Abstract
Dimethoate is an organophosphate (OP) pesticide used to control a wide variety of insects on agricultural crops and ornamentals. To ensure that dimethoate is used safely, it is important to determine exposure levels that protect against adverse effects at all life stages, including the developing fetus, infant, and child. Based on an analysis of a developmental neurotoxicity (DNT) study, a cholinesterase (ChE) sensitivity study, a cross-fostering study, and several single- and multigenerational reproductive toxicity studies, two potential critical endpoints for dimethoate were identified: brain ChE inhibition (ChEI) in adult females, and pup mortality. An initial evaluation concluded that pup mortality was a preferable endpoint, based on an increased number of pup deaths born to dams dosed with ≥3 mg/kg dimethoate via oral gavage. Closer examination, however, revealed that the pup deaths were clustered in a small number of litters in which the dams providing postnatal care exhibited maternal care deficits. When the data were analyzed using the dam as the unit of statistical significance, a significant increase in the average litter proportion of pup deaths was observed only when the dams were dosed postnatally with 6 mg/kg dimethoate while they were raising the pups. Gestational exposure (i.e., during pregnancy only) to 6 mg/kg dimethoate exerted no effect on pup survival. This leads to the conclusion that it is postnatal exposure of the nursing dams that is associated with pup mortality. Furthermore, a previous benchmark dose (BMD) meta-analysis approach revealed that BMDL10 for adult females (the lower 95% bound of the dose resulting in a 10% reduction in the parameter of interest) for ChEI was > 3-fold lower than the BMDL10 for pup mortality (0.19 and 0.68 mg/kg, respectively). Overall, this study underscores the importance of using the dam as the unit of statistical significance when assessing data collected in the perinatal period, and it is concluded that adult brain ChEI is the correct critical endpoint for assessing risk of dimethoate toxicity.
Prediction of whether exposure to a given agent can produce detrimental effects, and estimation of the intensity thereof, is accomplished through a set of procedures that comprise risk assessment. In CitationHolson et al. (2000), some of the authors of the present article described in detail the general procedures and special considerations relating to the use of appropriate animal models and routes of administration for the assessment of risks during prenatal development. Development, of course, is not limited to the prenatal period; in various mammalian species, development occurs with differing chronologies such that some organ systems acquire significant maturation prenatally in certain species while the same systems mature mainly in the postnatal period in others (CitationMorford et al., 2004). The understanding that development is a continuum that temporally spans birth has led to the realization that assessment of children's health risks requires life-stage specific determination of susceptibility as it relates to activities, ingestion patterns, and behaviors (CitationDaston et al., 2004), in addition to selection of an appropriate animal model that mimics to the extent possible the human condition of the organ system under study at the time of exposure (CitationMorford et al., 2004).
The purpose of the present study was to assess the safety in offspring of exposure to the organophosphate (OP) insecticide dimethoate. New data from a series of animal experiments that examined various developmental, neurodevelopmental, and reproductive effects are presented, focusing primarily on the critical effects of pup mortality and cholinesterase (ChE) inhibition, which were the strongest and most sensitive effects measured in the developmental neurotoxicity study. In addition, this study evaluates the influence of maternal toxicity on offspring toxicity through review of a cross-fostering study and more careful evaluation of clinical observations of dams and offspring during the perinatal period. The focus of this evaluation is for the purpose of risk assessment rather than full characterization of the toxicology of the chemical.
SELECTING A CRITICAL ENDPOINT FOR USE IN RISK ASSESSMENT
The critical endpoint for use in risk assessment is that adverse effect that is produced reproducibly at the lowest dose or exposure level. For dimethoate, two potential critical endpoints in animal studies have been identified: brain cholinesterase inhibition (ChEI) in adults and offspring, and pup mortality. The dose at which the critical endpoint is produced is considered a point of departure (POD) for risk calculations. It is important to identify an appropriate POD that will ensure protection against all other potential relevant adverse effects. For many OP pesticides, maternal brain ChEI was shown to be a more sensitive endpoint than effects on pups (CitationSheets, 2000). In such cases, it is reasonable to consider the use of adult brain ChEI as a POD for risk assessment for both adults and children. This would permit use of dermal and inhalation adult toxicity studies in assessing risks to human dermal and inhalation exposures. However, brain ChEI in adult studies can be used as a POD for children's exposure only if it is sufficiently protective of all relevant toxic endpoints for these different sub-populations. Furthermore, if developmental effects in offspring appear to be closely linked to maternal toxicity, then there is greater confidence that risk assessments based on sensitive endpoints in adults will be protective of developmental effects. In the case of dimethoate, this requires a close examination of the pup mortality data. Addressing the question of whether or not pup mortality is the suitable critical endpoint for dimethoate must start with determination of the appropriate unit for analysis.
THE LITTER IS THE MOST APPROPRIATE UNIT OF ANALYSIS FOR EARLY PREWEANING PUP MORTALITY DATA
It is important to recall the crucial influence of the pregnant and lactating dam on offspring well-being. During gestation, the maternal mammal is clearly the dominant influence on the health of her fetus(es). She is the sole source of nutrients, electrolytes, and oxygen. Further, she controls homeostatic mechanisms regulating fluids and temperature, and provides means for the elimination of metabolic wastes. If a pregnant animal is exposed to an environmental insult at such a level that it results in overt maternal toxicity (e.g., reductions in food intake, reductions in body weight, increases in body core temperature, the induction of an acute-phase response), changes in maternal metabolism secondary to maternal toxicity can contribute to fetal or pup toxicity (CitationDeSesso, 1987), even when the insult itself exerts minimal, if any, direct effects on the fetus or pup (CitationKeen et al., 2003a, Citation2003b). The use of the litter as the appropriate unit of analysis has been reflected in U.S. Environmental Protection Agency (EPA) guidance from the Office of Research and Development (ORD) as well as in a key report by the International Life Sciences Institute (CitationILSI, 1999).
Mammals in general are characterized by an extended period of maternal care, and accordingly, in the rat, the close relationship between mother and offspring is not immediately curtailed following parturition. In the rat, the dam remains the sole source of nutrition via lactation until approximately the beginning of wk 3 of lactation (CitationTyl et al., 2008), and she is vital for physical protection and thermal homeostasis early in a pup's life. A qualitative characterization of the estimated extent of pre- and postnatal maternal dependence based on the authors' experience in the rat is presented in .
TABLE 1 Estimated Contribution of Maternal Influence to Offspring Well-Being
Thus, when considering the developmental toxicity associated with a maternally administered substance in studies using the rat as the experimental model, this close relationship between the dam and the pup during gestation and lactation should be recognized such that the litter, and not the fetus or the young pup, should be considered as the relevant unit to evaluate potentially adverse effects.
While it is acknowledged that the pup is less dependent upon the dam following parturition in terms of certain processes, such as respiration and waste elimination, the pup remains sufficiently dependent upon the dam so that it cannot be considered an independent statistical unit, especially during the first 2 wk of postnatal life. Although these dependencies naturally decrease with age, aspects of them remain detectable even after weaning. Furthermore, in maternal gavage studies the dam is the only potential source of exposure for the pup.
If one were to consider individual pups as the unit for assessment without consideration of the litter as the primary unit of assessment, misleading conclusions could be drawn, as will be discussed. In fact, experimental data have been utilized to show that the use of the pup as the unit of analysis, when the effect is actually on the litter as a whole, can seriously exaggerate the significance level (CitationHaseman & Hogan, 1975). Detailed review of this issue is discussed further by CitationReiss and Gaylor (2005).
CASE STUDY: DIMETHOATE
Dimethoate is an organophosphate (OP) pesticide that is used to control a wide variety of insect pests on a range of agricultural crops and ornamentals. Thus, the oral route (food and water) is the primary route of exposure for the general population, while dermal exposure is the primary pathway of concern for occupationally exposed workers, including mixers, loaders, applicators, and re-entry workers.
Relevant Information Concerning Human Exposures
A search of the open literature regarding exposure of humans to dimethoate, especially during pregnancy and lactation, failed to identify any epidemiology studies. A few reports of poisonings in agricultural situations or as self-inflicted exposures were identified, but most of these reported the effects of acute poisoning and none investigated the impact on pregnant or nursing women and their offspring (CitationEddleston et al., 2005; CitationDavanzo et al., 2004; CitationBu et al., 2001; CitationTsatsakis et al., 1996). In the absence of relevant data regarding human exposures, the risk assessment must be based on well-conducted safety tests performed on lab animals.
Results from Animal Studies
Numerous animal toxicity studies were conducted on dimethoate using different routes of exposure for various durations. Ideally, risk assessments for humans should be based on toxicity studies that were conducted using a relevant route of exposure in order to minimize uncertainties in extrapolating from one route of exposure to another. It is also important to select the most sensitive POD that will protect against the different adverse effects for all life stages including the developing fetus, infants, and children.
Developmental Neurotoxicity (DNT) Study
Developmental neurotoxicity (DNT) studies are designed to assess the potential impact on neurological function among progeny that experienced pre- and postnatal exposure to test agents. In a DNT study of dimethoate (CitationMyers, 2003), groups of 24 parent female Crl:CD BR rats received dimethoate (99.1% a.i.) by gavage at dose levels of 0, 0.1, 0.5, or 3 mg/kg body weight (bw)/d from gestation day 6 through postnatal day 10; offspring were exposed directly by gavage to the same doses from postnatal day 11 to postnatal day 21, inclusive.
Maternal animals were evaluated twice a day for mortality/moribundity and daily cage-side observations. Gross observations of the dams were conducted daily as follows: prior to treatment, as each animal was returned to the cage, at the end of dosing for each group, between 1 and 2 h after completion of dosing, and as late as possible during the work day. A functional operational battery (FOB) was performed on 10 dams/dose prior to dosing on gestation days 12 and 18 and lactation days 4 and 10 by observers unaware of treatment level.
The day of completion of parturition was designated as postnatal (or lactation) day 0. Live pups were counted, sexed, and weighed individually for each litter on postnatal days 1, 4, 7, 11, 14, 17, and 21. Daily throughout lactation, offspring were examined cage-side for gross signs of mortality or morbidity. Any gross signs of toxicity in the offspring were recorded as they were observed, including the time of onset, degree, and duration.
Offspring were evaluated as follows: age-appropriate functional observation battery on d 4, 11, 21, 35, 45, and 60, automated motor activity on d 13, 17, 22, and 59, assessment of auditory startle response on d 23/24 and 60/61, assessment of learning and memory (Morris water maze) at postnatal days 23/24 and at postnatal days 61/62 (separate groups), brain weights on d 11, 21, and 65, and brain histopathology and morphometrics on d 21 and 65. Pup physical development was assessed by body weight, and sexual maturation of females was assessed by age at vaginal opening. Maturation of males was assessed by age at completion of balanopreputial separation.
Occurrence of Pup Deaths in the DNT Study
There was no group difference in number of litters born (23–24), number of pups per litter at birth, postimplantation survival index, or sex ratio. There were no group differences in pup body weight, food consumption, auditory startle parameters, learning and memory evaluations, brain weights and morphometric measurements, or histopathological evaluations at weaning or at postnatal day (PND) 60. There were no significant effects on motor activity or rearing at PND 13, 22, or 59. At PND 17, there were statistically nonsignificant increases in motor activity in males at all dose levels that appear to be due to low control values. In fact, the treated male pups exhibited the commonly measured ontogenic pattern of increase in activity and then decrease or plateau in motor activity levels from d 13 to d 22. In contrast, the control males decreased in activity level at PND 17 compared to PND 13; therefore, the apparent increases in activity are not biologically significant. In female pups, there was a quantitative decrease in activity at 3 mg/kg/d at PND 17 but not at PND 13, 22, or 59. This change in motor activity is equivocal given the high variability that is often associated with activity measurements on PND 13 and 17 in DNT guideline studies.
There was a decrease in pup survival in the 0.5- and 3-mg/kg/d dose groups () that was most pronounced between PND 1 and PND 10, when the pups are most dependent on maternal care. No excess deaths were noted during the direct dosing period (PND 11–21). Pup mortality is the strongest most sensitive finding in the DNT study and is the primary focus of this study.
TABLE 2 Summary of Pup Mortality Data from Dimethoate Developmental Neurotoxicity Study
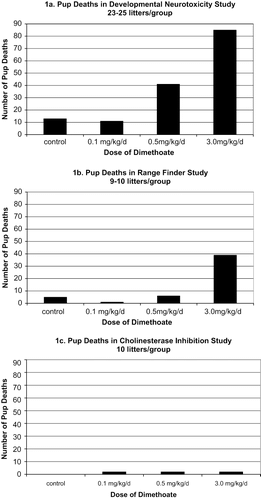
Although it is possible that the pups' dependence on the dam during the first 12 d of the lactation period requires the use of the litter as the statistical unit, analysis of the distribution of the absolute number of pup deaths was performed in part to further examine whether dimethoate influences pup death in a per pup or per litter manner. In the dose range finder study, and particularly in the DNT study, if one looks only at the total number of pup deaths, there seems to be a dose-response relationship between maternal administration of dimethoate and the total number of pup deaths up to PND 10 ().
FIG. 1. Number of pup deaths during PND 1–11 in the (a) developmental neurotoxicity (DNT), (b) range finder, and (c) relative ChE sensitivity studies in which dams were dosed with dimethoate by oral gavage from GD 6 to PND 10. Note that the number of litters in the DNT study is more than twice the number in the range finder and relative ChE sensitivity studies.
In the dimethoate-treated groups, pup deaths are clustered in a small number of litters while most other litters have few to no deaths (). In the control and low-dose groups, the total number of deaths in the groups was 13 and 11, respectively, and the incidence of pup deaths per litter ranged from 0 to 3. In the mid- and high-dose dimethoate groups, substantially more total pup deaths occurred (41 and 84, respectively), but as the graphs illustrate, a majority of the deaths are found in a few litters. In the mid-dose group, 32 of the total 41 (78%) deaths are in 3 litters; in the high-dose group, 68 of the total 88 (77%) deaths occurred in 5 litters. This demonstrates that the effect of treatment is not uniform and suggests that a few dams or litters react differently from other similarly treated dams or litters.
FIG. 2. Distribution of pup deaths in the developmental neurotoxicity study indicating dams with maternal care issues. The shaded area depicts the greatest number of pup deaths in a control litter (three pups).
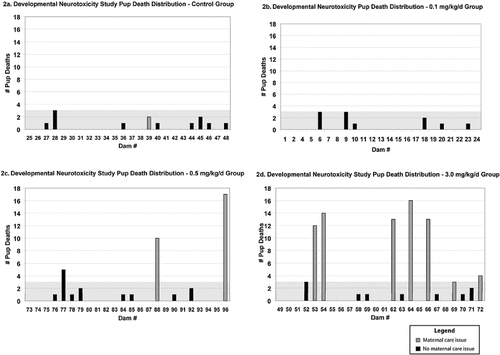
There were no treatment-related maternal body weight changes over the entire period of gestation or lactation. However, there was a nearly 30% decrease in body weight gain in the high-dose pups from PND 1–4 during early lactation. There were no treatment-related behavioral effects noted on the detailed clinical and functional observation of dams, which was conducted prior to daily dosing. Dose-related clinical signs that can be associated with maternal care issues were observed in the dam and the litter. The behavior and clinical signs among the dams in the various groups were analyzed in order to determine whether some aspect of treatment to the dams could be associated with the excessive deaths. As part of our initial analysis, dams were identified as displaying “maternal care issues” if there were two or more occurrences of inadequate maternal care. A single day in which one or more of the following related maternal care parameters was observed was scored as one occurrence: scattering of pups, dams ignoring their litters, no milk in stomach of pups, offspring cool to the touch, or inactive mammary tissue. General observations such as poor pup weight gain were also scored as an occurrence. For the DNT study, the dams with two or more occurrences of maternal care issues are identified in by the light shaded bars and are also listed in . While there is a striking correlation between dams with maternal care issues and litters with three or more pup deaths among the dimethoate-treated groups, the correlation is not perfect:
TABLE 3 Maternal Care Issues Observed in Individual Dams in the Developmental Neurotoxicity Study
| • | A litter from one control dam with maternal care issues had two pup deaths. | ||||
| • | Litters of two high-dose dams with maternal care issues had relatively few (three and five) pup deaths. | ||||
The reasons for the lack of complete correlation are not clear and may stem from biological variability, as well as the inability to monitor all dams at all times for quality of maternal care. (Recall that the assessment for maternal care issues was a post hoc undertaking.) It is also difficult to separate maternal care issues from pup toxicity based on the DNT design. The likelihood that maternal care issues contributed to pup mortality will be discussed in greater detail later in the discussion of the cross-fostering study. Here it is worthwhile to emphasize how the data convey the concept that the dam/litter is an important and relevant unit upon which to base the analysis of pup deaths.
Closer analysis, as demonstrated by , finds that the majority of pup deaths are clustered in a small number of litters, an important point that is missed by simply tallying the number of pup deaths per group (). Irrespective of the maternal toxicity correlation, this clustering of pup deaths illustrates the concept that dimethoate affects rats in a per-litter manner.
Pup Mortality Patterns in Related DNT Studies
In addition to the main DNT study, two related studies were conducted by the same laboratory using the same strain of rats and same dosing regimen. The pup mortality data in these two studies are relevant in evaluating the weight of evidence for the effects of dimethoate. The first study was a range-finding study conducted approximately 5 mo prior to the start of the DNT study in which dams (8–10/dose) were dosed from GD6 to PND 10 at 0, 0.2, 3, or 6 mg/kg/d, via gavage. Pups were dosed directly, using the same doses (on a milligrams per kilogram basis) administered to their dams, from PND 11 until weaning on PND 21. The second study was a comparative ChE study conducted approximately 9 mo after the start of the DNT study, using the same dosing and exposure regimen as the main DNT study with 8–10 dams/dose.
Among rodent litters, a few pup deaths are expected to occur regardless of treatment. Some pup mortality was observed in all dimethoate studies in which dams were dosed by gavage, including the range-finder study and the relative ChE sensitivity study (CitationMyers, 2001), as presented in .
Unlike in the DNT study, there were no excess deaths at 3 mg/kg/d in the range-finding study or in the relative ChE sensitivity study. Taking the data in all three studies into consideration, the distribution of pup deaths was characterized. Further, there is a substantial amount of study-to-study variation in the number of pup deaths attributed to specific doses of dimethoate administered to dams. Comparison of the number of pup deaths in litters in which dams treated with 3 mg/kg/d by oral gavage (range finder, relative ChE sensitivity study, and DNT studies) indicates that the number of pup deaths only significantly exceeds control levels in the DNT study. Many more pup deaths are seen at the 3-mg/kg/d group in the DNT study than in either the range finder or the relative ChE sensitivity study.
Dimethoate Exposure During Gestation, During Lactation, and Following Direct Dosing, as Measured by Brain Cholinesterase Activity
Brain, plasma and red blood cell (RBC) ChE levels were measured following exposure to repeated doses of dimethoate in both the comparative ChE study and the dose range-finding study. Only brain ChEI is reported here because it was more sensitive to effects of dimethoate gavage than plasma and RBC ChE. For the comparative ChE study, dams were administered daily gavage doses of 0.1, 0.5, and 3 mg/kg/d from GD 6 until PND 10 and pups directly from PND 11 to PND 21 as in the DNT study. Brain ChEI was measured in dams and fetuses on GD 20 and in pups on PND 4, 11, 21, and 60. Dam and pups were sacrificed by carbon dioxide inhalation on GD 20 (3 h after dosing), PND 21 (2 h after dosing), and PND 60. On PND 4 (4 h after dosing) and PND 11 (2 h after dosing) animals were killed by decapitation. Fetuses were removed from the uterus on GD 20, sexed, and placed immediately onto a cooling plate. Individual brains from dams, pups, and fetuses were removed from the skull, wrapped in aluminum foil (brains from fetuses were pooled together for each litter), and snap frozen in liquid nitrogen, then stored in dry ice prior to transfer to storage at −80°C. All ChE assays were performed on a Hitachi 911 clinical analyzer at 410 nm. Brain ChE activity was measured by following the action of thiocholine on 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) to form a colored product. Sample size was 8–9 dams, litters (pooled fetuses for each litter), or pups (1 pup/sex/litter). For the range-finding study, doses of 0, 0.2, 3, or 6 mg/kg/d were administered to dams (GD 6–PND 10) and pups (PND 11–21). Brain ChEI was measured in dams and fetuses on GD 20 and in pups on PND 21. At GD 20, the sample size was 5 dams and 5 litters of fetuses (pooled together for each litter). At PND 21 the sample size was 14–20, with each sample comprising as many as 2 pups/litter.
The ChE data indicate that at the 3-mg/kg/d dose level, there are no biologically significant effects at PND 4, but significant inhibition (22–33%) in male and female fetuses at GD 20 (). At PND 21 there is marked inhibition of 42–45% following direct dosing to pups from PND 11–21 and previous exposure via the dams from GD 6–PND 10. Data suggest that there is much less exposure to dimethoate during early lactation, indicating that pup deaths occurring during this time period are unlikely to be related to postnatal lactational exposure to dimethoate.
TABLE 4 Brain Cholinesterase Activity after Repeated Oral Gavage Doses to Dams and Pups Following DNT-Study Exposures
Cholinesterase inhibition can be compared using benchmark dose estimates of data from both the comparative ChE study and the range-finding study combined (). The BMD 10 values for GD 20 fetuses, PND 4 pups, and PND 21 pups are 1, 3.8–4, and 0.64, respectively (CitationReiss & Gaylor, 2005). The highest dose level tested for PND 4 pups was 3 mg/kg/d. This indicates that the BMD estimate of 3.8–4 for PND 4 pups is an uncertain estimate because it had to be extrapolated above the experimental dose range. These data provide further quantitative support that at the 3-mg/kg/d dose level, there was likely less exposure of the offspring to dimethoate during early lactation compared to late gestation on the definitive DNT study. This finding is significant because pup mortality occurred primarily between PND 1 and 4 (), with no excess deaths following direct dosing to pups, and little evidence of toxicity to the litters at birth.
TABLE 5 Selected BMD Estimates for Brain Cholinesterase Inhibition and Pup Mortality after Repeated Exposures
Cross-Fostering Study
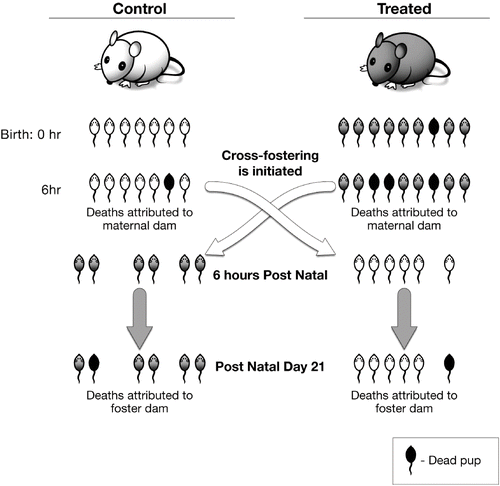
To address concerns about whether the pup mortality in the DNT study was maternally mediated or due to a direct effect on pups, a cross-fostering study was conducted. The study determined the relative impact of gestational and lactational exposures to dimethoate on the health of the pups. In addition, more frequent systematic observations were reported for the dams and litters, which can be used to infer whether pup deaths were the likely result of a direct effect of dimethoate on the pups or were secondary to dimethoate effects on the dams.
In the cross-fostering study, dimethoate was administered to dams at doses of 0, 3, or 6 mg/kg, from GD 6 through PND 11, in a regimen similar to that used in the main DNT study. The number of presumed pregnant females exposed to 0, 3, or 6 mg/kg dimethoate was 100, 25, and 50, respectively. On PND 1, pups from dams treated with dimethoate at 3 or 6 mg/kg were cross-fostered to control dams, and control pups were cross-fostered to dimethoate-treated dams; pups from 2 additional groups (control or 6-mg/kg dams) were not cross-fostered. Cross-fostering allows maternal animals to rear unrelated offspring. In this study half of the litters containing 12 or more pups from dams receiving 0 and 6 mg/kg/d were cross-fostered to different dose groups whereas all of the litters containing 12 or more pups from dams receiving 3 mg/kg/d were cross-fostered. The control group was not cross-fostered so that the control dams were kept with their own litter ().
TABLE 6 Pup Mortality in Cross-fostering Study without Data from Stillbirths, PND 1 Deaths, and Abnormal Dams
Dams and offspring were observed for maternal behavior and clinical signs 5 times each day beginning on PND 1. Observations were made at 6:00, 9:00, 12:00, 15:00, and 18:00. The 9:00 check was the detailed assessment for clinical signs. At the other four times, dams and pups were not handled but visual assessments included the following:
| • | Did the interactions between dams and offspring appear normal? | ||||
| • | Were offspring scattered about cage? | ||||
| • | Did the dams appear restless? | ||||
| • | Did the dam appear to ignore her offspring? | ||||
| • | Was there reason to suspect there were dead pups? | ||||
| • | Were there signs of physical abuse of pups? | ||||
| • | Was milk visible in pup stomachs? | ||||
Signs consistent with physical abuse of pups and the presence/absence of milk in the stomach were also recorded at the time of necropsy.
It is important to review the purpose and features of the cross-fostering study (). The cross-fostering experiment is designed to assess the influence of maternal exposure to dimethoate during gestation and the postnatal period on offspring survival. In addition, the study evaluates the effect of maternal influence (i.e., nurturing) and pup–dam interaction on the postnatal development and health of the pups. The experiment requires that females be administered the test agent beginning at the time of implantation (gestational day ∼6 in rats) and continuing until study termination at the end of lactation. Pups born to control and treated dams must be of the same age. That is to say that their entire litters must be switched within ∼6–8 h after birth. After the switching of litters, observations of the litters begin. While it is important to observe what occurs throughout gestation and at birth, the experiment begins at the time of the cross-fostering. Stillbirths or deaths in the first hours after birth (while pups are with their birth mothers) cannot be attributed to postnatal nurturing effects. It is not appropriate to include data collected prior to the crossing as part of the analysis that assesses the nurturing that occurs after crossing. Thus, if a treated dam has a litter of 9 pups, including 1 stillbirth and 2 that die within the first 6 h, her 6 live pups will be cross-fostered to a control dam. Clearly, it is not appropriate to include the three early deaths in the data that analyze the nurturing of the control dam. Thus, in the case just described, for cross-fostering purposes, the control dam starts with 6 live pups and no dead pups, not 6 live and 3 dead pups. All pup deaths that occur after cross-fostering until study termination should be charged to the foster dams.
Two dams in the cross-fostering study of dimethoate should be considered as outliers. The first is a control dam that received no treatment and fostered pups from a dam exposed to 6 mg/kg/d dimethoate during gestation. This control dam exhibited aberrant restless behavior, signs of pup abuse (injured pups), and scattering of her pups throughout the cage, likely contributing to pup deaths (see , which includes the control dam number 19). Furthermore, she lost more weight during PND 1–4 than any other control dam in this study. This illustrates the importance of evaluating the data on a per-litter basis, because shows that the numerical increase in pup mortality in the 0/6 group is associated with the one dam that had signs of maternal care issues. The second outlier dam (dam number 139) was exposed to 6 mg/kg/d dimethoate during gestation and raised her own offspring. She gave birth to a litter of 23 pups, a number that greatly exceeds the number of nipples and overtaxes the dam such that some pups would be expected to die. In fact, she lost 7 pups. When these outlier dams are eliminated from the analysis (), the overall conclusion is that the increased mean percentage of pup deaths per litter is associated with a strong maternal influence that occurs when dams are exposed to dimethoate.
FIG. 4. Distribution of pup deaths by litter in the cross-fostering study. Doses are presented as the dose to nursing dam/dose to birth dam. Maternal care issues were determined as follows. Multiple observations of poor maternal nurturing behavior (as described in the text) made on the same day were collectively scored as one occurrence. Dams that had four or more occurrences (days) of poor nurturing behavior were identified as having maternal care issues.
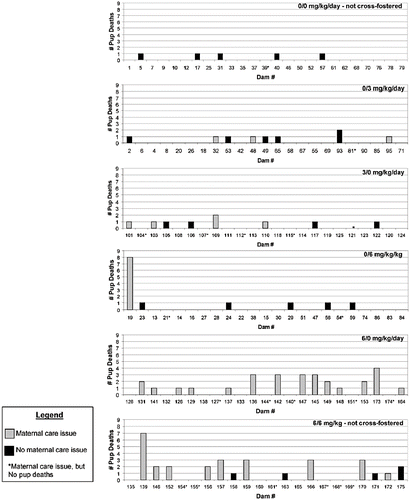
TABLE 8 Simple Group Comparisons of Litter Proportions of Pup Mortality for PND1–10
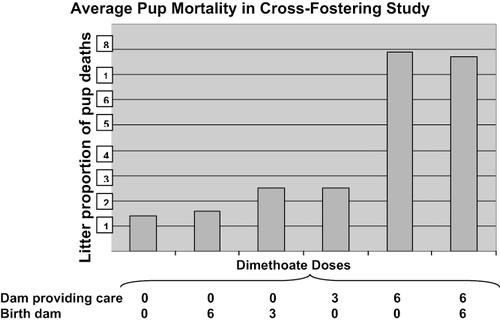
When the adjusted data in are inspected, the pup deaths seem to be linked to lactational, but not gestational, exposure to dimethoate. This is clearly illustrated in , which displays the mean litter proportions of pup deaths by treatment. This suggests that pup deaths are enhanced after postnatal exposures of the dams. Furthermore, most deaths occurred during the first 11 d of life, when the pups are most dependent upon the dams. Taken together, data indicate a strong maternal influence suggesting that the pup deaths are related to postnatal exposure to dams rather than gestational exposure to dams. This is consistent with the lack of effects noted in pups at birth or other parameters such as litter size, post implantation loss, and pup birth weight.
FIG. 5. The cross-fostering study demonstrates a dose-response relationship between the dose of dimethoate given to the dam providing care with the percent pup mortality per litter.
Clinical observations of the dam and pup during early lactation indicate that there was an increase in total number of observations related to maternal care issues in dams exposed to dimethoate (). The clinical observations can be combined into a single analysis by defining inadequate maternal care as four or more occurrences (days) of scattering of offspring, restlessness of the dam, absence of milk in the stomach of at least one pup, pup cold to touch, or signs of physical abuse based on examination of pups. The cutoff of 4 d was selected because control dams had a range of 0–4 d of “inadequate” maternal care observations and because there were more than twice as many daily observations made on the definitive DNT study.
TABLE 7 Observations in Dams and Pups Made in the Cross-Fostering Study
Based on the criterion of > 4 d of maternal care issues, data indicate that maternal care issues are well correlated with pup deaths among the litters raised by treated dams regardless of the treatment status of the dam that carried the pups during gestation ().
The distribution of pup deaths during lactation in the 6-mg/kg/d groups requires additional discussion. A greater number of pups died during PND 1–4 in litters where exposures to 6 mg/kg/d dimethoate took place during both gestation and lactation (6/6 group), compared to the number of pups that died in the group that was exposed only during the period of lactation (group 6/0). In addition, the latter group had more litters with pup losses from PND 4–11. While it might be argued that gestational exposure to 6 mg/kg/d in and of itself does not increase the mean percentage of pup deaths per litter, prenatal exposure might contribute to early pup loss when combined with postnatal maternal exposure. It is noted, however, that the total number of pup deaths in each of these groups did not differ significantly from control values, with or without inclusion of the dam in the 6/6 group that had 23 pups.
To further evaluate the relative impact of gestational versus lactational exposure to dimethoate, the litter proportions for pup deaths in the cross-fostering study were statistically analyzed using the Wilcoxon signed-rank test, an appropriate test for nonnormally distributed data (CitationWilcoxon, 1945). The results of this analysis indicate that pup mortality is unlikely to be due to a direct effect on the pups. The evidence for this is the observation that gestational-only exposure did not result in a statistically significant impact on pup mortality (groups 0/3 and 0/6 vs. 0/0), while at a sufficient dose level (6 mg/kg/d), mortality was higher in the group where exposed dams were nursing unexposed pups (6/0 group) relative to both control (group 0/0) and the group of pups born to exposed dams and nursed by control dams (0/6 group) (). (This is true regardless of whether the control dam with maternal care issues (dam number 19) or the dam with 23 pups (dam number 139) is included or excluded from the statistical analyses.) There was also no difference in survival when pups were exposed both gestationally and lactationally (6/6 group) relative to those that were only exposed lactationally (6/0 group), again indicating that in utero exposure did not adversely affect pup survival. This is important given that the fetus would be expected to receive a much higher dose of dimethoate due to placental transfer than pups would receive via lactational exposure. The concept that exposure via the milk is low is supported by findings in the relative ChE sensitivity study that indicate that ChEI in PND 4 pups was lower than that seen in GD 20 fetuses just 5 d earlier.
In summary, the cross-fostering study demonstrates that increased pup mortality is correlated with dose of the nursing dams and not to the dose given to the dams during gestation. Furthermore, maternal care issues are well correlated with pup deaths among the litters raised by treated dams regardless of the treatment status of the dam that carried the pups during gestation.
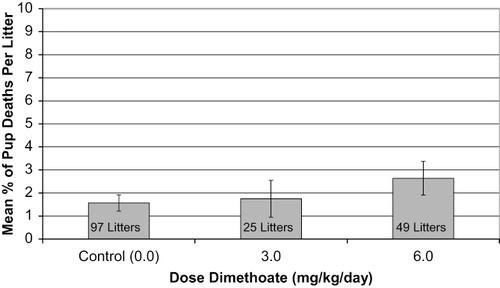
Pre-Cross-Fostering Pup Deaths
Performance of a cross-fostering study requires mating and delivery of numerous litters of all treatment groups in the hope that litters of the different treatment groups will be delivered nearly simultaneously. This means that there was a pool of litters that exceeded the number needed to populate each of the groups (0, 3, and 6 mg dimethoate/kg/d) that were included in the cross-fostering study. Each of the litters in the pool was observed for pup mortality during the first 6–8 h after birth. The early pup death data from all litters in the original pool (both the cross-fostered litters and those that were ultimately discarded) were examined for potential differences. The original pool consisted of 97 control litters (0 mg/kg/d), 25 low-dose litters (3 mg/kg/d), and 49 high-dose litters (6 mg/kg/d). The mean proportion of pup deaths per litter in the original pool did not differ among the groups. The data are displayed graphically in . Statistical analysis of the data revealed no differences among the mean litter proportions for the three groups.
DISCUSSION
Several factors suggest that the observed pup deaths are likely the result of poor maternal care by the dams treated with the high dose of dimethoate. First, pup deaths in the DNT study were clustered in a small number of litters and, in many cases for both the DNT and cross-fostering studies, dams rearing the affected litters exhibited maternal care issues ( and ). Second, the majority of pup deaths observed in the DNT study occurred during the time period when the pup is most dependent on the dam for survival, up to PND 4 (). The incidence of pup deaths decreases as the pup becomes more independent. Between PND 11 and 21, there is no relationship between dimethoate dose and mortality when analyzed using either the pup or the litter as the unit of analysis. This suggests that pup mortality was linked to maternal care. Third, the cross-fostering study shows that high incidences of pup deaths occurred in those litters whose dams were exposed during lactation, whether or not gestational exposure occurred (, , and ). This reinforces the notion that exposure to the dam during lactation is the most important time period impacting pup mortality. Finally, it is unlikely that lactational exposure to dimethoate via the milk contributes to pup deaths because several pups that died during lactation were found to have no milk in their stomachs at the time of necropsy. Furthermore, there is a decline in brain ChEI in PND 4 pups (7–13% relative to GD 20 fetuses (22–33% ChEI) at 3 mg/kg/d. The average BMD estimates for brain ChEI at PND 4 and GD 20 in the relative ChE sensitivity study are greater than 3.8 (above highest dose level tested) and 1 mg/kg/d dimethoate, respectively. A possible explanation for this is that young pups receive a much reduced dose of dimethoate during nursing compared to that received during gestation. There does appear to be a dose-response relationship between dimethoate and brain ChE inhibition in pups, but the difference between the 0.1- and 0.5-mg/kg/d groups is slight, and these levels of inhibition are all less than those reported in the scientific literature to be associated with increased pup mortality (CitationTang et al., 2003).
An alternative explanation could be that pups were sick as a result of gestational exposure so the maternal behavior is a reaction to the sick pups; however, this cannot be the case as the cross-fostering study demonstrated that in utero-only exposure exerted no effect on pup mortality (; 0/3 and 0/6 groups vs. 0/0; 6/0 group vs. 6/6).
Several studies (e.g., CitationKhera, 1987, Citation1991; CitationKeen et al, 2003a; CitationDeSesso & Goeringer, 1990; CitationCarey et al., 2000, Citation2003; CitationDeSesso, 1987; CitationDaston, 1994; CitationTaubeneck et al., 1994; CitationDuffy et al., 1997) illustrate the necessity to consider maternal toxicity as a common contributing cause to abnormal fetal development or death. Similarly, maternal toxicity can, and does, influence the development of the young during the postnatal period (CitationKeen et al, 2003b; CitationWeaver et al., 2004). When statistically analyzed as individual unrelated parameters, the numbers of observations of maternal restlessness, scattering of pups, and lack of milk in the stomachs of pups were increased in the 3/0, 6/0, and 6/6 groups but not the 0/0, 0/3, or 0/6 groups. In addition, there was a significant decrease in maternal body weight gain in the 3/0, 6/0, and 6/6 groups but not in the 0/0, 0/3, or 0/6 groups during the early period of lactation (PND 1–7). First, these data suggest that the maternal behavior cannot be attributed to sickness resulting from in utero exposure. Second, although the raw number of maternal care issue observations was increased in the 3/0 group, our analysis indicates that the number of dams exhibiting maternal care issues for 4 or more days was considerably higher in the 6/0 and 6/6 groups compared to the 3/0 group, which is consistent with the pattern of increased pup mortality in the 6/0 and 6/6 groups but not in the 3/0 group (; last row of ). Further, in evaluating these data, it should be noted that cross-fostering itself might lead to a rise in the number of maternal care issues observed, such that the number of dams exhibiting maternal care issues for 4 or more days in the 0/0 and 6/6 groups (which were not cross-fostered) is likely to be less than if these groups had been cross-fostered.
It is worthwhile to note that the correlation between dams with maternal care issues and pup mortality in is not perfect. There are some dams with maternal care issues that had no pup mortality. There are also dams with no maternal care issues with pup mortality. This expected biological variability should not detract from the overall pattern that indicates an increased incidence of maternal care issues associated with pup mortality in dams from the 6/6 and 6/0 groups compared to the other groups. It should also be noted that all the dams in the cross-fostering study, including the outliers (control dam number 19 [0/6] with maternal care issues and 6/6 dam number 139 with 23 pups) are included in .
Taken together, these data indicate that there is a strong maternal influence on pup mortality in the dimethoate studies, emphasizing the importance of evaluating the data using litter as the biological unit and providing additional supportive evidence that protection against toxicity to adults will be protective of offspring effects.
This is further supported by data from three dietary reproduction studies in which dams were exposed prior to gestation, as well as throughout gestation and lactation. These include 2 rat multigeneration reproduction studies in which dietary exposure to approximately 6 mg/kg/d dimethoate did not result in treatment-related effects on pup mortality in spite of substantial (> 60%) fall in maternal brain ChEI (CitationMellert et al, 2003; CitationBrooker et al, 1992). In addition, a one-generation range-finding study (CitationBrooker & Stubbs, 1990) resulted in increased pup mortality at 5.8 and 7.5 mg/kg/d, which was also associated with overt maternal toxicity (tremors, decrease body weight gain, and ChE inhibition).
These data support the conclusion that dimethoate produced pup mortality only at maternally toxic doses, and that risk assessments based on the no-obsesrved-adverse-effect level (NOAEL) for maternal ChE inhibition will be protective of pup mortality.
CONCLUSIONS
In conclusion, pup mortality and brain ChE inhibition are the two critical effects observed in the dimethoate DNT-related studies in which doses were administered by oral gavage. In evaluating pup mortality in the DNT study, the litter should be considered the primary unit of analysis for both qualitative and quantitative evaluation because (a) 68/85 pup deaths (80%) were clustered in 5 of the 25 litters, and (b) signs of maternal neglect were correlated with those litters with excess pup losses (losses of 3 or more pups). Assessment of pup mortality emphasizing the individual pup as the unit of analysis will provide misleading conclusions regarding dose-response relationships, and from a statistical standpoint is indefensible. Exposure to dimethoate in the diet does not exert a consistent effect on pup mortality similar to that observed in the DNT and related gavage studies, suggesting that the pharmacokinetic differences between gavage and dietary exposure may be contributing significantly to the toxicity of dimethoate in the DNT studies.
The cross-fostering study revealed that pup mortality was primarily associated with maternal exposure that occurred during lactation rather than gestation. This indicates that there is a major postnatal maternal influence on pup mortality. The data from the cross-fostering study support the hypothesis that there is a maternal influence on pup mortality, although a specific mechanism for pup mortality cannot be determined.
Based on a quantitative dose-response assessment conducted by CitationReiss and Gaylor (2005), ChE inhibition in adults is an appropriate POD for risk assessment and is protective of pup mortality by two- to threefold. This allows full use of the dimethoate database, including ChE data from inhalation and dermal adult toxicity repeated-dose studies, which are relevant to the inhalation and dermal human occupational exposures.
Acknowledgments
The authors acknowledge Cheminova for funding and for providing dimethoate study report.
Notes
International Life Sciences Institute. 1999. An evaluation and interpretation of reproductive endpoints for human health risk assessment, Report prepared by an expert panel. Washington, DC: ILSI.
REFERENCES
- Brooker , A. J. and Stubbs , A. 1990 . Dimethoate: Dietary range finding study in mature male and female rats and their juvenile offspring , Huntingdon, Cambridgeshire, England : Huntingdon Research Center Ltd .
- Brooker , A. J. , Homan , B. A. , Parker , C. A. , Offer , J. M. , Anderson , A. and Dawe , I. S. 1992 . The effect of dimethoate on reproductive function of two generations in the rat , Huntingdon, Cambridgeshire, England : Huntingdon Research Center Ltd .
- Bu , J. , Yan , L. , Chen , Y. , Chu , J. X. , Xie , X. F. and Chen , T. P. 2001 . Prospective study of lethal blood concentrations of organophosphorous in humans . Fa Yi Xue Za Zhi , 17 : 21 – 24 .
- Carey , L. C. , Berbee , P. L. , Coyle , P. , Philcox , J. C. and Rofe , A. M. 2003 . Zinc treatment prevents lipopolysaccharide-induced teratogenicity in mice . Birth Defects Res. A Clin. Mol. Teratol. , 67 : 240 – 245 .
- Carey , L. C. , Coyle , P. , Philcox , J. C. and Rofe , A. M. 2000 . Maternal ethanol exposure is associated with decreased plasma zinc and increased fetal abnormalities in normal but not metallothionein-null mice . Alcohol Clin. Exp. Res. , 24 : 213 – 219 .
- Daston , G. P. 1994 . “ Relationships between maternal and developmental toxicity ” . In Developmental toxicology , 2nd , Edited by: Kimmel , C. A. and Buelke-Sam , J. 189 – 201 . New York : Raven Press .
- Daston , G. , Faustman , E. , Ginsburg , G. , Fenner-Crisp , P. , Olin , S. , Sonawane , B. , Bruckner , J. and Breslin , W. 2004 . A framework for assessing risks to children from exposure to environmental agents . Environ. Health Perspect. , 112 : 238 – 256 .
- Davanzo , F. , Settimi , L. , Fraaoni , L. , Maiozzi , P. , Travaglia , A. and Marcello , I. 2004 . Agricultural pesticide-related poisonings in Italy: Cases reported to the Poison Control Centre of Milan in 2000–2001 . Epidemiol. Prev. , 28 : 330 – 337 .
- DeSesso , J. M. 1987 . Maternal factors and developmental toxicity . Teratogen. Carcinogen. Mutagen. , 7 : 225 – 240 .
- DeSesso , J. M. and Goeringer , G. C. 1990 . Developmental toxicity of hydroxylamine: An example of a maternally mediated effect . Toxicol. Ind. Health , 6 : 109 – 121 .
- Duffy , J. Y. , Baines , D. , Overmann , G. J. , Keen , C. L. and Daston , G. P. 1997 . Repeated administration of alpha-hederin results in alterations in maternal zinc status and adverse developmental outcome in the rat . Teratology , 56 : 327 – 334 .
- Eddleston , M. , Eyer , P. , Worek , F. , Mohammed , F. , Senarathna , L. , von Meyer , L. , Juszczak , E. , Hittarage , A. , Azher , S. , Dissanayake , W. , Sheriff , M. H. R. , Szinicz , L. , Dawson , A. H. and Buckley , N. A. 2005 . Differences between organophosphorous insecticides in human self-poisoning . Lancet , 366 : 1452 – 1459 .
- Haseman , J. K. and Hogan , M. D. 1975 . Selection of the experimental unit in teratology studies . Teratology , 12 : 165 – 171 .
- Holson , J. F. , DeSesso , J. M. , Jacobson , C. F. and Farr , C. H. 2000 . Appropriate use of animal models in the assessment of risk during prenatal development: An illustration using inorganic arsenic . Teratology , 62 : 51 – 71 .
- International Life Sciences Institute. 1999. An evaluation and interpretation of reproductive endpoints for human health risk assessment, Report prepared by an expert panel. Washington, DC: ILSI.
- Keen , C. L. , Clegg , M. S. , Hanna , L. A. , Lanoue , L. , Rogers , J. M. , Daston , G. P. , Oteiza , P. and Uriu-Adams , J. Y. 2003a . The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications . J. Nutr. , 133 : 1597S – 1605S .
- Keen , C. L. , Hanna , L. A. , Lanoue , L. , Uriu-Adams , J. Y. , Rucker , R. B. and Clegg , M. S. 2003b . Developmental consequences of trace mineral deficiencies in rodents: Acute and long-term effects . J. Nutr. , 133 : 1477S – 1480S .
- Khera , K. S. 1987 . Maternal toxicity in humans and animals: Effects on fetal development and criteria for detection . Teratogen. Carcinogen. Mutagen. , 7 : 287 – 295 .
- Khera , K. S . 1991 . Chemically induced alterations in maternal homeostasis and histology of conceptus: Their etiologic significance in rat and fetal anomalies . Teratology , 44 : 259 – 297 .
- Mellert , W. , Hellwig , J. , Gembardt , C. , Deckert , K. and van Ravenzwaay , B. 2003 . Dimethoate—Two-generation reproduction toxicity study in Wistar rats: Administration in the diet , Ludwigshafen/Rhein, Germany : Experimental Toxicology and Ecology, BASF Aktiengesellschaft .
- Morford , L. L. , Henck , J. W. , Breslin , W. J. and DeSesso , J. M. 2004 . Hazard identification and predictability of children's health risk from animal data . Environ. Health Perspect. , 112 : 266 – 271 .
- Myers , D. P. 2001 . Dimethoate effects on cholinesterase in the CD rat (adult and juvenile) by oral gavage administration , Cambridgeshire, UK : Huntingdon Life Sciences .
- Myers , D. P. 2003 . Dimethoate developmental neurotoxicity study in the CD rat by oral gavage administration (Final report with consolidation of amendments 1 and 2) , Cambridgeshire, UK : Huntingdon Life Sciences .
- Reiss , R. and Gaylor , D. 2005 . Use of benchmark dose and meta-analysis to determine the most sensitive endpoint for risk assessment for dimethoate . Regul. Toxicol. Pharmacol. , 43 : 55 – 65 .
- Sheets , L. 2000 . A consideration of age-dependent differences in susceptibility to organophosphorous and pyrethroid insecticides . Neurotoxicology , 21 : 57 – 63 .
- Tang , J. , Carr , R. L. and Chambers , J. E. 2003 . The effects of repeated oral exposures to methyl parathion on rat brain cholinesterase and muscarinic receptors during postnatal development . Toxicol. Sci. , 76 : 400 – 406 .
- Tsatsakis , A. M. , Aguridakis , P. , Michalodimitrakis , M. N. , Tsaklov , A. K. , Alegakis , A. K. , Koumantakis , E. and Troulakis , G. 1996 . Experiences with acute organophosphate poisonings in Crete . Vet. Hum. Toxicol. , 38 : 101 – 107 .
- Taubeneck , M. W. , Daston , G. P. , Rogers , J. M. and Keen , C. L. 1994 . Altered maternal zinc metabolism following exposure to diverse developmental toxicants . Reprod. Toxicol. , 8 : 25 – 40 .
- Tyl , R. W. , Crofton , K. , Moretto , A. , Moser , V. , Sheets , L. P. and Sobotka , T. J. 2008 . Identification and interpretation of developmental neurotoxicity effects: A report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints . Neurotoxicol. Teratol. , 30 : 349 – 381 .
- Weaver , I. C. , Cervoni , N. , Champagne , F. A. , Alessio , A. C. , Sharma , S. , Seckl , J. R. , Dymov , S. , Szyf , M. and Meaney , M. J. 2004 . Epigenetic programming by maternal behavior . Nat. Neurosci. , 7 : 791 – 792 .
- Wilcoxon , F. 1945 . Individual comparisons by ranking methods . Biometrics Bull. , 1 : 80 – 83 .