, et al. HIV/AIDS stigma-associated attitudes in a rural Ethiopian community: characteristics, correlation with HIV knowledge and other factors, and implications for community intervention. BMC Int Health Hum Rights. 2012;12:6.
- Hargreaves JR, Stangl A, Bond V, et al. HIV-related stigma and universal testing and treatment for HIV prevention and care: design of an implementation science evaluation nested in the HPTN 071 (PopART) cluster-randomized trial in Zambia and South Africa. Health Policy Plan. 2016;31(10):1342–1354.10.1093/heapol/czw071
- Treves-Kagan S, Steward WT, Ntswane L, et al. Why increasing availability of ART is not enough: a rapid, community-based study on how HIV-related stigma impacts engagement to care in rural South Africa. BMC Public Health. 2016;16:87.
