Abstract
Nasopharyngeal carcinoma (NPC) is an endemic tumor with a relatively high incidence in Southern China and Southeast Asia. Paclitaxel combination chemotherapy has been used for treatment of advanced NPC. However, treatment failure often occurs due to development of acquired paclitaxel resistance. In this study, we first established a paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines by treating the parental CNE-1, HNE-2 and 5–8F cells with increasing doses of paclitaxel for about 5 months, respectively. Then, microRNA arrays were used to screen differentially expressed miRNAs between the CNE-1/Taxol cells and the parental CNE-1 cells. We found 13 differentially expressed miRNAs, of which miR-1204 was significantly downregulated in the paclitaxel-resistant CNE-1/Taxol cells. We restored miR-1204 expression in the CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells and found that restoration of miR-1204 re-sensitized the paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells to paclitaxel both in vitro. Finally, we demonstrated that restoration of miR-1204 in significantly inhibits tumor growth in vivo. Thus, our study provides important information for the development of targeted gene therapy for reversing paclitaxel resistance in NPC.
Abbreviations
| NPC | = | nasopharyngeal carcinoma |
| EBV | = | Epstein-Barr virus |
| miRNAs | = | MicroRNAs |
| Lv-miR-1204 | = | Lentiviruses containing miR-1204 |
Introduction
Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV) strongly associated tumor, with a relatively high incidence in Southern China and Southeast Asia such as Guangdong province, Hong Kong and Singapore.Citation1,2 Fortunately, NPC cells are highly sensitive to both radiotherapy and chemotherapy. Paclitaxel is one of the widely used chemotherapeutic agents against many types of human cancers,Citation3,4 and has also been used for advanced NPC.Citation5-8 Although patients with advanced NPC benefit from paclitaxel-based combined chemotherapy, treatment failure often occurs due to development of acquired paclitaxel resistance. Thus, it is important to understand the underlying molecular mechanisms responsible for the development of paclitaxel resistance in NPC cells.
MicroRNAs (miRNAs) are a class of small non-coding RNAs that play critical roles in a variety of biological processes such as cellular proliferation, differentiation, apoptosis, metabolism and oncogenesis.Citation9 Increasing evidence has revealed that aberrant expression of specific miRNAs is closely associated with the pathologies of specific cancers.Citation10 Recently, the evidence of miRNAs involvement in drug resistance has been emerging.Citation11 On one hand, during chemotherapy, specific cancer cells initially sensitive to chemotherapy eventually develop drug resistance, accompanied by changes of specific miRNAs expression profile. For example, let-7e expression is significantly reduced in cisplatin-resistant human epithelial ovarian cancer cells.Citation12 On the other hand, some miRNAs have been found to modulate sensitivity to anticancer treatment. For example, miR-34a restoration induces sensitivity to the antitumor effect of sorafenib in human hepatocellular carcinoma cells,Citation13 while miR-125b inhibition enhances the chemosensitivity of glioblastoma stem cells to temozolomide.Citation14 Considering the variation exists in different kinds of cancers, the comprehensive high-throughput identification of miRNAs regulating cancer specific drug sensitivity has a clinical significance.
In the current study, we first established a paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell subline by treating the parental CNE-1, HNE-2 and 5–8F cells with increasing doses of paclitaxel for about 5 months, respectively. Then, miRNA microarray analysis was performed in CNE-1 cells and CNE-1/Taxol cells to reveal miRNAs associated with paclitaxel resistance in nasopharyngeal cancer cells. Among 13 differentially expressed miRNAs, miR-1204 was validated to be downregulated in the paclitaxel-resistant CNE-1/Taxol cells suggesting that loss of miR-1204 may contribute to paclitaxel resistance in nasopharyngeal cancer cells. Then, we restored miR-1204 in the paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells and investigated the role of miR-1204 in regulating paclitaxel sensitivity in nasopharyngeal cancer cells in vitro. Finally, nude mouse transplantation tumor experiment was performed to explore the effect of miR-1204 in vivo.
Results
Establishment of paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines
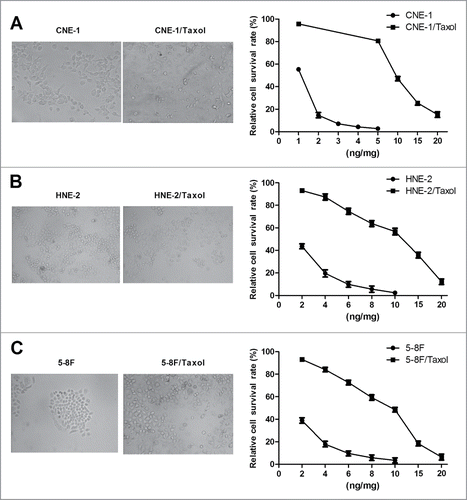
In this study, we first established paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines by treating CNE-1, HNE-2 and 5–8F cells with increasing doses of paclitaxel for about 5 months, respectively. Under an inverted microscope, although the paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells were all similar to the parental CNE-1, HNE-2 and 5–8F cells (), MTT assay indicated that the established CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells were all high resistant to paclitaxel with the IC50 value of nearly 10 ng/ml ().
Figure 1. Establishment of paclitaxol-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines. Cell morphology of the parental CNE-1, HNE-2 and 5–8F cells and paclitaxol-resistant CNE-1/Taxol (A), HNE-2/Taxol (B) and 5–8F/Taxol (C) cell sublines (left panel). Paclitaxel sensitivity was estimated by MTT assay (right panel). The established CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines were all high resistant to paclitaxel. (*P value < 0.05).
Differential expression of miRNAs in the paclitaxel-resistant CNE-1/Taxol cell subline
To profile differential expression of various miRNAs in the paclitaxel-resistant CNE-1/Taxol cell subline, we performed miRNA microarray analysis in CNE-1/Taxol cells, as well as the parental CNE-1 cells. After normalizing the expression data with bioinformatical methods, the differential miRNA expression profiles were plotted in volcano plot. We performed fold change filtering between the data for CNE-1/Taxol cells and the parental CNE-1 cells. The threshold for both the upregulated and downregulated miRNAs was at least fold2- and the intensity of the hybridization signal was at least 500. Overall, compared to the parental CNE-1 cells, 7 upregulated and 6 downregulated miRNAs were found in CNE-1/Taxol cells ().
Figure 2. Expression of miR-1204 is significantly different in the paclitaxel-resistant nasopharyngeal carcinoma cell sublines. (A) Volcano plot showing differences in the miRNAs detected by microarray for the paclitaxel-resistant CNE-1/Taxel cells (n=3 , N2, N4, N6) and the parental CNE-1 cells (n=3 , Q1, Q3, Q5). The -Log10 (p-value) is plotted against the fold change difference in expression, the vertical lines correspond to fold2- up and down, respectively, and the horizontal line represents a p-value of 0.05, so the red points in the plot represent the differentially expressed miRNAs with statistical significance. (B) Comparion of miR-1204 expression levels between CNE-1, HNE-2 and 5–8F cells and CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells. (*P value < 0.05).
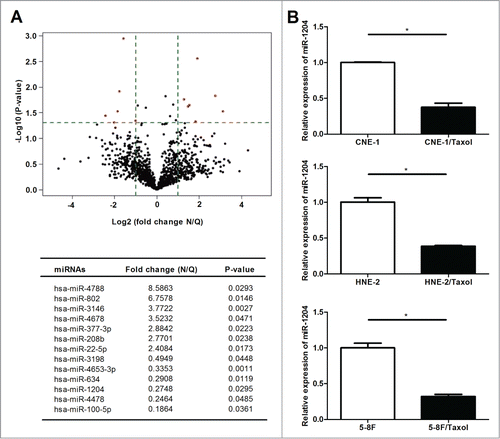
Among 13 significant differentially expressed miRNAs deteted by microarray, miR-1204 was most significantly downregulated in the paclitaxel-resistant CNE-1/Taxol cells compared with the parental CNE-1. The qRT-PCR results showed that the expression levels of miR-1204 was also remarkably lower in CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells than that in the parental CNE-1, HNE-2 and 5–8F cells (), and our qRT-PCR data confirmed the microarray data.
MiR-1204 sensitizes nasopharyngeal carcinoma cells to paclitaxel in vitro
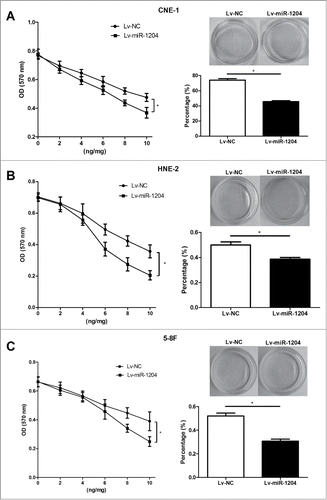
Based on this observation, we postulated that miR-1204 may play an important role of regulating paclitaxel sensitivity in NPC cells. To test this hypothesis, we exogenously up-regulated miR-1204 expression in CNE-1/Taxol , HNE-2/Taxol and 5–8F/Taxol cells via lentiviral infection and observed its impact on paclitaxel sensitivity using MTT assay and colony formation assay. As shown in , Lv-miR-1204 stably infected CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells were established, and had a significantly upregulated miR-1204 expression level compared with the negative controls. Furthermore, the drug sensitivity was determined with MTT assay at 72 h with different paclitaxel doses (0, 2, 4, 6, 8 and 10 ng/ml). Paclitaxel sensitivities were all significantly increased after forced overexpression of miR-1204 in CNE-1/Taxol cells, HNE-2/Taxol and 5–8F/Taxol compared with negative controls. Colony formation assay also revealed the similar result, in which paclitaxel (10 ng/ml) treatment for 24 h resulted in significant decreased colony formation percentages of CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells infected with Lv-miR-1204 lentivirus compared with the negative control (). Taken together, our findings suggest that miR-1204 may modulate the sensitivity to paclitaxel in NPC cells.
Figure 3. Up-regulation of miR-1204 expression in CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells was successful. The CNE-1/Taxol (A), HNE-2/Taxol (B) and 5–8F/Taxol (C) cells were infected with Lv-miR-1204 (Lv- miR-1204) or negative control (Lv-NC) labeled with GFP gene sequences, respectively. GFP expression was observed under a fluorescence microscope after being infected for 6 d (left panel). Real-time qRT-PCR was performed to determinate expression levels of miR-1204 in stably infected CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells (right panel), Lv-NC groups were taken as the control. (*P value< 0.05).
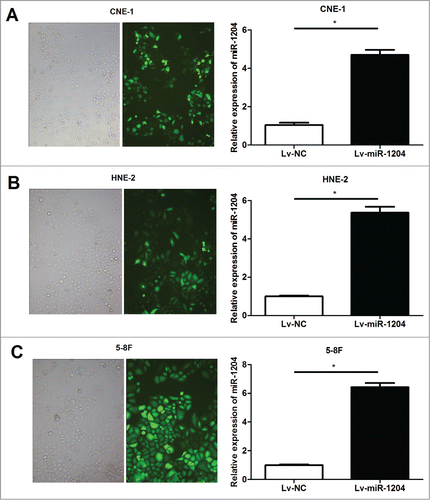
Figure 4. MiR-1204 sensitizes CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells to paclitaxel in vitro. The impacts of miR-1204 on drug sensitivity of CNE-1/Taxol (A), HNE-2/Taxol (B) and 5–8F/Taxol (C) cells at different paclitaxel doses (0, 2, 4, 6, 8 and 10 ng/ml) were determined by MTT assay. The CNE-1/Taxol (A), HNE-2/Taxol (B) and 5–8F/Taxol (C) cells were treated with a final concentration of 10 ng/ml paclitaxel for 24 h, and the impact of miR-1204 on drug sensitivity was determined by colony formation assay. (*P value < 0.05).
MiR-1204 sensitizes nasopharyngeal carcinoma cells to paclitaxel in vivo
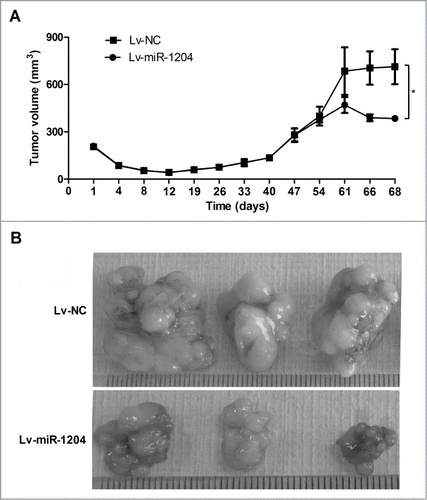
To further investigate the role of miR-1204 in regulating paclitaxel sensitivity in vivo, we injected subcutaneously Lv-miR-1204 infected CNE-1/Taxol cells, as well as the negative control Lv-miR-NC infected CNE-1/Taxol cells into 3- to 5-week female BALB/C nude mice, respectively. 61 d after CNE-1/Taxol cells injection, the mice were treated with the paclitaxel (10 mg kg-1) once a day for 5 days, and euthanized 2 d later. All mice developed palpable orthotopic tumors, and the volume of tumor was calculated. As shown in , tumor growth had no notable difference between miR-1204 overexpression group and negative control group before paclitaxel administration. Upon 5-day paclitaxel treatment, tumor growth was more significantly inhibited in miR-1204 overexpression group than that in negative control group (). Thus, ourfinding in vivo suggests that miR-1204 may enhance paclitaxel sensitivity in nasopharyngeal cancer cells.
Figure 5. MiR-1204 sensitizes CNE-1/Taxol cells to paclitaxel in vivo. BALB/C nude mice were subcutaneously inoculated with CNE-1/Taxol cells with overexpressed miR-1204 (Lv-miR-1204, n=5 ) and negative controls (Lv-miR-NC, n=5 ), respectively. After 61 days, paclitaxel (10 mg kg-1) was then intravenously injected into mice once a day for 5 d (A) Tumor volume (mm3) was calculated every 4 d (B) Two days after complete paclitaxel treatments, all mice were euthanized and the tumors were excised and imaged under a light microscope. (*P value < 0.05).
Discussion
Despite paclitaxel has been widely used either as single agent or in combination with other anticancer agents in many kinds of cancer since it was firstly approved for clinical use in advanced ovarian cancer in 1992, drug resistance is a major obstacle to successful cancer treatment, which ultimately leads to relapse and poor prognosis. Thus, it is necessary to discover specific molecules involved in paclitaxel resistance development and regulating paclitaxel sensitivity for providing important therapeutic targets. Recently, the involvement of miRNAs in developing drug resistance has been reported.Citation15-17 Some miRNAs have been reported to be involved in paclitaxel sensitivity or resistance of different cancers, such as miR-200c in endometrial cancer cells,Citation18 miR-125b in breast cancer cells,Citation19 miR-21 in glioblastoma cells,Citation20 miR-337–3p in non-small cell lung cancer cells,Citation21 and miR-34a and miR-148a in prostate cancer cells.Citation22,23 Manipulation of specific miRNA expression, upregulated by miRNA mimics, or silenced by miRNA inhibitor mimics, has been reported to alter drug sensitivity of cancer cells. For example, Ren, et al. reported the miR-21 inhibitor could enhance the chemosensitivity of human glioblastoma cells to taxol, suggesting a combination of miR-21 inhibitor and taxol could be an effective therapeutic strategy for controlling the growth of glioblastoma cancer.Citation20 Thus miRNAs represent a promising tool to overcome cancer drug resistance.
In the present study, to discover paclitaxel resistance associated miRNAs in PNC cells, we first established a paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell subline by treating the parental CNE-1, HNE-2 and 5–8F cells with increasing doses of paclitaxel for about 5 months, respectively. Using miRNA microarray, we identified 13 differentially expressed miRNAs in acquired paclitaxel-resistant CNE-1/Taxol cells. These miRNAs probably play a crucial role in the development of paclitaxel resistance in nasopharyngeal carcinoma cells.
Among these miRNAs, miR-1204 was most significantly down-regulated. MiR-1204 is one of the 6 annotated miRNAs of the non-protein coding PVT1 locus,Citation24 which can be stimulated by p53, a prominent tumor suppressor gene.Citation25 Besides a potential role of miR-1204 in cell differentiation and senescence,Citation24,26 recently, Bisio, et al. has reported that miR-1204 is one of p53 target miRNAs, and ectopic expression of miR-1204 leads to increased p53 levels and causes cell death in a partially p53-dependent manner.Citation27 The limited published data indicate that miR-1204 might function as an important regulator. Given the strong association of loss of miR-1204 expression and developing paclitaxel resistance in NPC cells, we performed a further investigation for the role of miR-1204 in regulating paclitaxel sensitivity in NPC cells in vitro and in vivo. Here, we found that re-expression of miR-1204 in the paclitaxel-resistant CNE-1/Taxol cells by lentiviral infection inhibited cell proliferation as well as colony formation, thus re-sensitized paclitaxel-resistant CNE-1/Taxol cells to paclitaxel in vitro. Similar results were also found in another 2 NPC cell lines (HNE-2 and 5–8F). In xenograft mouse model, re-expression of miR-1204 in CNE-1/Taxol cells had no marked effect on tumor growth before paclitaxel treatment. However, tumor growth was more significantly inhibited in miR-1204 re-expression group than that in negative control group upon paclitaxel treatment. Taken together, our data suggest that miR-1204 may regulate paclitaxel sensitivity of NPC cells both in vitro and in vivo, and a combination of miR-1204 and paclitaxel may be an effective therapeutic strategy for overcoming drug resistance during paclitaxel based clinical therapy.
In summary, to our knowledge, this study is the first genome-wide screen for aberrantly expressed miRNAs involved in paclitaxel resistance development in nasopharyngeal carcinoma cells. Paclitaxel down-regulates miR-1204 expression and restored miR-1204 consequently enhances the sensitivity to paclitaxel in nasopharyngeal carcinoma cells in vitro and in vivo. MiR-1204 might be a therapeutic target in paclitaxel-resistant nasopharyngeal carcinoma.
Materials and Methods
Cell line and culture
The human nasopharyngeal carcinoma CNE-1, HNE-2 and 5–8F cell Lines were obtained from Cancer Research Institute of Central South University. Cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin (Life Technologies, USA) in a humidified incubator at 37℃ with 5% CO2.
Generation of paclitaxel-resistant CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines
The paclitaxel-resistant nasopharyngeal carcinoma CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines were established by exposing CNE-1, HNE-2 and 5–8F cells to increased concentrations of paclitaxel (Cytoskeleton, USA) as previous described.Citation28 Briefly, cells were inoculated in a 10-ml cell culture flask and cultivated for 24 h in culture medium containing a low concentration of paclitaxel (0.1ng/ml). Subsequently, cells were continuously cultured without paclitaxel exposure until cell growth was in the logarithmic phase. Then, cells were collected and re-inoculated in a 10-ml culture flask in culture medium containing an elevated concentration (1.5- to fold2- of the previous dose) or at a previous concentration. This procedure was repeated until the cells exhibited stable growth and proliferation in a culture medium with 40ng/ml paclitaxel. A period of about 5 months was required to establish CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cell sublines. The level of drug resistance was determined using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay.
Paclitaxel sensitivity MTT assay
Exponentially growing parental NPC cells and paclitaxel-resistant CNE-1/Taxol, , HNE-2/Taxol and 5–8F/Taxol cells were seeded at 10,000 cells (100 μl culture medium) per well in 96-well plates and incubated for 12 h. The cells were then exposed to different concentrations of paclitaxel for 72 h, then 20 μl of MTT (Sigma Chemicals, St. Louis, MO, USA; 5 mg/ml in PBS) was added to each well, and the cells were cultured for an additional 4 h. Subsequently, 200 μl of DMSO was added to each well to dissolve the crystals. The values of the optical density at 570 nm were then measured using a microplate ELISA reader. Paclitaxel sensitivity were estimated by the IC50 value (paclitaxel concentration resulting in 50% reduction in absorbance compared with the control).
MiRNA microarray analysis
The parental CNE-1 cells (n=3 , Q1, Q3, Q5) and corresponding established paclitaxel-resistant CNE-1/Taxol cells (n=3 , N2, N4, N6) were sent to KangChen Bio-tech company (Shanghai, China) for miRNA isolation, quality control, chip hybridization, and microarray data analysis. In KangChen Bio-tech company, the samples were labeled using the miRCURY™ Hy3™/Hy5™ Power labeling kit and hybridized on the miRCURY™ LNA Array (version 18.0) which contains 3100 capture probes, covering all human microRNAs annotated in miRBase 18.0. Following the washing steps, the slides were scanned by the Agilent Scanner G2505C and scanned images were then imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. Replicated miRNAs were averaged and miRNAs with more than 50 intensities in all samples were chosen for calculating normalization factor. Expressed data were normalized using the median normalization. After normalization, significant differentially expressed miRNAs were identified through Volcano Plot filtering.
Quantitative reverse transcribed PCR (qRT-PCR)
Total RNAs from cells were extracted using Trizol (Invitrogen, USA) according to the manufacturer's instruction. About 500 ng of total RNAs was reversely transcribed into cDNA using the Primer Script RT reagent Kit (TaKaRa Bio, Japan) according to the manufacturer's instructions. Real-time qRT-PCR was performed on ABI 7500 Sequence Detection System (Life Technologies, USA) using SYBR Green real-time PCR master mix (Toyobo Co., Japan) with a primer concentration of 200 nM under the conditions of 95℃ for 1 min, followed by 40 cycles of 95℃ for 15 sec, 60℃ for 15 sec, 72℃ for 20 sec. The small nuclear U6 was used as internal control. The specific primers for miRNA-1024 and U6 were purchased from Guangzhou RiboBio (Guangzhou RiboBio Co., Ltd., Guangzhou, China). All experiments were performed in triplicate. Relative expression levels were calculated using the 2-ΔΔCt method.
Lentiviral stable infection
Lentiviruses containing miR-1204 (Lv-miR-1204) and negative control (Lv-NC) were purchased from GeneChem Company (Shanghai, China). To get stably infected CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells, the cells were cultured to about 70% of the plates, and then added by a concentration of 1.0 × 104 TU/well Lv-miR-1204 or negative control lentivirus. Real-time qRT-PCR was performed to determinate expression levels of miR-1204 after being infected for 6 d The stably infected CNE-1/Taxol, HNE-2/Taxol and 5–8F/Taxol cells were expanded and harvested for further experiments.
Colony formation assay
For the colony formation assay, Five hundred Lv-miR-1204 or Lv-NC stably infected CNE-1/Taxol cells were placed in complete growth media in each 35 mm dish and allowed to grow for 6 h. A final concentration of 10 ng/ml paclitaxel was then added to each dish. After 24 h treatment, paclitaxel was removed by adding fresh complete growth media, and cells were allowed to grow until visible colonies formed (2 weeks). Cell colonies were fixed with methanol, stained with Giemsa, washed, air dried, photographed and counted.
Animal treatments
Female nude mice of 3–5 weeks old, 17.9 ± 0.82 g in weight, were purchased from Shanghai Laboratory Animal Center (SLAC, Shanghai, China), and maintained under specific pathogen-free conditions. The parental CNE-1 cells and paclitaxel-resistant CNE-1/Taxol cells overexpressing miR-1204 were harvested, re-suspended in serum-free medium, and 1000,000 cells in 200 μl of cell suspension were injected into the proximal tibia of each anesthetized nude mice (n = 5 animals per group). Every 4 d post inoculation, individual orthotopic tumor from each mouse was measured with calipers, and the volume (mm3) of orthotopic tumor was calculated according to the formula: 1/2 × length x width2. 61 d after inoculation, the mice developed palpable tumors, the paclitaxel (10 mg kg-1) was intraperitoneally injected once a day for 5 d Two days after complete paclitaxel treatments, all of the mice were euthanized and the tumors were excised and imaged under a light microscope.
Statistical analysis
The experiments were repeated at least 3 times, and the data are shown as the mean ± SD. Student's t-test was used to analyze the differences in the experiments. Statistical analyses were performed using the SPSS 11.0 software, and P<0.05 was considered to indicate a statistically significant difference.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81372140, 81301688, 81272192, 81171882); Ph.D. Programs Foundation of Ministry of Education of China (No. 20130162110050 and 20130162120093); Post-doctoral Foundation of Central South University (No. 131425); China Postdoctoral Science Foundation (2014M552167); Natural Science Foundation of Hunan Province (Grant No. 12JJ4088); Technology Project of Hunan Province (2012SK3229 and 2013FJ6003); Research foundation of Health Department of Hunan Province ( B2012–100);125 Talent Project of the Third Xiangya Hospital of Central South University.
References
- Fahraeus R, Fu HL, Ernberg I, Finke J, Rowe M, Klein G, Falk K, Nilsson E, Yadav M, Busson P, et al. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer 1988; 42:329-38; PMID:2843473; http://dx.doi.org/10.1002/ijc.2910420305
- Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a "Cantonese cancer"? Chin J Cancer 2010; 29:517-26; PMID:20426903; http://dx.doi.org/10.5732/cjc.009.10329
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4:253-65; PMID:15057285; http://dx.doi.org/10.1038/nrc1317
- Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Ann Rev Med 1997; 48:353-74; PMID:9046968; http://dx.doi.org/10.1146/annurev.med.48.1.353
- He XY, Hu CS, Ying HM, Wu YR, Zhu GP, Liu TF. Paclitaxel with cisplatin in concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma. Eur Arch Oto-Rhino-Laryngol 2010; 267:773-8; PMID:19820959; http://dx.doi.org/10.1007/s00405-009-1112-7
- Leong SS, Wee J, Rajan S, Toh CK, Lim WT, Hee SW, Tay MH, Poon D, Tan EH. Triplet combination of gemcitabine, paclitaxel, and carboplatin followed by maintenance 5-fluorouracil and folinic acid in patients with metastatic nasopharyngeal carcinoma. Cancer 2008; 113:1332-7; PMID:18615622; http://dx.doi.org/10.1002/cncr.23687
- Leong SS, Wee J, Tay MH, Toh CK, Tan SB, Thng CH, Foo KF, Lim WT, Tan T, Tan EH. Paclitaxel, carboplatin, and gemcitabine in metastatic nasopharyngeal carcinoma: a Phase II trial using a triplet combination. Cancer 2005; 103:569-75; PMID:15611975; http://dx.doi.org/10.1002/cncr.20804
- Tan EH, Khoo KS, Wee J, Fong KW, Lee KS, Lee KM, Chua ET, Tan T, Khoo-Tan HS, Yang TL, et al. Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol 1999; 10:235-7; PMID:10093695; http://dx.doi.org/10.1023/A:1008390929826
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281-97; PMID:14744438; http://dx.doi.org/10.1016/S0092-8674(04)00045-5
- Li M, Li J, Ding X, He M, Cheng SY. microRNA and cancer. AAPS J 2010; 12:309-17; PMID:20422339; http://dx.doi.org/10.1208/s12248-010-9194-0
- Raza U, Zhang JD, Sahin O. MicroRNAs: master regulators of drug resistance, stemness, and metastasis. J Mol Med 2014; 92:321-36; PMID:24509937; http://dx.doi.org/10.1007/s00109-014-1129-2
- Cai J, Yang C, Yang Q, Ding H, Jia J, Guo J, Wang J, Wang Z. Deregulation of let-7e in epithelial ovarian cancer promotes the development of resistance to cisplatin. Oncogenesis 2013; 2:e75; PMID:24100610; http://dx.doi.org/10.1038/oncsis.2013.39
- Yang F, Li QJ, Gong ZB, Zhou L, You N, Wang S, Li XL, Li JJ, An JZ, Wang DS, et al. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol Cancer Res Treat 2014; 13:77-86; PMID:23862748
- Chen J, Fu X, Wan Y, Wang Z, Jiang D, Shi L. miR-125b inhibitor enhance the chemosensitivity of glioblastoma stem cells to temozolomide by targeting Bak1. Tumour Biol 2014; 35(7):6293-302; PMID:24643683; http://dx.doi.org/10.1007/s13277-014-1821-4
- Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen J, Su F, Yao H, Song E. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem 2011; 286:19127-37; PMID:21471222; http://dx.doi.org/10.1074/jbc.M110.216887
- Holleman A, Chung I, Olsen RR, Kwak B, Mizokami A, Saijo N, Parissenti A, Duan Z, Voest EE, Zetter BR. miR-135a contributes to paclitaxel resistance in tumor cells both in vitro and in vivo. Oncogene 2011; 30:4386-98; PMID:21552288; http://dx.doi.org/10.1038/onc.2011.148
- Shen Y, Wang P, Li Y, Ye F, Wang F, Wan X, Cheng X, Lu W, Xie X. miR-375 is upregulated in acquired paclitaxel resistance in cervical cancer. British J Cancer 2013; 109:92-9; PMID:23778521; http://dx.doi.org/10.1038/bjc.2013.308
- Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther 2009; 8:1055-66; PMID:19435871; http://dx.doi.org/10.1158/1535-7163.MCT-08-1046
- Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi Y, Xiong W, Li G, Lu J, Fodstad O, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem 2010; 285:21496-507; PMID:20460378; http://dx.doi.org/10.1074/jbc.M109.083337
- Ren Y, Zhou X, Mei M, Yuan XB, Han L, Wang GX, Jia ZF, Xu P, Pu PY, Kang CS. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer 2010; 10:27; PMID:20113523; http://dx.doi.org/10.1186/1471-2407-10-27
- Du L, Subauste MC, DeSevo C, Zhao Z, Baker M, Borkowski R, Schageman JJ, Greer R, Yang CR, Suraokar M, et al. miR-337-3p and its targets STAT3 and RAP1A modulate taxane sensitivity in non-small cell lung cancers. PloS one 2012; 7:e39167; PMID:22723956; http://dx.doi.org/10.1371/journal.pone.0039167
- Fujita Y, Kojima K, Ohhashi R, Hamada N, Nozawa Y, Kitamoto A, Sato A, Kondo S, Kojima T, Deguchi T, et al. MiR-148a attenuates paclitaxel resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem 2010; 285:19076-84; PMID:20406806; http://dx.doi.org/10.1074/jbc.M109.079525
- Singh S, Chitkara D, Mehrazin R, Behrman SW, Wake RW, Mahato RI. Chemoresistance in prostate cancer cells is regulated by miRNAs and Hedgehog pathway. PloS one 2012; 7:e40021; PMID:22768203; http://dx.doi.org/10.1371/journal.pone.0040021
- Huppi K, Volfovsky N, Runfola T, Jones TL, Mackiewicz M, Martin SE, Mushinski JF, Stephens R, Caplen NJ. The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res 2008; 6:212-21; PMID:18314482; http://dx.doi.org/10.1158/1541-7786.MCR-07-0105
- Barsotti AM, Beckerman R, Laptenko O, Huppi K, Caplen NJ, Prives C. p53-Dependent induction of PVT1 and miR-1204. J Biol Chem 2012; 287:2509-19; PMID:22110125; http://dx.doi.org/10.1074/jbc.M111.322875
- Marasa BS, Srikantan S, Martindale JL, Kim MM, Lee EK, Gorospe M, Abdelmohsen K. MicroRNA profiling in human diploid fibroblasts uncovers miR-519 role in replicative senescence. Aging 2010; 2:333-43; PMID:20606251
- Bisio A, De Sanctis V, Del Vescovo V, Denti MA, Jegga AG, Inga A, Ciribilli Y. Identification of new p53 target microRNAs by bioinformatics and functional analysis. BMC Cancer 2013; 13:552; PMID:24256616; http://dx.doi.org/10.1186/1471-2407-13-552
- Peng X, Li W, Tan G. Reversal of taxol resistance by cisplatin in nasopharyngeal carcinoma by upregulating thromspondin-1 expression. Anti-Cancer Drugs 2010; 21:381-8; PMID:20051827; http://dx.doi.org/10.1097/CAD.0b013e3283363980
