Abstract
New Approach Methodologies (NAMs) are being widely used to reduce, refine, and replace, animal use in studying toxicology. For respiratory toxicology, this includes in silico and in vitro alternatives using air:liquid interface (ALI) exposures to replace traditional in vivo inhalation studies. In previous studies using 1,3-dichloropropene (1,3-DCP), a 5-day 4 h repeat exposures of MucilAir™ nasal cell culture models caused, dose-dependent cytotoxicity, depletion of GSH, changes in differential gene expression and histopathological transitions in cellular morphology from pseudostratified columnar epithelium to squamous epithelium. In this report we attempted to extend these studies using 15-day 1,3-DCP 4 h exposures to using MucilAir™ nasal cultures as outlined by an US EPA recent task order (US EPA Citation2023). For the 15-day repeat exposure, there were severe histopathologic changes in the MucilAir™ nasal mock-treatment (air-only) VITROCELL® chamber controls compared to incubator controls preventing any further analysis. The histopathological transitions in cellular morphology from pseudostratified columnar epithelium to squamous epithelium observed in the air only control in this study and previously with 1,3-DCP in MucilAir™ nasal cultures is also a hallmark of chemically induced cytotoxic responses in vivo in the respiratory tract. Histopathology assessments of 3D respiratory tract models used in ALI exposures can provide the linkage between in vitro to in vivo outcomes as part of the validation efforts of ALI use in regulatory toxicology. This report indicates that importance of histopathological assessments of incubator and mock-treatment (air-only) controls from each ALI exposure experiment along with exposed cell based model.
Introduction
New Approach Methodologies (NAMs) using in silico and in vitro biological assays using human-relevant test systems are being developed to reduce, refine, and replace animal use (Schmeisser et al. Citation2023). Although regulatory toxicology relies on animal studies for use in risk assessments, there is an ongoing shift to non-animal test methods and the use of genomic tools in the safety assessment of new drug candidates and environmental chemicals that is revolutionizing the practice of toxicology. There are several reasons for switching to a non-animal test that include social concerns, regulatory mandates, company/corporate decisions and just as importantly, there is an ongoing revolution of cell culture methods enabling safety testing in human relevant 3D biological test systems that mimic the anatomical architecture, physiology and functions of organs that include liver, lung, skin, and kidney (Stucki et al. Citation2022).
Of relevance to this report are NAMs-based safety assessments using respiratory tract models with Air:Liquid Interface (ALI) exposures aimed at reducing the reliance on rodent inhalation studies (Braakhuis et al. Citation2023). The human respiratory tract is in direct contact with the atmosphere and a portal of entry to the systemic circulation for airborne chemicals, which include aerosols (particulate suspensions in air), vapors/gases (molecular solutions in air). Inhalation of toxic chemicals may cause adverse effects at the point of contact (POC), i.e. in the airway epithelial tissue at the interface of the atmosphere and the body or systemically affecting other target organs (Hayes and Bakand Citation2010; Leikauf Citation2015). Inhalation is the primary route of exposure to airborne chemicals, which include aerosols (particulate suspensions in air) and vapors/gases (molecular solutions in air). Risk assessments of aerosol exposures to humans over the last several decades have relied upon histopathological observations in 90-day whole-body and chronic rodent inhalation studies. Among the National Toxicology Program (NTP) Toxicology and Carcinogenesis studies, the lung is second to the liver as the most common target site of neoplasia of chemical carcinogens in male and female mice (Dixon et al. Citation2008). However, rodent inhalation studies are very costly, require multidisciplinary expertise to conduct either whole-body or nose-only inhalation studies, have significant animal welfare concerns, are associated with poor predictive value to humans, and current timelines exceed more than 1 year to conduct regulatory compliant 90-day studies and 3–4 yrs for a chronic inhalation study. The current approach cannot meet the throughput needed for testing of potentially thousands of environmental chemicals and the regulatory demands by the US EPA to provide data in support of risk assessments for either groups of compounds or specific compounds (National Research Council Citation2007; National Academies of Sciences et al. Citation2022). Secondly, well-known differences in rodent respiratory tract anatomical, biochemical, and biological responses compared to humans particularly at high exposures used for regulatory testing can make rodent to human extrapolations questionable (Meyerholz et al. Citation2017). Although animal testing has served as the gold standard in assessing potential risk of chemicals to humans this approach with high cost, long timelines, and use of large numbers of animals cannot meet the backlog of chemicals that lack safety assessments requiring animal testing using the inhalation route of exposure (Cao et al. Citation2021; Hargrove et al. Citation2021; Singh et al. Citation2021;Ramanarayanan et al. Citation2022; Moura et al. Citation2023).
Highly standardized and controlled inhalation studies are required to fulfill general regulatory requirements for the registration of new drugs, pesticides, or notification of chemicals (OECD Citation2018). Histopathology assessment of lung tissue is the primary endpoint used to assess impact of xenobiotic exposures in rodents exposed by inhalation exposure. The National Toxicology Program has established the ‘Nonneoplastic Lesion Atlas A guide for standardizing terminology in toxicologic pathology for rodents’ that contains high resolution photomicrographs of unexposed controls and a catalog of well-known lesions of the respiratory tract from the nose to the alveolar space (https://ntp.niehs.nih.gov/atlas/nnl/respiratory-system/lung).
A 5-day repeat exposures to 1,3-dichloropropene (1,3-DCP) causes a progression of nasal lesions observed in rodents exposed to 1,3-dichloropropene (Paudel et al. Citation2023). These were captured as a dose-dependent changes in cellular morphology of 1,3-DCP exposed MucilAir™ nasal cultures: transition of pseudostratified columnar epithelium to squamous epithelium at cytotoxic exposure concentration (Paudel et al. Citation2023). Inhalation studies that cause respiratory tract toxicity follow a well characterized response of regenerative proliferative and a transition from respiratory epithelium to squamous epithelium. For initial ‘validation’ of respiratory tract toxicity assessments with well-known prototypic toxicants using respiratory tract cell culture models, histopathology assessment of respiratory tract cell cultures can serve as phenotypic anchor and a bridge for direct extrapolation of histopathological lesions observed rodent respiratory tract for extrapolation of cellular, biochemical, and genomics endpoints by IVIVE modeling for human risk assessments (Paudel et al. Citation2023). These histopathologic changes observed in the nasal cultures are significant since they mimic known responses in the nose of rats exposed to well-known nasal cytotoxicants including 1,3-DCP, providing a direct linkage from in vitro exposures to know in vivo outcomes. It is of importance for the qualification of respiratory tract cell based NAMs to accurately predict responses that occur in traditional regulatory testing and more importantly predict what can occur in humans. We suggest that respiratory tract cell culture model histopathological changes can provide a direct linkage from in vitro to in vivo to qualify novel cell based respiratory tract models for human risk assessments.
In an effort to expand these studies, we initiated a 15-day repeat exposure with 1,3-DCP using a limited set of exposure levels, mock-treatment (air- only) VITROCELL® chamber control and incubator control. After completion of the experiment, histopathological changes were observed in the MucilAir™ mock treatment cultures compared to incubator controls that precluded assessment of 1,3-DCP exposed cultures. These changes were not observed in the mock air-only control cultures from a 5-day repeat exposure with 1,3-DCP (Paudel et al. Citation2023). This report emphasizes the need for histopathological assessment of respiratory tract cell based models as a linkage to known histopathological changes observed from in vivo cytotoxic exposures and a requirement for parallel air-control mock treatment cultures and incubator controls for every ALI exposure.
Methods
Cell culture and maintenance
MucilAir™ cultures of nasal origin were obtained from Epithelix Sarl (Switzerland). These cultures were derived from 14 pooled donors and seeded into Transwell permeable supports (6.5 mm diameter polyester membranes with 0.4 µm pores) and differentiated by the manufacturer prior to delivery to the ScitoVation laboratory. Upon receipt, cultures were maintained at the ALI with 700 µL of the proprietary medium supplied by the manufacturer (MucilAir™ Culture Medium-EP05MM) in the basolateral chamber. The medium was exchanged every 2–3 days, and the apical chamber was washed once a week to wash off the mucus. For the cell culture wash, 200 µLof PBS was added to the apical chamber and incubated at 37 °C for 30 min. After the incubation, the apical chamber was washed by pipetting up and down and the PBS was aspirated gently.
VITROCELL® 12/12 exposure system
The cultures were exposed to 1,3-DCP vapor using a 12/12 VITROCELL® exposure system designed as described in detail in our previous publication (Moreau et al. Citation2022). For this study, we used a 6.5 mm transwell permeable support, 2 mL/minute flow rate, and 2 mm trumpet height. The exposure was run with clean air and 2 different concentrations of 1,3-DCP for 4 h with a set of control cultures that remained in the incubator throughout (incubator controls) as was done in our previous work (Moreau et al. Citation2022). No analytical methods were used to quantify exposure concentrations in the chamber of air outlet. The cells were allowed to recover for 20 h in a humidified incubator at 37 °C. Immediately before each exposure, mucus was washed from the apical surface of each culture with D-PBS+Ca2+/Mg2+. Immediately after each exposure cell cultures were returned to plastic well plates containing 700 µL of fresh basal medium and incubated at 37 °C for 20 h. This was done for 15 days.
Histology
MucilAir™ nasal cultures were fixed at 4% paraformaldehyde in pH 7.4 phosphate buffered saline for 20 h and transferred to 70% ethanol/water and shipped to Cerba Research (Montpellier, France). For further processing, tissue samples were incubated with 10% neutral buffered formalin (NBF), various alcohol dilutions, xylene, and paraffin for a total of one and a half hours. After processing samples, tissues were embedded in paraffin for a permanent state of preservation. Tissues were sectioned at 4 µm, with 1 section per slide. After sectioning, samples were placed in an oven for additional adhesion. Slides were stained with hematoxylin and eosin (H&E) using reagents from Leica and Statlab™. After staining, sections were dehydrated, and the film cover slipped using a Tissue-Tek Film™ automated Coverslipper. The whole slide scanning (×40 objective) was performed on an Aperio AT2 digital whole slide scanner (Leica Biosystems). Scanned images of slides for the MucilAir™ nasal mock-treatment (air-only) VITROCELL® chamber controls were compared to incubator controls from 5-day and 15-day studies. Images were viewed using Hamamatsu NDP.view2 image viewing software and evaluated by a board-certified veterinary pathologist (S Elmore).
Lactate dehydrogenase in media to assess cytotoxicity
All media was removed and collected from all cell cultures at the end of each daily exposure. Cells were replenished with fresh test article-free each day media until the next exposure. The media was then diluted at least 1:20 with LDH storage buffer (200 mM Tris-HCl, pH 7.3, 10% glycerol, 1% BSA) and stored at −20 °C until LDH analysis. This was repeated for 15 days. Prior to analysis samples were thawed, and LDH analysis was performed using the LDH-Glo Assay Kit (Promega) according to the manufacturer’s instructions. A 12-point LDH standard curve was included on every plate run, and test samples were further diluted as necessary such that all samples fell within the linear range of the standard curve. Using GraphPad Prism software, standards were fit by a linear model with 1/Y2 weighting (to minimize the sum of the squares of the relative distance of the points from the curve), excluding the highest concentration standards that clearly deviated from the linear relationship. LDH concentrations in test samples were interpolated from the standard curve, and then total LDH in the relevant source solution was calculated based on dilution factors and total sample volumes. Cytotoxicity is expressed as a decrease in LDH as compared to incubator controls. Pairwise ANOVA was used to assess statistical significance of LDH in media.
Results
Mock-treatment air-only compared to incubator controls: 5-day compared to 15-day repeat exposures
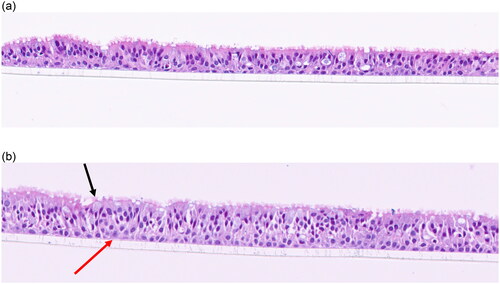
The 5-day incubator controls and mock-treatment (air-only) VITROCELL® chamber controls () consist of well-defined pseudostratified ciliated epithelium cells with scattered mucin producing (goblet) cells and underlying basal cells. Larger vacuoles consisting of stippled blue mucin are present in some samples (). There is a range in the thickness of the cell layers in some samples () as well as scattered disruption of epithelia along the scaffold (processing artifact) in certain samples. There were no histological differences observed between 5-day incubator control samples () compared to the 5 day mock-treatment (air-only) VITROCELL® chamber control samples ().
Figure 1. (a) 5-day incubator control. Note the well-defined pseudostratified ciliated epithelium with goblet cells and underlying basal cells. (b). 5- day mock treatment (air only), 20x. There are 1–2 layers of basal cells with basophilic nuclei, small nucleoli, and amphophilic cytoplasm (red arrow). Up to ∼6 layers of pseudostratified ciliated epithelial cells (asterisk) with darker nuclei and indistinct cytoplasmic borders. There are occasional mucin-producing cells with light blue stippled material (back arrow).
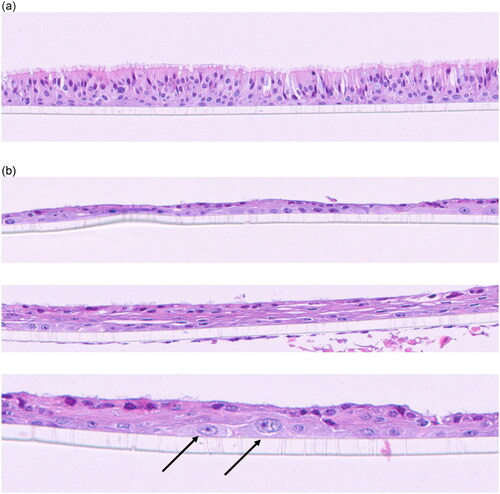
The 15-day incubator control samples () are similar to the 5-day clean air and incubator samples with well-defined pseudostratified epithelia, goblet cells, and basal cells (). However, the 15-day mock-treatment (air-only) VITROCELL® chamber controls have respiratory epithelium that is thinner with darker, more flattened nuclei compared to the 15-day incubator control samples (). In many areas the respiratory epithelium is only one cell thick, and cilia is either missing or scant. Goblet cells are not present. Basal cells, sometimes reactive, are present adjacent to the scaffold. The dark eosinophilic surface of the respiratory epithelium is indicative of keratin production. There are also focal areas of hyperplasia and hypertrophy of basal cells with disorganized overlying epithelium (). There is a combination of minimally ciliated cells as well as scattered degenerating cells. These changes are indicative of mucosal injury that results in hyperplastic or squamous epithelium as a protective mechanism.
Figure 2. (a). 15-day incubator control. Well-defined pseudostratified ciliated epithelium with goblet cells and underlying basal cells. (b) 15-day mock treatment (air only) control. The respiratory epithelium in the upper panel is thinner (one layer in this image) and the nuclei are darker and more flattened indicative of squamous metaplasia. The dark eosinophilic surface is indicative of keratin production. Basal cells are present adjacent to the scaffold and appear reactive. The Middle panel has more layers of squamous epithelial cells with keratin production and scattered ciliated cells. The lower panel (20+ magnification) illustrates reactive basal cells (arrows) that are larger with paler chromatin and prominent nucleoli.
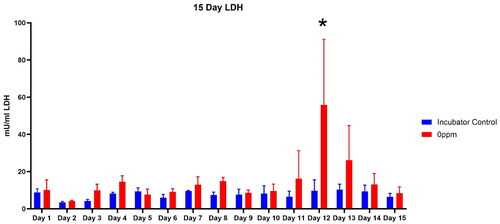
Lactate Dehydrogenase Release into the Media: Incubator controls compared to mock-treatment (air-only) VITROCELL® chamber controls
After the histopathological observations in the mock-treatment (air-only) VITROCELL® chamber controls compared to incubator controls was reported, media samples collected each day to assess lactate dehydrogenase (LDH) release into media to assess cytotoxicity were thawed then analyzed (). As shown in , there was small day to day variability in media LDH over the fifteen day experiment. However, on Day 12, three days prior to sample collection for histopathology, there was a sharp approximately 5 fold increase in LDH release (p < 0.01). Although we are uncertain as to the cause of the increased LDH release on Day 12, this may have been a signal of the initial injury leading to the histopathological changes observed in the mock-treatment (air-only) VITROCELL® chamber controls.
Discussion
Human airway organoids possess cell polarity and several of the cell types found in airway epithelium. The use of ALI exposures allows direct exposure to inhaled substances. Primary human airway epithelial cells cultured at ALI develop a linear pseudostratified epithelium. Using H&E, the basal, ciliated, and mucus-producing (goblet) cells were easily identified. Besides having a realistic structure, this pseudostratified model also has barrier properties and metabolic functions, thus effectively mimicking the human nasal epithelium (Moura et al. Citation2023).
There are four main histological layers within the respiratory system: respiratory mucosa, which includes epithelium and supporting lamina propria, submucosa, cartilage and/or muscular layer, and adventitia. Respiratory epithelium is ciliated pseudostratified columnar epithelium found lining most of the respiratory tract, including the nasal passages and as observed in MucilAir™ nasal cultures. The epithelium classifies as pseudostratified even though it is a single layer of cells along the basement membrane; the alignment of the nuclei is not in the same plane and appears as multiple layers. The role of this unique type of epithelium is to function as a barrier to pathogens and foreign particles; however, it also operates by preventing infection and tissue injury via the use of the mucociliary elevator.
An acute response to injury of 3D respiratory tract models may cause squamous metaplasia as in the 15-day mock-treatment (air-only) VITROCELL® chamber controls and as observed with 5 day 1,3-DCP repeat exposures (Paudel et al. Citation2023), where the adjacent basal cells are recruited, and squamous cells form to cover the denuded epithelium. Basal cells are the undifferentiated progenitor cells that proliferate to produce new epithelial cells (either respiratory or squamous). This change to squamous cell morphology, along with keratin production, helps to prevent future injury. Keratins and associated filaments provide a scaffold for epithelial cells and tissues to sustain mechanical stress, maintain their structural integrity, ensure mechanical resilience, and to protect against variations in hydrostatic pressure and establish cell polarity (Dixon et al. Citation2008).
In terms of this organoid study, the main findings were in the 15-day mock-treatment (air-only) VITROCELL® chamber controls. The 15-day incubator controls show no change, as expected. However, the other 15-day mock-treatment (air-only) VITROCELL® chamber controls show evidence of acute injury characterized by squamous metaplasia and foci of hyperplasia with keratin production. The 15-day mock-treatment (air-only) VITROCELL® chamber control samples have areas consistent with squamous metaplasia with underlying reactive basal cells as well as focal areas consistent with hyperplasia and reactive basal cells. The disorganized overlying epithelium has scattered degenerating cells. The only difference between the incubator controls and the 0 ppm samples was that the control samples stayed in the incubator all the time while the 0 ppm samples were taken from the incubator, put on the vapor exposure system for four hours a day, then put back in the incubatorfor 15 days.
Histopathology assessments of 3D respiratory tract models is perhaps the closest biological endpoint that can be directly compared to respiratory tract toxicity in animal studies using chemical exposures. Our study showed the change in cellular morphology from pseudostratified columnar/cuboidal to cuboidal and finally squamous epithelium observed in mock-treatment (air-only) VITROCELL® chamber controls from this study parallels histopathological observations with 1,3-DCP exposure in MucilAir™ nasal cultures and are consistent histopathological observations known to occur in nasal mucosa of a broad range of compounds that induce non-neoplastic lesions in the nose of rats (Monticello et al. Citation1990). Nasal biopsy of individuals with occupational exposure to formaldehyde indicate the normal respiratory epithelium changing to stratified cuboidal epithelium with loss of ciliated epithelium and finally stratified squamous epithelium (Holmström et al. Citation1989). These data indicate that the MucilAir™ nasal cultures capture the known histopathological changes in nasal tissues observed in rodents and in humans following cytotoxic exposures. Histopathology assessments of 3D respiratory tract models can be a bridge to validate these models since they mimic the known vivo effects of cytotoxic exposures to the respiratory epithelium.
The vapor exposure system was at 37° C and cell media contained HEPES to maintain pH. The VITROCELL® system consists of two components: a vapor generating system and a cell exposure module. The vapor generation system has a controller for adjusting the flow of clean air and system temperature and a digital syringe drive for injecting test articles at a predefined speed. Given that the temperature was held constant at 37° C, the only other variables to consider would be how the extended time 10 days (15 days versus 5 days) combined with the flow setting of clean air may have affected these samples. Although a ‘spike’ in LDH release was observed on day 12 that preceded histopathology assessments of the MucilAir™ cultures, we are uncertain as to the cause and whether this was an initial signal of cell injury during the 15 days repeat exposure regimen.
Human 3D respiratory tract NAMs are being developed and undergoing qualification trials needed to reduce, refine, or replace the need for costly inhalation exposure studies in rodents. Due to the significance of alveolar macrophages in mediating cell injury from particualt exposure certain investigators have employed respiratory tract 3D culture with added macrophages (Moura et al. Citation2023). Recent 3D models undergoing development include a tri-culture model of the alveolar-microvascular interface used to assess diesel exhaust particulate (DEP) exposures (Vitucci et al. Citation2024). However, as these models become more complex the throughput decreases and increased scientific and biological expertise is needed to maintain three cell-based models alone then combine them for exposure in an ALI system. This can become a significant limitation of these test systems since many do not contain all of the cell types that make up a region of the respiratory tract under investigation. Overall, a major limitation for 3D respiratory tract models concerns dosimetry and exposure to the test system. Although toxicokinetic modeling may overcome this concern, there is limited ability for real time sampling of delivered dose during exposures to the 3D respiratory tract cultures.
Although ALI and respiratory tract cell culture models are maturing limited repeat exposures studies for 15-days as mandated in a US EPA Citation2023 task order for ALI testing. Secondly, certain regions of the respiratory tract such as alveolar slices may be more resilient than the nasal cultures used here (Patel et al. Citation2023). This data indicates that histopathological assessments and mock-treatment (air-only) chamber controls should be included in every ALI respiratory tract safety assessment. Since the health status of 3D cultures is difficult to assess by simple microscopy during the conduct of these studies’ other ‘non-invasive’ monitors of health status should be considered during the conduct of extended repeat dose ALI studies rather than wait until the experiment is completed wasting valuable resources and time.
Disclosure statement
The authors of this manuscript do not have any conflict of interest with the contents of this manuscript.
Data availability statement
Data available effective July 15, 2024.
Additional information
Funding
References
- Braakhuis HM, Gremmer ER, Bannuscher A, Drasler B, Keshavan S, Rothen-Rutishauser B, Birk B, Verlohner A, Landsiedel R, Meldrum K, et al. 2023. Transferability and reproducibility of exposed air-liquid interface co-culture lung models. NanoImpact. 31:100466. doi:10.1016/j.impact.2023.100466.
- Cao X, Coyle JP, Xiong R, Wang Y, Heflich RH, Ren B, Gwinn WM, Hayden P, Rojanasakul L. 2021. Invited review: human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. In Vitro Cell Dev Biol Anim. 57(2):104–132. doi:10.1007/s11626-020-00517-7.
- Dixon D, Herbert RA, Kissling GE, Brix AE, Miller RA, Maronpot RR. 2008. Apr Summary of chemically induced pulmonary lesions in the National Toxicology Program (NTP) toxicology and carcinogenesis studies. Toxicol Pathol. 36(3):428–439. doi:10.1177/0192623308315360.
- Hargrove MM, Parr-Dobrzanski B, Li L, Constant S, Wallace J, Hinderliter P, Wolf DC, Charlton A. 2021. Use of the Mucilair airway assay, a new approach methodology, for evaluating the safety and inhalation risk of agrochemicals. Appl In Vitro Toxicol. 7(2):50–60. doi:10.1089/aivt.2021.0005.
- Hayes A, Bakand S. 2010. Inhalation toxicology. In: Luch A, editor. Molecular, clinical, and environmental toxicology: volume 2: clinical toxicology. Basel: Birkhäuser Basel; p. 461–488.
- Holmström M, Wilhelmsson B, Hellquist H. 1989. Histological changes in the nasal mucosa in rats after long-term exposure to formaldehyde and wood dust. Acta Otolaryngol. 108(3–4):274–283.
- Leikauf GD. 2015. Chapter 15: “Toxic responses of the respiratory system”. In: Klaassen CD, WatkinsIIIJB, editors. Casarett & Doull’s essentials of toxicology. 3rd ed. New York: McGraw Hill.
- Meyerholz DK, Suarez CJ, Dintzis AM, Frevert CW. 2017. In: Respiratory system” comparative anatomy and histology a mouse, rat, and human atlas. 2nd ed. Treuting PM, Dintzis SM, Montine KS, editors. New York: Academic Press.
- Monticello T M, Morgan KT, Uraih L. 1990. Nonneoplastic nasal lesions in rats and mice. Environ Health Perspect. 85:249–274.
- Moreau M, Fisher J, Andersen ME, Barnwell A, Corzine S, Ranade A, McMullen PD, Slattery SD. 2022. NAM-based prediction of point-of-contact toxicity in the lung: a case example with 1,3-dichloropropene. Toxicology. 481:153340.
- Moura JA, Meldrum K, Doak SH, Clift MJD. 2023. Alternative lung cell model systems for toxicology testing strategies: current knowledge and future outlook. Semin Cell Dev Biol. 147:70–82. doi:10.1016/j.semcdb.2022.12.006.
- National Academies of Sciences, Engineering, and Medicine. 2022. New approach methods (NAMs) for human health risk assessment: proceedings of a workshop–in brief. Washington, DC: The National Academies Press. doi:10.17226/26496.
- National Research Council. 2007. Toxicity testing in the 21st century: a vision and a strategy. Washington, DC: The National Academies Press.
- OECD. 2018. Test no. 408: repeated dose 90-day oral toxicity study in rodents, OECD guidelines for the testing of chemicals, Section 4. Paris: OECD Publishing.
- Patel VS, Amin K, Wahab A, Marimoutou M, Ukishima L, Alvarez J, Battle K, Stucki AO, Clippinger AJ, Behrsing HP. 2023. Cryopreserved human precision-cut lung slices provide an immune competent pulmonary test system for "on-demand" use and long-term cultures. Toxicol Sci. 191(2):253–265. PMID: 36617185; PMCID: PMC9936202. doi:10.1093/toxsci/kfac136.
- Paudel I, Barutcu AR, Samuel R, Moreau M, Slattery SD, Scaglione J, Recio L. 2023. Increasing confidence in new approach methodologies for inhalation risk assessment with multiple end point assays using 5-day repeated exposure to 1,3-dichloropropene. Toxicology. 499:153642. doi:10.1016/j.tox.2023.153642.
- Ramanarayanan T, Szarka A, Flack S, Hinderliter P, Corley R, Charlton A, Pyles S, Wolf D. 2022. Application of a new approach method (NAM) for inhalation risk assessment. Regul Toxicol Pharmacol. 133:105216. doi:10.1016/j.yrtph.2022.105216.
- Schmeisser S, Miccoli A, von Bergen M, Berggren E, Braeuning A, Busch W, Desaintes C, Gourmelon A, Grafström R, Harrill J, et al. 2023. New approach methodologies in human regulatory toxicology - not if, but how and when!. Environ Int. 178:108082. Epub 2023 Jul 4. PMID: 37422975; PMCID: PMC10858683. doi:10.1016/j.envint.2023.108082.
- Singh AV, Romeo A, Scott K, Wagener S, Leibrock L, Laux P, Luch A, Kerkar P, Balakrishnan S, Dakua SP, et al. 2021. Emerging technologies for in vitro inhalation toxicology. Adv Healthcare Mater. 10(18):2100633. doi:10.1002/adhm.202100633.
- Stucki AO, Barton-Maclaren TS, Bhuller Y, Henriquez JE, Henry TR, Hirn C, Miller-Holt J, Nagy EG, Perron MM, Ratzlaff DE, et al. 2022. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front Toxicol. 4:964553. doi:10.3389/ftox.2022.964553.
- US EPA. 2023. Trifluoro(trifluoromethyl)oxirane order under section 4 of the toxic substances control act (TSCA) January 4, 2023. In vitro respiratory tract epithelial toxicity in primary human cell culture (see Appendix E).
- Vitucci ECM, Simmons AE, Martin EM, McCullough SD. 2024. Epithelial MAPK signaling directs endothelial NRF2 signaling and IL-8 secretion in a tri-culture model of the alveolar-microvascular interface following diesel exhaust particulate (DEP) exposure. Part Fibre Toxicol. 21(1):15. doi:10.1186/s12989-024-00576-8.