Abstract
In order to use endothelial progenitor cells (EPCs) as a therapeutic and imaging probe to overcome antiangiogenic resistance for gliomas, how to enhance proliferation and targeting ability of transplanted EPCs is a high priority. Here, we confirmed, for the first time, the expression of P2X7 receptors in rat spleen-derived EPCs. Activation of P2X7 receptors in EPCs by BzATP promoted cells proliferation and migration, rather than apoptosis. In vivo, the homing of transplanted EPCs after long-term suppression of P2X7 receptors by persistent BBG stimulation was evaluated by MRI, immunohistochemistry and flow cytometry. Compared to the group without BBG treatment, less transplanted EPCs homed to gliomas in the group with BBG treatment, especially integrated into the vessels containing tumor-derived endothelial cells in gliomas. Moreover, western blot showed that CXCL1 expression was downregulated in gliomas with BBG treatment, which meant P2X7 receptors suppression inhibited the homing of EPCs to gliomas through down-regulation of CXCLl expression. Further, effects of P2X7 receptors on C6 glioma cells or gliomas were evaluated at the same dose of BzATP or BBG used in EPCs experiments in vitro and in vivo. MTT assay and MRI revealed that P2X7 receptors exerted no significant promoting effect on C6 glioma cells proliferation, gliomas growth and angiogenesis. Taken together, our findings imply the possibility of promoting proliferation and targeting ability of transplanted EPCs to brain gliomas in vivo through P2X7 receptors, which may provide new perspectives on application of EPCs as a therapeutic and imaging probe to overcome antiangiogenic resistance for gliomas.
Abbreviations
| DCE | = | dynamic contrast enhanced |
| ECs | = | endothelial cells |
| EPCs | = | endothelial progenitor cells |
| GSCs | = | glioma stem-like cells |
| HSCs | = | hematopoietic stem cells |
| MRI | = | magnetic resonance imaging |
| SDF-1α | = | Stromal derived factor 1α |
| VEGF | = | vascular endothelial growth factor |
Introduction
Endothelial progenitor cells (EPCs), a subpopulation of pluripotent haematopoietic stem cells (HSCs), have high proliferation potential and can differentiate to mature endothelial cells (ECs).Citation1 A large body of literature has substantiated that EPCs could migrate actively to gliomas,Citation2-4 incorporating directly into the neovasculature with high specificity, which is referred as vasculogenesis.Citation5-7 Recently, emerging evidence has convincingly demonstrated that exogenous EPCs integrated into the vessels containing the tumor-derived ECs,Citation8 which may be one of mechanisms proposed to explain resistance to anti-VEGF therapy.Citation9-11 Based on that, EPCs may be a best vehicle to deliver the therapeutic genes, targeting tumor-derived ECs more directly.
In order to evaluate efficacy and appropriateness of cell based therapy, it is critically important to track the migration, localization, engraftment efficiency, and functional capability of EPCs following transplantation in vivo. Magnetic resonance imaging (MRI) can be used both to non-invasively follow dynamic spatio-temporal patterns of the EPCs targeting allowing for the optimization of treatment strategies and to assess efficacy of the therapy.Citation12 However, our previous study found that with the extension of time, MRI failed to clearly detect the transplanted EPCs. The lower concentration of USPIO caused by the death of EPCs may be responsible for that. Therefore, how to maintain these transplanted cells activity in vivo still pose a great challenge for their implantation as a targeting probe.
Nowadays, CXCL12 is considered to play an important role in the mobilization and recruitment of EPCs, with high expression of CXCR4, to gliomas.Citation13,14 However, glioma stem-like cells (GSCs) were also reported to highly express CXCR4.Citation15,16 If we increased EPCs homing to gliomas by elevating the level of CXCL12, it could promote GSC-initiated glioma growth and angiogenesis by stimulating VEGF production.Citation17 Therefore, it is suggested that searching for new molecules that can regulate EPCs proliferation and migration without promoting glioma cells proliferation will be an important target. P2X7 receptors, a unique family of extracellular ATP-activated plasma membrane ion channels, express in cells of the haematopoietic lineage, epithelium and endothelium.Citation18,19 The formation of cellular prolongations and cell migration are found to be dramatically increased in the context of P2X7 receptors stimulation through the activation of Ca2+-activated potassium channels SK3.Citation20 It is proposed that low levels of ATP in the extracellular milieu lead to the basal activation of P2X7 receptors that couple to signaling pathways to promote cell survival.Citation21 Meanwhile, high concentration of extracellular ATP, the endogenous ligand of P2X7 receptors, was reported in gliomas.Citation22 Therefore, it is possible that EPCs express P2X7 receptors, activation of which could promote the proliferation and targeting ability of EPCs. However, few studies have been investigated the functional expression of P2X7 receptors in EPCs, the role of P2X7 receptors in EPCs proliferation still remains unknown.
In the present study, we found the previously unreported expression of P2X7 receptors in rat spleen-derived EPCs. Activation of P2X7 receptors could enhance the proliferation and migration of EPCs, with no promoting effect on C6 glioma cells. Moreover, inhibition P2X7 receptors through using antagonist of P2X7 receptors could suppress the homing of EPCs to gliomas, especially integrating into the vessels containing the tumor-derived ECs in gliomas. Our work may provide useful support for the possibility of promoting proliferation and targeting ability of transplanted EPCs, to be a therapeutic and imaging probe, to brain gliomas in vivo through P2X7 receptors.
Results
Identification of EPCs
To identify the characteristics of EPCs, immunocytochemistry was performed to detect the surface markers and function of EPCs. EPCs were found to express high amount of CD34, CD31, vWF and Flk1 (). Most adherent cells showed uptake of DiI-acLDL and binding of FITC-UEA-1 ().
Figure 1. Identifying the characteristics of rat spleen-derived endothelial progenitor cells (EPCs). (A–D) Representative images of the markers on rat spleen-derived EPCs. (A) for CD34 (red); (B) for CD31 (green); (C) for vWF (red); (D) for Flk1 (red). Scale bar: 25 μm. (E) Representative images of EPCs uptake of DiI-acLDL and binding of FITC-UEA-1. Scale bar: 100 μm.
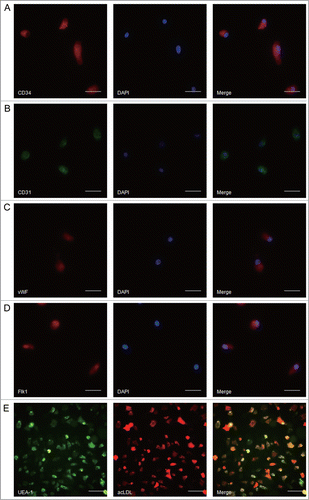
P2X7 receptors expressed in rat spleen-derived EPCs
To examine the expression of P2X7 receptors in rat spleen-derived EPCs, immunofluorescent staining was carried out using monoclonal antibody against P2X7 receptors. Representative results were presented in , which revealed the location of P2X7 receptors in the cytoplasm of EPCs. Next, to further validate the finding of immunofluorescent staining and find out whether the P2X7 receptors expression is changing in time, P2X7 receptors protein of EPCs at different cultured time were determined by Western blot assay. As shown in , P2X7 receptors protein were presented in EPCs and the 7-days cultured EPCs had the highest immunoreactivity for P2X7 receptors.
Figure 2. P2X7 receptors expressed in rat spleen-derived EPCs. (A) The P2X7 receptors located in the cytoplasm of EPCs. Scale bar: 25 μm. (B) The protein expression of P2X7 receptors in EPCs with different cultured time. Bars represent mean ± SD of the quantitated bands independently obtained in triplicate.##P < 0.01 vs. 5 days or 3 days. **P < 0.01 vs. 3 days.
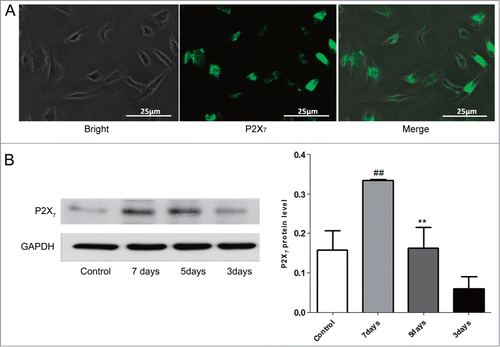
P2X7 receptors activation elevating proliferation rather than apoptosis in EPCs, without promoting proliferation of C6 glioma cells
P2X7 receptors are related to ATP-induced cell death in various cell types through mechanism that involves apoptotic features including phosphatidylserine (PS) externalization and shrinking.Citation23,24 In the present study, the apoptosis of EPCs treated with or without BzATP, an agonist of P2X7 receptors, were examined using flow cytometry and Western blot assay. As shown in , although there were more PI or Annexin-V staining positive cells in the presence of BzATP, the difference between the groups were not significant. Moreover, Western blot analysis revealed that stimulation of EPCs with BzATP had no effect on increasing the expression of caspase-3 protein (). These findings indicated that P2X7 receptors activation could not trigger apoptosis of EPCs. Next, MTT assay was used to determine the effect of P2X7 receptors on the proliferation of EPCs. Interestingly, the results showed that BzATP markedly increased the absorbance compared with untreated EPCs. This suggested that P2X7 receptors activation could promote the growth of EPCs. In addition, treatment of EPCs using BBG, an antagonist of P2X7 receptors, along with BzATP significantly reversed the P2X7 receptors activation – induced cell proliferation ().
Figure 3. Effects of P2X7 receptors activation on the apoptosis, proliferation and migration of EPCs and proliferation of C6 glioma cells. (A) Flow cytometry of apoptotic cells in EPCs cultured with or without P2X7 receptors agonist (BzATP) in the presence or absence of P2X7 receptors antagonist (BBG). Values are presented as mean ± SD from 6 separate experiments. (B) Western blot analysis of caspase-3 expression in EPCs with or without BzATP treatment in the presence or absence of BBG. GAPDH blot serves as loading control. Values are presented as mean ± SD from 6 separate experiments. (C) Proliferation of EPCs cultured with or without BzATP in the presence or absence of BBG was measured using MTT assay. Values are presented as mean ± SD from 6 separate experiments.##P < 0.01 vs. BzATP. (D) Proliferation of C6 glioma cells cultured with or without BzATP in the presence or absence of BBG was measured using MTT assay. Values are presented as mean ± SD from 6 separate experiments. (E) EPCs were cultured with or without BzATP in the presence or absence of BBG. Then, transwell migration assay was applied to detect the migratory cells in different groups. Quantification of migration was expressed as the number of migrating cells per high-power field (HPF; ċ20; bottom). Values are presented as mean ± SD from 6 separate experiments.##P < 0.01 vs. Control or BzATP + BBG.
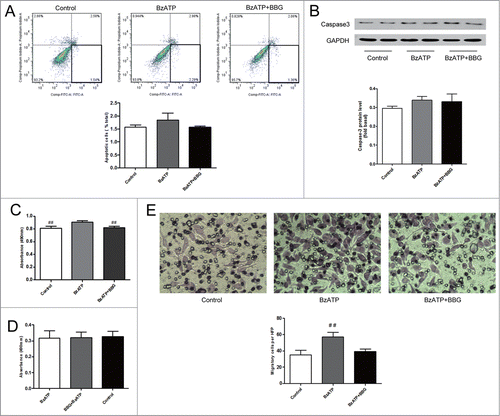
To study the role of P2X7 receptors in C6 glioma cells proliferation, we exploited MTT to monitor the cells proliferation. As shown in , MTT assay demonstrated that lower absorbance of cells with BzATP treatment in the absence or presence of BBG than the control, but the difference among these 3 groups was not significant. This suggested that activation of P2X7 receptors in C6 glioma cells could not promote cells proliferation.
Activation of P2X7 receptors in EPCs enhancing cell migration
To verify the role of P2X7 receptor in the migration of EPCs, BzATP, was used to treat EPCs. The migration of EPCs with or without BzATP treatment was investigated using transwell migration assay. In addition, the migration of BzATP treated EPCs in the presence or absence of BBG was also observed. After 24 h incubation, a few migrated EPCs were observed in control group (cultured with serum-free medium).While, the number of migrated cells in BzATP treated group was much more than the control, which was increased about 63%. This BzATP-induced migration was partially inversed in the presence of BBG (), suggesting that P2X7 receptors may play an important role in EPCs migration.
Suppression of P2X7 receptors inhibiting homing of exogenous EPCs to gliomas
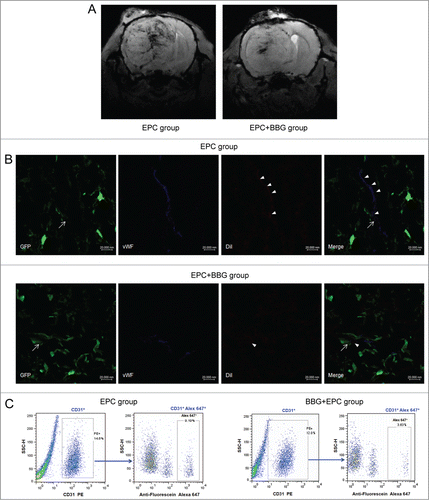
Before EPCs transplantation, EPCs were labeled with fluorescein and USPIO which were confirmed by Prussian blue staining. As shown in , USPIO-labeled EPCs showed blue iron particle in the cytoplasm. All the rats were performed MR scanning at 10 d post-injection of C6 glioma cells to verify the growth of gliomas, which showed circular mass with high intensity in T2-weighted imaging (T2WI) and obvious contrast enhancement. To track the distribution of USPIO-EPCs, the rats were performed MR examination at 1, 3, 5 d after EPCs transplantation. Immediately at 1 day after EPCs transplantation, T2WI detected the USPIO-labeled EPCs which exhibited dotted or patchy low-intensity at the periphery of tumor (). To quantify the USPIO-labeled EPCs homing to gliomas in vivo, T2 maps were performed (), which was reported the linear correlation between the number of labeled cells detected by flow cytometry and corresponding △R2 value in T2 maps.Citation8 Signal intensity on the T2WI in EPC group was lower than that in EPC + BBG group each time point after transplantation, with significant difference ().
Figure 4. P2X7 receptors suppression inhibited the homing of USPIO-labeled EPCs to gliomas. (A) Representative imaging of in vitro Prussian blue staining revealing the morphology of USPIO-labeled EPCs. Scale bar: 50 μm (left), 25 μm (right). (B) Coronal T2-weighted imaging (T2WI) showing the USPIO-labeled EPCs (yellow arrow). (C) Representative T2 maps of glioma-bearing rats in EPC group and EPC + BBG group. Hypointensity accumulated in the region of USPIO-labeled EPCs. Red and yellow represent relative higher- and lower-value, respectively. (D) Changes of signal intensity on T2WI in EPC group and EPC + BBG group were analyzed. Data are mean ± SD from 6 independent experiments. **, P < 0.01. (E) Representative Prussian blue staining revealed the accumulation of USPIO-labeled EPCs at the periphery of gliomas (black arrows; left). The blue-stained cells were quantified (right).##P < 0.01 vs. Control. (F) The relationship between host macrophages and iron-positive cells. Multiple localized host macrophages (F4/80-positive cells, dotted ring) were observed (left up). However, the area of macrophage accumulation did not correspond to the site of incorporated iron-positive cells (arrows). No iron-positive cells are seen at the site of macrophage migration (right up), and no host macrophages (left down) are seen at the site of iron-positive cells (right down; arrows). Scale bar: 100 μm.
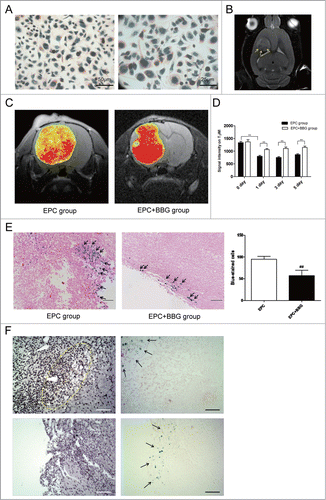
To verify the findings of MRI, histopathological analysis was performed. Prussian blue staining demonstrated that numerous iron-positive cells were found in the tumor tissue, mainly located at the periphery of the tumor (). Quantification of the blue-stained cells in glioma tissue found that BBG treatment notablely decreased the number of cells positive for Prussian blue staining (). Moreover, corresponding consecutive sections revealed none of the host macrophages (rF4/80-positive cells) infiltrating into the large tumors was positive for iron oxide nanoparticles on Prussian blue staining () which indicated that these iron-positive cells were USPIO-labeled EPCs, not host macrophages. Taken together, these findings suggest that suppression of P2X7 receptors may inhibit the homing of EPCs to gliomas.
P2X7 receptors modulating exogenous EPCs integrating into the vessels containing the tumor-derived ECs
To further study of the magnetically labeled EPCs integrating into the vessels in gliomas, SWI sequence was performed. SWI, a highly sensitive way of identifying iron storage,Citation25 can be used to depict both cerebral veins and arteries.Citation26 In EPC group, the hypointensity was obvious along the vessels on SWI, which showed more hyperintensity than that in EPC + BBG group (). Consistent with the MRI findings, immunofluorescence showed that more exogenous EPCs integrating into the vessels containing the tumor-derived ECs in EPC group than that in EPC + BBG treated group (). In addition, flow cytometry was performed to detect the presence of EPCs integration into the tumor vessels. About 8.06% of total ECs in tumor were positive for Alexa Fluor 647 in EPC group, some of which integrated into the vessels containing the tumor-derived ECs. While, about 3.45% of total ECs in tumor were positive for Alexa Fluor 647 in EPC + BBG group (). These results suggested that P2X7 receptors could modulate exogenous EPCs integrating into the vessels containing the tumor-derived ECs.
Figure 5. P2X7 receptors modulated exogenous EPCs integrating into the vessels containing the tumor-derived endothelial cells (ECs). (A) Representative susceptibility-weighted imaging (SWI) of glioma-bearing rats in EPC group or EPC +BBG group. Hypointensity accumulated in the region of rich-vessels. (B) Representative images of exogenous EPCs, tumor-derived ECs and regular ECs lined the vessel lumen in gliomas of groups with or without BBG treatment. EPCs (arrowheads) were labeled with DiI (red) but also expressed EC marker vWF (blue). In contrast, tumor derived-ECs (arrows) expressed both the GFP (green) and vWF, and regular ECs only expressed vWF. Scale bar: 20 μm. (C) Representative results of flow cytometry for dissociated glioma tissue in groups with or without BBG treatment. In EPC group, ECs were CD31+ and constituted 14.6% of the whole tumor (left), and CD31+ Alex 647+ (exogenous EPCs) represented 9.19% of total ECs (right). While, in EPC + BBG group, ECs were CD31+ and constituted 12.0% of the whole tumor (left), and CD31+ Alex 647+ (exogenous EPCs) represented 3.63% of total ECs (right).
P2X7 receptors inhibition suppressing the homing of transplanted EPCs to gliomas through downregulation of CXCLl expression
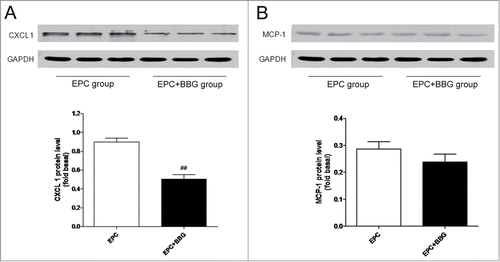
Previous studies have reported that EPCs express CXCR2 in both human and rodent. Furthermore, CXCR2 and its ligand, CXCL1 are essential for the recruitment of EPCs after artery injury,Citation27 suggesting that CXCL1 may be a candidate factor mediating EPCs recruitment. Given this, we explored and compared the protein expression of CXCL1 in glioma-bearing rats with or without BBG treatment. Western blot analysis demonstrated a dramatic decrease in CXCL1 expression in glioma tissue from EPC + BBG group (). It is reported that P2X7 receptors could regulate the expression of MCP-1,Citation28,29 which plays a key role in the recruitment of progenitor cells.Citation30 So, we also detected the expression of MCP-1 in BBG treated or untreated group. Inconsistent with expected, although BBG treatment slightly down-regulated the protein expression of MCP-1 compared with BBG untreated group, the difference was not significant (). All these results suggest that the anti-homing effect of P2X7 receptors inhibition on EPCs may through downregulation of CXCL1 expression in glioma tissue.
Figure 6. P2X7 receptors suppression decreased the expression of CXC ligand-1 (CXCL1) rather than monocyte chemoattractant protein-1 (MCP-1). (A–B) Western blot analysis of CXCL1 (A) and MCP-1 (B) protein expression in gliomas in EPC group and in EPC + BBG group, respectively (top). GAPDH blot serves as loading control. Relative protein levels were shown in bar graph (bottom). Data are presented as mean± SD from 6 separate experiments.##P < 0.01 vs. Control.
Effect of P2X7 receptors inhibition on rat in situ glioma growth, neovascularization and vascular function
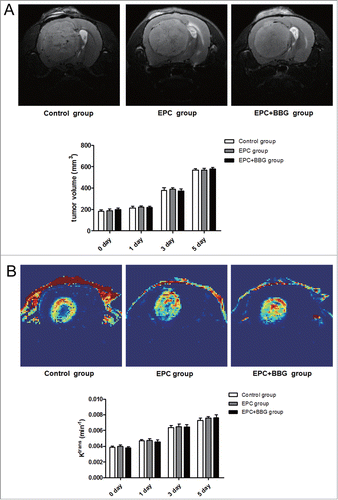
To determine the role of P2X7 receptors in glioma growth in vivo, the volume of tumors was measured by MRI. As showed, no significant difference was found about the tumor volume in these 3 groups at each time point. Given that neovascularization plays a key role in tumor growth, dynamic contrast enhanced MRI (DCE-MRI), which can be useful in assessing tumor vascular parameters and in predicting tumor angiogenesis and tumor response following antiangiogenic therapy,Citation31-33 was performed on the glioma-bearing rats. Ktrans value was obtained to determine the tumor neovascularization levels and the vascular function such as permeability in the different groups. Representative Ktrans map images were shown in . Compared to EPC group, Ktrans value in EPC + BBG group showed no significant difference at each time point. Taken together, these results suggested that P2X7 receptors inhibition exerts no promoting effect on C6 glioma growth, neovascularization and the vascular function. Additionally, no significant differences about tumor volume and Ktrans value were also found among groups with or without EPCs transplantation, regardless of BBG treatment. This indicated that exogenous EPCs may not exert pro-growth and pro-angiogenesis effect on gliomas.
Figure 7. Effects of P2X7 receptors inhibition on rats in situ glioma growth and neovascularization. (A) Representative axial T2-weighted imaging (T2WI) images of rats with glioma at 5 d post-transplantation of EPCs obtained from different groups (Control group, EPC group, EPC + BBG group). Glioma was region with high intensity on T2WI images (top). Moreover, to evaluate the effect of BBG on tumor growth, tumor volume of different groups was determined using Advantage Workstation (bottom). (B) Representative Ktrans maps of rats with glioma at 5 d post-transplantation of EPCs obtained from different groups. Red and blue represent relative higher- and lower-value, respectively (top). Changes of Ktrans value in these 3 groups were analyzed (bottom). Data are mean ± SD from 6 independent experiments.
Discussion
Here we describe, for the first time, the expression of P2X7 receptors in rat spleen-derived EPCs. Activation of P2X7 receptors in EPCs could enhance the proliferation and migration of EPCs, inhibition P2X7 receptors through using antagonist of P2X7 receptors could suppress the homing of EPCs to gliomas. These data imply the possibility of promoting proliferation and targeting ability of exogenous EPCs, to be a therapeutic and imaging probe, to brain gliomas in vivo through P2X7 receptors.
Previous studies have reported that P2X7 receptors activation is related to apoptotic or necrotic cell death. Indeed, ECs exhibit necrotic cell death in the context of exogenous millimolar levels of ATP, which is associated with chronic activation of P2X7 receptors in ECs.Citation21 On the contrary, increasing evidence also presents for the pro-growth effect of P2X7 receptors. In central nervous system, ATP exposure induces proliferation of neural stem cells (NSC) through the PI3k-kinase-dependent P70S6 kinase signaling pathway.Citation34 In some different cancer types, upregulated expression of P2X7 receptors in tumor cells is closely related to tumor growth, such as breast cancer,Citation20 lymphoid neoplasm,Citation35 bone-related cancersCitation36 and so on. The present data showed that activation of P2X7 receptors in spleen-derived EPCs using BzATP, an analog of ATP, contributed to enhanced cells proliferation. Although BzATP induced more cells exhibiting apoptotic feature, the difference between the treated and untreated cells was not significant. In addition, protein gel blot also found no significant difference of caspase-3 expression in the EPCs with or without BzATP. These finding may suggest that activation of P2X7 receptors in spleen-derived EPCs enhance cells proliferation rather than induce cells apoptosis. Moreover, that may further support that P2X7 receptors exerting a pro-survival or pro-death effect depends on the mode of activation.Citation21
In brain gliomas, due to the highly aggressive, the interface between the glioma and normal appearance brain parenchyma is difficult to be truly recognized. With the invasion of glioma cells, the adjacent brain tissue is injured and ruined, these damaged area of gliomas has high concentration of extracellular ATP.Citation37 ATP, acting as an extracellular ligand specifically on purinergic receptors of the plasma membrane, takes an important place in several cellular activities. Besides pro-proliferation, activation of P2X7 receptors in other cells, such as breast cancer cells, also contributes to cells migration, which is critical for the formation of metastases. In the present study, the migration of EPCs was also increased when these cells were stimulated with the agonist of P2X7 receptors. Meanwhile, this pro-migration phenomenon was partially inhibited in the presence of the antagonist of P2X7 receptors. On the other hand, in vivo study, T2 maps of MRI, Prussian blue staining, and flow cytometry demonstrated decreased homing of exogenous EPCs in the brain glioma-bearing rats with BBG treatment. Further, SWI on high field small animal MR scanner was used to track the incorporation of these EPCs into vessels in gliomas. Interestingly, SWI revealed that the number of exogenous EPCs, integrating into the vessels containing tumor derived ECs that might be one of the resistance mechanisms against anti-VEGF therapy, in EPC + BBG group was fewer than that in EPC group, which was also confirmed by immunofluorescence analysis. There has been concern that host macrophage would phagocytose apoptotic or dead USPIO-labeled EPCs within the tumorCitation4, and thereby confound the interpretation of the decrease in intensity observed on MRI after transplantation of USPIO-labeled EPCs. Similar to the previous results reported by Arbab et al.Citation4,38, we did not observe any uptake of USPIO-labeled EPCs by host macrophages, which indicates that the low signal intensity areas in the tumors seen on MRI are due to migrated EPCs incorporating into the neovasculature. Collectively, these data indicated that activated P2X7 receptors in EPCs could facilitate cells migration. Moreover, the targeting ability of exogenous EPCs to gliomas in vivo, especially integrating into the vessels containing the tumor-derived ECs in glioma, might be modulated be P2X7 receptors.
The enhanced migration of cells induced by P2X7 receptors activation may be attributed to morphological changes leading to the acquisition of a pro-morphological and no membrane blebbing.Citation18 Some researchers believe that environment changes, resulting from the P2X7 receptors activation, also contribute to the increased migration of cells through regulating the release of some cytokines.Citation35 Stromal derived factor 1α (SDF-1α/CXCL12)/CXCR4 has been extensively illustrated in the recruitment and homing of EPCs to contribute to neovascularization of tumor or ischemic tissue.Citation39-41 Besides, other subtypes of CXC chemokines, such as CXCL1 and CXCL2, also show their important roles in EPCs recruitment. As previous studies have been revealed that CXCL1/CXCR2 takes an important place in the recruitment of EPCs to the sites of artery injury and lung diseases, we speculated that CXCL1 may also contribute to EPCs homing to gliomas. Here we showed that the number of administrated EPCs was notably decreased in gliomas with BBG treatment, concomitant with markedly downregulation of CXCL1 expression in the glioma tissue. In addition, we also observed the changes of MCP-1 expression. Unlike CXCL1, BBG only slightly downregulated the expression of MCP-1. These findings suggest that BBG exerts the inhibiting effect on homing of EPCs to gliomas probably through suppressing the chemotaxis of CXCL1.
Further, we evaluated the effect of P2X7 receptors on proliferation of C6 glioma cells or glioma growth at the same dose of BzATP or BBG used in EPCs experiments in vitro and in vivo. MTT assay found no significant difference among these 3 groups (BzATP group, BzATP + BBG group, control group) in vitro. Meanwhile, gliomas growth and angiogenesis were monitored noninvasively by MR imaging. MR imaging-measured changes in vascular permeability/flow (i.e., Ktrans) and in tumor volume revealed no significant difference between the groups with or without BBG treatment. Unlike SDF-1α/CXCR4, which could promote GSC-initiated glioma growth and angiogenesis by stimulating VEGF production,Citation17 P2X7 receptors may become a prospective molecular that could regulate EPCs proliferation and migration without promoting glioma cells proliferation and gliomas growth, neovascularization and the vascular function. Additionally, no significant differences about tumor volume and Ktrans value were also found among groups with or without EPCs transplantation, regardless of BBG treatment, which indicated that exogenous EPCs could not exert pro-growth and pro-angiogenesis effect on gliomas. This phenomenon may be explained by which exogenous EPCs may be not a necessity for glioma growth and neovascularization, which may provide useful support for the future application of EPCs as a therapeutic and imaging probe. However, this hypothesis needs to be confirmed in future.
There are several limitations to this study. One potential criticism is that BzATP, an analog of ATP which is too expensive, was not used to investigate the effect of activation of P2X7 receptors on homing of exogenous EPCs in vivo. However, we confirmed this pro-migration effect with BzATP treatment on EPCs in vitro. Moreover, BBG, the antagonist of P2X7 receptors, was used to suppress homing of exogenous EPCs, which may imply the role of P2X7 receptors in EPCs targeting ability reversely.
In conclusion, the study presented here demonstrates a previously unreported functional expression of P2X7 receptors in rat spleen-derived EPCs. Activation of P2X7 receptors could enhance the proliferation and migration of EPCs, with no progrowth effect on C6 glioma cells. Moreover, inhibition P2X7 receptors through using antagonist of P2X7 receptors could suppress the homing of EPCs to gliomas, especially integration into the vessels containing the tumor-derived ECs in gliomas. These data imply the possibility of promoting proliferation and targeting ability of transplanted EPCs to gliomas in vivo through P2X7 receptors, which may provide new perspectives on application of EPCs as a therapeutic and imaging probe to overcome antiangiogenic resistance for gliomas.
Materials and Methods
Cell culture and characterization
C6 glioma cells were obtained from cell bank of Chinese Academy of Sciences (Shanghai, China). C6 glioma cells transduced by pGeenPuro virus were done as described.Citation42 Cells were incubated in DMEM/F12 medium (Gibco, Carlsbad, CA) supplemented with 10% FBS and 100 units/ml penicillin (Hyclone, Logan, AR) at 37°C in a humidified atmosphere containing 95% air and 5% CO2.
Spleen-derived EPCs were obtained as previously describedCitation43 by isolating mononuclear cells using Ficoll density-gradient centrifugation from healthy Sprague-Dawley rats (obtained from the Experimental Animal Center of Daping Hospital, Chongqing, China). Freshly prepared EPCs were incubated in DMEM, supplemented with 20% fetal bovine serum (FBS), 50 ng/ml VEGF, 1 ng/ml bFGF, and 2 ng/ml IGF-1 (Sigma-Aldrich, St. Louis, MO). Initially cells were suspended in media at 1 × 106 per ml and grown in 5% CO2/95% air at 37°C in a humidified atmosphere, with fresh media added every third day. After 7 days, EPCs were identified via uptake DiI-labeled acetylated low-density lipoprotein and binding of FITC-labeled lectin-1 (Sigma-Aldrich, St. Louis, MO). To determine cell surface markers, cells were fixed with 4% paraformaldehyde, incubated with antibodies CD34 (Abnova, Taiwan, China), CD31 (Millipore, Bedford, MA), vWF (Millipore, Bedford, MA), and Flk1 (Abcam, Cambridge, UK). Digital images were acquired using a TE200-U Nikon eclipse microscope.Citation44
Proliferation assay
To study the role of P2X7 receptors in proliferation of EPCs and C6 glioma cells, cells were cultured with BzATP (100 μmol/L) in the presence or absence of BBG (100 μmol/L). The proliferation of cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. EPCs and C6 glioma cells were plated in 96-well plates at a density of 3 × 104 cells/well and 5 × 103 cells/well, respectively. Medium was removed prior to adding MTT (Sigma-Aldrich, St. Louis, MO) in DPBS at a final concentration of 5 mg/ml, then cells were incubated at 37°C for 4 h. After incubation, MTT was removed, 150 μl dimethyl sulfoxide (DMSO) was added to lyse the cells and the absorbance of each well was measured at 490 nm.
In vitro migration assay
The effect of P2X7 receptors on migration of EPCs was assayed by using transwell (diameter, 5.0 mm; pore, 8.0 μm; BD Biosciences, San Jose, CA). EPCs were detached when cells were confluent, then about 2 × 104 cells suspended in 100 μl serum-free DMEM media were seeded in the upper chamber of each transwell plate. Cells were cultured with serum-free DMEM for 12 h, prior to treating with BzATP (100 μmol/L) in the presence or absence of BBG for 12 h. DMEM containing 20% FBS in the lower chamber was acted as chemotactic agent. After overnight incubation at 37°C in a humidified atmosphere containing 5% CO2/95% air, the membrane was fixed with 4% paraformaldehyde for 10 min in room temperature prior to staining with crystal violet (Sigma Aldrich, St. Louis, MO). The nonmigrating cells in the upper chamber and residual matrigel were removed using cotton swabs. EPCs migration was quantified by counting the number of stained cells in 10 random high power fields photographed for each chamber and each experiment was replicated 3 times.
EPCs labeling
EPCs were labeled with USPIO (P7228; Guerbet Asia Pacific, Hong Kong, China) as described previously.Citation45 After USPIO labeling, some of these cells were labeled with fluorescent dye DiI (Invitrogen, Grand Island, NY), others were labeled with CellTracker™ Green CMFDA (Invitrogen, Grand Island, NY) according to the manufacturer's protocol, washed, resuspended at 1 × 106 cells per ml. Cells labeled with CMFDA were prepared for transplantation to the glioma-bearing rats which were used for flow cytometry.
Establishment and treatment of animal model
The use of laboratory animals was in compliance with the guideline of National Institute of Health. All animal experiments were performed according to a protocol approved by the Animal Use Subcommittee. To establish the in situ brain glioma model, 72 healthy adult male Sprague-Dawley rats were anesthetized with 3% pentobarbital (1 ml/kg; Sigma–Aldrich, St. Louis, MO) and C6 glioma cells were implanted into brain parenchyma as previously.Citation42
Animals were randomized to the control group (24 rats), EPC group (24 rats), EPC + BBG group (24 rats). Rats in EPC group were transplanted with double labeled EPCs (1 × 106) suspended in 1 ml DPBS via tail vein on 10 d after the in situ glioma established. At the same time, rats in the control group were administrated equivalent volume of DPBS. Rats in the EPC + BBG group were injected intraperitoneally at a dose of 10 mg/kg immediately after double labeled EPCs transplantation, and then once every other day.
In vivo magnetic resonance imaging
All rats were performed magnetic resonance imaging (MRI) with a Bruker BioSpec 7 T/20 cm system (Bruker, Ettlingen, Germany), using a head surface coil, after C6 glioma cells transplanted. The sequences used in the scanning covered as follows: T1-weighted image (T1WI; repetition time = 1500 ms, echo time = 8.0 ms, field of view = 35 mm × 35 mm, slice thickness = 0.5 mm, NEX = 4, flip angle = 90°); T2-weighted image (T2WI; repetition time = 2500 ms, echo time = 45 ms, field of view = 35 mm ×35 mm, slice thickness = 0.5 mm, NEX=4, flip angle = 90°); T2 maps sequence (repetition time = 2000 ms; echo time = 9, 18, 27, 36, 45, 54, 63, 72, 81,90, 99, 108, 117, 126, 135 ms; field of view = 35 mm × 35 mm; slice thickness = 1.0 mm; slice distance = 1.0 mm); Susceptibility-weighted image (SWI; repetition time = 700 ms; echo time = 18 ms; field of view = 35 mm × 35 mm; slice thickness =0.5 mm; flip angle = 40.0°; NEX = 8). All the images were analyzed by Image Display and Processing Software in the ParaVision 6.0 workstation (Bruker, Ettlingen, Germany). Absolute tumor volume was calculated after integration of the area measurements from every slice.
To generate T1 maps from precontrast images, DCE FLASH images with multiple flip angles of 5°, 10°, 15°, 20 ° and 25° were acquired. The following parameters were used to acquire the DCE FLASH images: TR = 52.823 ms, TE = 1.765 ms using a 128 × 128 matrix, FOV = 35 mm × 35 mm, and NEX = 1. Effective slice thickness was 0.5 mm. To obtain dynamic postcontrast DCE FLASH images, a fixed flip angle of 20° was used, with 6.761s for 540.907s (80 time points). Acquisition of DCE FLASH images started before the administration of contrast to have baseline T1 signals. When the third dynamic loop finished, 0.5 mmol/ml Omniscan (GE Healthcare, Co. Cork, Ireland) was administered at a dosage of 0.1 mmol/kg body weight by hand push within 4s. All images were transferred to an independent workstation for quantitative analysis using an in-house program coded in Matlab2009 (MathWorks, Natick, MA, USA). The Tofts model was used to calculate the forward transfer constant Ktrans.
Immunohistochemistry and Prussian blue staining
Immediately following MR examination, rats were sacrificed by overdose anesthesia and perfused via left ventricle with 250–300 ml of physiological saline and 200–250 ml of 4% paraformaldehyde to drain blood. Entire brain tissue was divided in half for frozen sections, and paraformaldehyde fixation with embedding in paraffin. Eight micron sections were cut from tumors embedded in paraffin, and then stained with Prussian blue. To determine whether host macrophages had phagocytosed some of the iron-positive cells, resulting in a low signal intensity on MRI, consecutive sections were stained with ant-rat F4/80 antibody (specific for rat macrophages; Santa Cruz Biotechnology Inc., Santa Cruz, CA). Consecutive sections were also used as negative control in which the primary antibody was omitted, but all other procedures were performed in an identical manner. Immunohistochemistry was performed with standard procedures using horseradish peroxidase (HRP) –tagged secondary antibodies.
Immunofluorescent staining of cytospins and tumor sections
For cytospins, to detect the P2X7 receptors expression in rat spleen-derived EPCs, cells grown on plastic coverslips were fixed with 4% paraformaldehyde at room temperature for 20 min, washed with DPBS for 3 times. Then, the coverslips were placed in 10% normal goat serum for 10 min and followed by incubation with rabbit anti-P2X7 receptors antibody (1:200, Santa Cruz Biotechnology Inc., Santa Cruz, CA) overnight at 4°C. After that cells were washed with DPBS for 3 times and incubated with fluorescent dye-labeled anti-rabbit IgG (1:500, Invitrogen, Grand Island, NY) at room temperature for 30 min. For negative staining control, primary antibody was omitted during the procedure. Images were digitized using a TE200-U Nikon eclipse microscope.
Tumors were collected and then divided for frozen section at 5 μm thickness. Sections were fixed in 4% paraformaldehyde and blocked with 10% goat serum. The primary antibodies used in this study were as follows: rabbit anti-vWF, rabbit anti-CD34, mouse anti-CD31. The secondary antibodies used were as follows (all from Invitrogen, Grand Island, NY): Alexa Fluor 647-rabbit anti-mouse IgG, Alexa Fluor 647-donkey anti-rabbit IgG. Antibodies were diluted in antibody diluent (Beyotime, Jiangsu, China), and incubations were done at room temperature. The images were captured by confocal laser scanning microscopy (Leica, Heerbrugg, Switzerland), and the obtained images were processed by Adobe Photoshop 7.0 (Adobe System Inc., San Jose, CA).
Flow cytometry
For cellular apoptosis analysis, propidium iodide (PtdIns) in conjunction with Annexin V was used. EPCs were harvested and resuspended in 100 μl 1 × DPBS. Then, Annexin V-FITC, binding buffer and PI (BD Biosciences, San Jose, CA) were added into the tubes according to the manufacturer's recommendations and these tubes were incubated in the dark for 15 min at room temperature. The tubes without Annexin V-FITC and PtdIns acted as negative control.
The brain tumors were dissociated using a Neural Tissue Dissociation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). These cells were stained with the following antibodies according to the manufacturer's protocol: phycoerythrin (PE) anti-CD31 (BD Biosciences, San Jose, CA), anti-Fluorescein/Oregon Green mouse IgG (Invitrogen, Grand Island, NY), and Alexa Fluor 647-rabbit anti-mouse IgG (Invitrogen, Grand Island, NY). All samples were then analyzed on a BD LSR I flow cytometer (BD Biosciences, San Jose, CA).
Western blot analysis
Total proteins from cultured EPCs and rat brain glioma tissues were extracted and Western blot analysis was done as previouslyCitation42. 10% SDS-PAGE was used to separate protein samples (20 μg), which were transferred to polyvinylidene difluoride membrane, probed with rabbit anti-P2X7 receptors (1:1000), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000), CXC chemokine ligand 1 (CXCL-1), monocyte chemoattractant protein-1 (MCP-1) and Caspase-3 antibody (all from Santa Cruz Biotechnology Inc., Santa Cruz, CA) overnight at 4°C, respectively. After incubation with secondary antibodies, the proteins expression was detected by enhanced chemiluminescence and quantified using a Gel Doc 2000 Imager (Bio-Rad, Hercules, CA). Each sample was processed at least 3 times.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis included student t-test or one-way analysis of variance using SPSS software, version 18.0 (SPSS Inc., Chicago, IL). Difference were considered significant at P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (Grant No. 81201139), the National Natural Science Foundation of China (Grant No. 81271626), and Natural Science Foundation Project of CQ CSTC (cstc2012jjB10028).
Reference
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964-7; PMID:9020076; http://dx.doi.org/10.1126/science.275.5302.964
- Ruzinova MB, Schoer RA, Gerald W, Egan JE, Pandolfi PP, Rafii S, Manova K, Mittal V, Benezra R. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell 2003; 4:277-89; PMID:14585355; http://dx.doi.org/10.1016/S1535-6108(03)00240-X
- Moore XL, Lu J, Sun L, Zhu CJ, Tan P, Wong MC. Endothelial progenitor cells' “homing” specificity to brain tumors. Gene Ther 2004; 11:811-8; PMID:15057261; http://dx.doi.org/10.1038/sj.gt.3302151
- Arbab AS, Pandit SD, Anderson SA, Yocum GT, Bur M, Frenkel V, Khuu HM, Read EJ, Frank JA. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells 2006; 24:671-8; PMID:16179427; http://dx.doi.org/10.1634/stemcells.2005-0017
- Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 2007; 21:1546-58; PMID:17575055; http://dx.doi.org/10.1101/gad.436307
- Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res 2009; 69:7243-51; PMID:19738068; http://dx.doi.org/10.1158/0008-5472.CAN-09-0167
- Zhang HR, Chen FL, Xu CP, Ping YF, Wang QL, Liang ZQ, Wang JM, Bian XW. Incorporation of endothelial progenitor cells into the neovasculature of malignant glioma xenograft. J Neuro-Oncol 2009; 93:165-74; PMID:19052696; http://dx.doi.org/10.1007/s11060-008-9757-4
- Chen X, Fang JQ, Wang SN, Liu H, Du XS, Chen JH, et al. A new mosaic pattern in glioma vascularization: exogenous endothelial progenitor cells integrating into the vessels containing tumor-derived endothelial cells. Oncotarget 2014; 5:1955-68; PMID:24722469
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells (vol 468, pg 824, 2010). Nature 2011; 477:238-; PMID:21102434; http://dx.doi.org/10.1038/nature10410
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010; 468:829-U128; PMID:21102433; http://dx.doi.org/10.1038/nature09624
- Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari S, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. P Natl Acad Sci U S A 2011; 108:4274-80; PMID:21262804; http://dx.doi.org/10.1073/pnas.1016030108
- Thu MS, Najbauer J, Kendall SE, Harutyunyan I, Sangalang N, Gutova M, Metz MZ, Garcia E, Frank RT, Kim SU, et al. Iron labeling and pre-clinical MRI visualization of therapeutic human neural stem cells in a murine glioma model. Plos One 2009; 4:e7218; PMID:19787043
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010; 120:694-705; PMID:20179352; http://dx.doi.org/10.1172/JCI40283
- Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res 2007; 1183:138-47; PMID:17976542; http://dx.doi.org/10.1016/j.brainres.2007.08.091
- Stevenson CB, Ehtesham M, McMillan KM, Valadez JG, Edgeworth ML, Price RR, Abel TW, Mapara KY, Thompson RC, et al. CXCR4 expression is elevated in glioblastoma multiforme and correlates with an increase in intensity and extent of peritumoral T2-weighted magnetic resonance imaging signal abnormalities. Neurosurgery 2008; 63:560-9; PMID:18812968; http://dx.doi.org/10.1227/01.NEU.0000324896.26088.EF
- Redjal N, Chan JA, Segal RA, Kung AL. CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin Cancer Res 2006; 12:6765-71; PMID:17121897; http://dx.doi.org/10.1158/1078-0432.CCR-06-1372
- Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC, Jiang T, Lin MC, Chen JH, Wang B, Zhang R, et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol 2011; 224:344-54; PMID:21618540; http://dx.doi.org/10.1002/path.2908
- Roger S, Pelegrin P. P2´7 receptor antagonism in the treatment of cancers. Expert Opin Inv Drug 2011; 20:875-80; http://dx.doi.org/10.1517/13543784.2011.583918
- North RA. Molecular physiology of P2X receptors. Physiol Rev 2002; 82:1013-67; PMID:12270951
- Jelassi B, Chantome A, Alcaraz-Perez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 2011; 30:2108-22; PMID:21242969; http://dx.doi.org/10.1038/onc.2010.593
- Thompson BA, Storm MP, Hewinson J, Hogg S, Welham MJ, MacKenzie AB. A novel role for P2´7 receptor signalling in the survival of mouse embryonic stem cells. Cell Signal 2012; 24:770-8; PMID:22120528; http://dx.doi.org/10.1016/j.cellsig.2011.11.012
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, et al. P2´7 receptor inhibition improves recovery after spinal cord injury. Nat Med 2004; 10:821-7; PMID:15258577; http://dx.doi.org/10.1038/nm1082
- Jitkaew S, Witasp E, Zhang S, Kagan VE, Fadeel B. Induction of caspase- and reactive oxygen species-independent phosphatidylserine externalization in primary human neutrophils: role in macrophage recognition and engulfment. J Leukocyte Biol 2009; 85:427-37; PMID:19106181; http://dx.doi.org/10.1189/jlb.0408232
- Lee SH, Meng XW, Flatten KS, Loegering DA, Kaufmann SH. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ 2013; 20:64-76; PMID:22858544; http://dx.doi.org/10.1038/cdd.2012.93
- Sharp DJ, Ham TE. Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol 2011; 24:558-63; PMID:21986682; http://dx.doi.org/10.1097/WCO.0b013e32834cd523
- Boeckh-Behrens T, Lutz J, Lummel N, Burke M, Wesemann T, Schopf V, Brückmann H, Linn J. Susceptibility-weighted angiography (SWAN) of cerebral veins and arteries compared to TOF-MRA. Eur J Radiol 2012; 81:1238-45; PMID:21466929; http://dx.doi.org/10.1016/j.ejrad.2011.02.057
- Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res 2007; 100:590-7; PMID:17272812; http://dx.doi.org/10.1161/01.RES.0000259043.42571.68
- Wei W, Ryu JK, Choi HB, McLarnon JG. Expression and function of the P2X(7) receptor in rat C6 glioma cells. Cancer Lett 2008; 260:79-87; PMID:18039556; http://dx.doi.org/10.1016/j.canlet.2007.10.025
- Fang KM, Wang YL, Huang MC, Sun SH, Cheng H, Tzeng SF. Expression of macrophage inflammatory protein-1 alpha and monocyte chemoattractant protein-1 in glioma-infiltrating microglia: involvement of ATP and P2X(7) receptor. J Neurosci Res 2011; 89:199-211; PMID:21162127; http://dx.doi.org/10.1002/jnr.22538
- Zhang F, Tsai S, Kato K, Yamanouchi D, Wang CJ, Rafii S, Liu B, Kent KC. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells. J Biol Chem 2009; 284:17564-74; PMID:19406748; http://dx.doi.org/10.1074/jbc.M109.013987
- Bagher-Ebadian H, Jain R, Nejad-Davarani SP, Mikkelsen T, Lu M, Jiang Q, Scarpace L, Arbab AS, Narang J, Soltanian-Zadeh H, et al. Model selection for DCE-T1 studies in glioblastoma. Magn Reson Med 2012; 68:241-51; PMID:22127934; http://dx.doi.org/10.1002/mrm.23211
- Ewing JR, Brown SL, Lu M, Panda S, Ding GL, Knight RA, Cao Y, Jiang Q, Nagaraja TN, Churchman JL, et al. Model selection in magnetic resonance imaging measurements of vascular permeability: Gadomer in a 9L model of rat cerebral tumor. J Cerebr Blood F Met 2006; 26:310-20; PMID:16079791; http://dx.doi.org/10.1038/sj.jcbfm.9600189
- Yang XY, Knopp MV. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol 2011; 2011:732848; PMID:21541193
- Burnstock G, Ulrich H. Purinergic signaling in embryonic and stem cell development. Cell Mol Life Sci 2011; 68:1369-94; PMID:21222015; http://dx.doi.org/10.1007/s00018-010-0614-1
- Ren SY, Zhang Y, Wang YJ, Lui YJ, Wei W, Huang XH, Mao W, Zuo Y. Targeting P2X(7) receptor inhibits the metastasis of murine P388D1 lymphoid neoplasm cells to lymph nodes. Cell Biol Int 2010; 34:1205-11; PMID:20722629; http://dx.doi.org/10.1042/CBI20090428
- Adinolfi E, Amoroso F, Giuliani AL. P2´7 receptor function in bone-related cancer. J Osteoporosis 2012; 2012:637863; PMID:22970409; http://dx.doi.org/10.1155/2012/637863
- Burnstock G, Arnett TR, Orriss IR. Purinergic signalling in the musculoskeletal system. Purinerg Signal 2013; 9:541-72; http://dx.doi.org/10.1007/s11302-013-9381-4
- Arbab AS, Janic B, Knight RA, Anderson SA, Pawelczyk E, Rad AM, Read EJ, Pandit SD, Frank JA. Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings. FASEB J 2008; 22:3234-46; PMID:18556461; http://dx.doi.org/10.1096/fj.07-105676
- Wang YB, Liu YF, Lu XT, Yan FF, Wang B, Bai WW, Zhao YX. Rehmannia glutinosa extract activates endothelial progenitor cells in a rat model of myocardial infarction through a SDF-1 alpha/CXCR4 Cascade. Plos One 2013; 8:e54303; PMID:23349848
- Zhao YH, Yuan B, Chen J, Feng DH, Zhao B, Qin C, Chen YF. Endothelial progenitor cells: therapeutic perspective for ischemic stroke. Cns Neurosci Ther 2013; 19:67-75; PMID:23230897; http://dx.doi.org/10.1111/cns.12040
- Mukherjee D, Zhao JH. The role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res 2013; 3:46-57; PMID:23359227
- Fang JQ, Chen X, Zhang LT, Chen JH, Liang Y, Li X, Xiang J, Wang L, Guo G, Zhang B, et al. P2X(7)R suppression promotes glioma growth through epidermal growth factor receptor signal pathway. Int J Biochem Cell B 2013; 45:1109-20; PMID:23523696; http://dx.doi.org/10.1016/j.biocel.2013.03.005
- Chen X, Yin J, Wu XN, Li R, Fang JQ, Chen R, Zhang B, Zhang W. Effects of magnetically labeled exogenous endothelial progenitor cells on cerebral blood perfusion and microvasculature alterations after traumatic brain injury in rat model. Acta Radiol 2013; 54:313-23; PMID:23528570; http://dx.doi.org/10.1258/ar.2012.120605
- Wang SA, Fang JQ, Zhang T, Wang B, Chen JH, Li X, Zhang S, Zhang W. Magnetic resonance imaging targeting of intracranial glioma xenografts by Resovist-labeled endothelial progenitor cells. J Neuro-Oncol 2011; 105:67-75; PMID:21523487; http://dx.doi.org/10.1007/s11060-011-0569-6
- Fang JQ, Wang SN, Chen JH, Zhang YL, Zhang B, Liang HP, Zhang W. The effects of magnetically labeled rat spleen-originated endothelial progenitor cells on growth of glioma in vivo: an experimental study. Acad Radiol 2011; 18:892-901; PMID:21543240; http://dx.doi.org/10.1016/j.acra.2011.02.017
