Dear Editor,
We read with great interest the recently published overview on the potential role of bevacizumab in cervical cancer and glioblastoma.Citation1 Results of the GOG 240 trial, demonstrating a significant survival advantage of adding the biological drug bevacizumab to standard chemotherapy in advanced cervical cancer have been recently published.Citation2 The trial has led to the FDA approval of bevacizumab for the treatment of advanced cervical cancer. In an era when targeted pharmacology and personalized medicine are literally ‘exploding’ in parallel with the cost needed to afford an adequate standard of care for patients with metastatic solid tumors, we believe that exploring alternative ways to quantify the real benefit of novel molecularly targeted (though costly) agents is of extreme value, especially if insights into possible predictive biomarkers are provided.
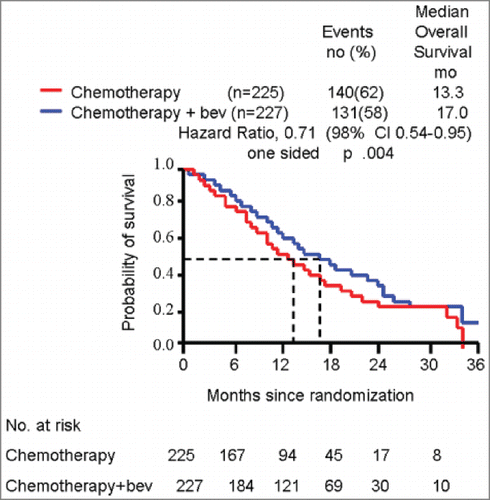
In the paper by Tewari et al.,Citation2 an increase in the median overall survival from 13 to 17 months (+4 months) was observed for bevacizumab-treated patients (, figures schematically depict the results published in the original paper by Tewari et al). However, simply looking at the figure shape, it is evident that the gain achieved with the new drug is produced around the 8th–10th month when the curves start separating and the ‘widest’ divergence is reached, quantifiable in an absolute increase of +10% of patients not dying with the bevacizumab administration and dying with standard chemotherapy. This ‘survival gap’ remains almost constant in the following months, meaning that the ‘pace’ at which deaths occur in this phase of the therapy is similar with and without bevacizumab. The curve divergence disappears around the 26th–28th month when more patients in the bevacizumab arm die in comparison to the standard treatment, and the 10% gain produced in the 8th–10th month is lost (). A provocative question arises: Is it completely incorrect to say that the real, net benefit of bevacizumab in this disease setting is actually to donate 18 months of life to 10% of patients with metastatic cervical cancer destined to die around the 8th-10th month? We acknowledge the simplicity of our dissertation and the criticisms linked to an ‘approximate’ examination of the data and the ‘absolute’ counting, instead of sophisticated and corrected statistical analysis. However, this simple way of drawing attention to this aspect of the results could, in our opinion, raise interesting issues: does it make sense to focus retrospectively (post-hoc), even at a molecular level (i.e. tumor tissue gene expression and proteomic profile), on patients dying around the 8th–10th month in the standard treatment arm and around the 26th–28th month in the bevacizumab arm to identify reliable predictive biomarkers of bevacizumab efficacy? We expect that the ‘perfect’ biomarker of bevacizumab activity in metastatic cervical cancer would be present in only 10% of patients!
Figure 2. survival curves schematically representing original results of GOG 240 trial with hypothetic quantification of real benefit from bevacizumab addition.
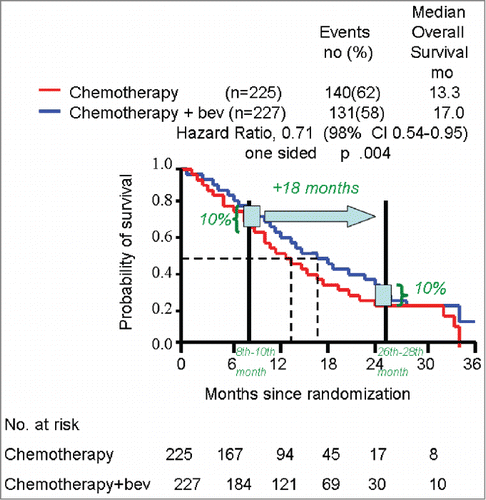
As human beings are destined to die sooner or later, there is always a time when the number of patients ‘saved’ earlier on by a given drug would pass away. Therefore, our ‘death pace’ point of view and biomarker discovery approach are potentially applicable to all randomized clinical trials involving cancer patients. We think that in an era where the cost of care in oncology is rising disproportionately and cost-effectiveness analyses are becoming an urgent need for regulatory agencies, a way to simplify the net benefit of new expensive targeted drugs is desirable as well as potentially helpful in orienting biomarker discovery.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Goey AK, Figg WD. Potential novel role of bevacizumab in glioblastoma and cervical cancer. Cancer Biol Ther 2014 Oct; 15(10):1296-8; Epub 2014 Jul 21; PMID:25046485; http://dx.doi.org/10.4161/cbt.29926
- Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014; 370:734-43; PMID:24552320; http://dx.doi.org/10.1056/NEJMoa1309748