Abstract
Background. RACK1 is known to be involved in tumor progression, and its prognostic value on many kinds of tumors has been identified. However, there are limited studies about the functional role of RACK1 in esophageal squamous cell carcinoma (ESCC). Patients and methods. RACK1 expression was examined in 100 ESCC tissue samples using immunohistochemistry staining. RACK1 was knocked-down in ESCC cell lines by shRNA. The effects on cell proliferation, invasion and migration were examined in ESCC cell lines and nude mouse model. Vimentin and E-cadherin were introduced to further study the association between RACK1 and EMT. Results. RACK1 expression was significantly associated with the tumor length (P = 0.012), diameter<3 cm (P = 0.047), T stage (P = 0.032), and lymph node metastasis (P = 0.038), respectively. Kaplan-Meier survival analysis and Cox analyses revealed RACK1 expression was an independent predictor for OS (P = 0.030) and DFS (P = 0.027) in ESCC. Down-regulation of RACK1 inhibited cell proliferation, along with invasion and migration in vitro and in vivo. A significant positive correlation between RACK1 expression and vimentin (P = 0.0190) and an inverse correlation between RACK1 expression and E-cadherin (P = 0.0047) were found. Conclusions. RACK1 predicted poor prognosis in ESCC, promoted tumor progression, and was involved in EMT of ESCC.
Abbreviations
| RACK1 | = | Receptor for Activated C Kinase 1 |
| ESCC | = | esophageal squamous cell carcinoma |
| EMT | = | epithelial-mesenchymal transition |
| IHC | = | immunohistochemistry |
| OS | = | overall survival |
| DFS | = | disease-free survival |
| M | = | male |
| F | = | female |
| R | = | radiotherapy |
| C | = | chemotherapy |
| CRT | = | chemoradiotherapy |
Introduction
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer death worldwide.Citation1 The incidence of esophageal cancer has remarkable regional differences. In Asian countries, the predominant histological type is squamous cell carcinoma (ESCC). Although great advances in diagnostic methods and treatments have been achieved, the mortality of esophageal cancer remains high. The reasons of poor prognosis include diagnosed at advanced stage, high recurrence rate after therapy, especially lack of liable markers to predict response to therapy. So it is urgent to identify effective and independent markers for clinical prognostic prediction.
RACK1, termed for Receptor for Activated C Kinase, was first reported as binding protein for Protein Kinase C (PKC) in 1991.Citation2 RACK1 is a 36-kDa scaffolding protein with 7 conserved WD-40 motifs and it shows high homology to the β subunit of G protein (G-β).Citation3 The conserved 7 blade propeller structure is intrinsic to RACK1s protein binding capacity and allows RACK1 to function as a signaling hub.Citation4 Through binding to diverse signaling and structural proteins, RACK1 can interact with multiple signal pathways, such as MAPK /ERK pathway,Citation5 Src,Citation6,7 PDE4D5,Citation8,9 which can affect cell motility, cell growth, and so on.
Currently more and more reports focus on correlation between RACK1 and malignant tumors. Previous studies have demonstrated that RACK1 function as an independent prognostic factor for patients with malignant tumors including oral squamous cell carcinoma,Citation10 breast carcinoma,Citation11 and pulmonary adenocarcinoma.Citation12 However, there is limited information regarding the predictive significance of RACK1 in ESCC.
Many studies have shown that RACK1 was associated with tumor progression in multiple malignant tumors, such as hepatocellular carcinoma,Citation13,14 breast cancer,Citation15 prostate cancer,Citation16 and so on. Recently Hu et al.Citation17 reported that RACK1 promoted the growth and migration of the cancer cells in the progression of ESCC.
EMT, epithelial-mesenchymal transition, is a process in which cells transit from an epithelial phenotype to a mesenchymal phenotype and cells become more invasive and migrated during this process. It is one of the most important process through which tumor cells acquires the ability of invasion and migration. Although RACK1 was already reported to be associated with tumor invasion and metastasis, whether it is involved in EMT still remains unclear. No reports about RACK1 regulating tumor invasion and migration through EMT come out yet.
In the current study, we carried out a retrospective immunohistochemistry to learn about the correlation between RACK1 expression with long-term postoperative outcome of ESCC. Next we employed shRNA to knockdown RACK1 in ESCC cell lines to examine the effects on cell growth, invasion and migration. At the end of the study, we preliminary explored how RACK1 regulated ESCC invasion and migration.
Results
Relationship between RACK1 expression and clinicopathological characteristics in ESCC
We observed RACK1 immunoreactivity was readily detected in the cytoplasm and occasionally in the nucleus. Representative immunostaining of RACK1 with positive and negative expression is shown in .
Figure 1. Representative immunostaining of RACK1 in ESCC tissues (Left, × 200; right, × 400): (A) ESCC tissue with positive expression; (B) ESCC tissue with negative expression.
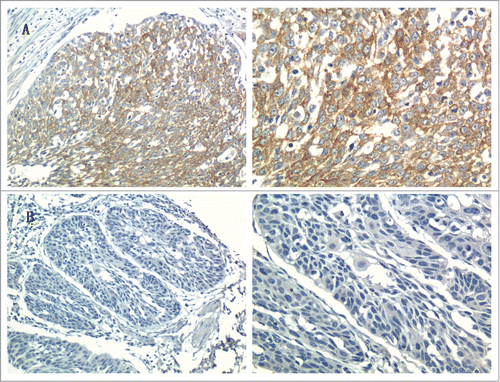
We systematically analyzed RACK1 expressions in 100 ESCC, among which 59 were RACK1 positive expressed and 41 was negative. The relationship between RACK1 expression and clinicopathological characteristics in ESCC is summarized in . The male to female ratio in RACK1 positive group and negative group was 47:12, 32:9 respectively. The mean age in positive and negative group was 61.9 (ranged 42–78), 60.4 (ranged 45–74) respectively. The expression was not significantly correlated with patients’ gender (P = 0.846) or age (P = 0.416). We found no significant differences in distribution according to tumor location, N stage, TNM stage, histological grade and treatment regimen between the 2 groups. However, the expression levels of RACK1 were significantly associated with the tumor length(P = 0.012), diameter<3 cm (P = 0.047), T stage (P = 0.032), and lymph node metastasis (P = 0.038), respectively.
Table 1. Relationship between RACK1 expression and clinicopathological characteristics in ESCC
Association of RACK1 expression with poor prognosis in ESCC patients
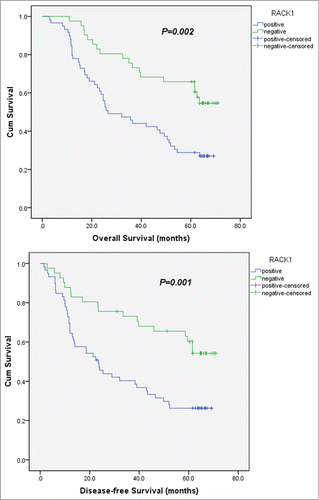
exhibits the overall and disease-free survival curves with respect to RACK1 expression. Kaplan-Meier survival analysis revealed a correlation between RACK1 expression levels and overall survival times. The OS rates were 27.1% in the RACK1 positive group and 56.1% in the RACK1 negative group. The DFS rates were 37.3% in the RACK1 positive group and 61.0% in the RACK1 negative group. Both overall and disease-free survival in patients positive for RACK1 expression were significantly shorter than those in patients who were negative (OS, P = 0.002; DFS, P = 0.001).
Figure 2. Kaplan-Meier survival analyses for 100 ESCC patients with or without RACK1 expression. Cox proportional hazards model was used for the analysis. Both overall and disease-free survival in patients positive for RACK1 expression were significantly shorter than those in patients who were negative (OS, P = 0.002; DFS, P = 0.001).
The results of univariate analyses are shown in . The log-rank test revealed that factors significantly correlated with OS and DFS included RACK1, T stage, N stage, lymph node metastasis, ratio of lymph node, TNM stage(all P < 0.05).
Table 2. Univariate analysis of factors associated with OS and DFS
Multivariate analyses were performed using Cox proportional-hazards regression. shows the results of multivariate analyses. With respect to OS, RACK1 expression was an independent predictor (P = 0.030). In addition, T stage(P = 0.031) and TNM stage(P = 0.036) could significantly influence the probability of poor outcome as well. With respect to DFS, RACK1 expression was also an independent predictor (P = 0.027), as well as TNM stage(P = 0.011).
Table 3. Multivariate analysis of factors associated with OS and DFS
Downregulation of RACK1 protein expression in ESCC cell lines
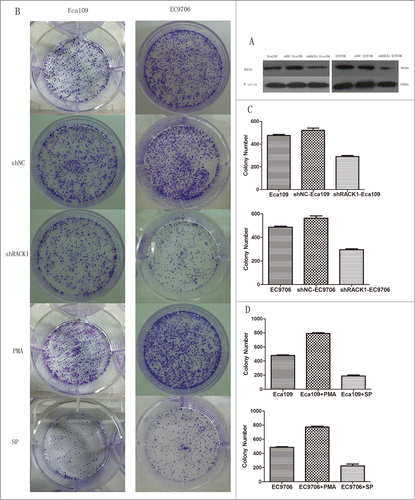
To investigate the function of RACK1 in ESCC, we used shRNA to specifically knockdown RACK1 expression in Eca109 and EC9706 cells. shRACK1 and the nonsense shRNA plasmids were successfully transfected into Eca109 and EC9706 cells. RACK1 protein expression in Eca109 and EC9706 cells was detected by western blotting. RACK1 protein was down-regulated after transfection by shRACK1. The results showed that RACK1 expression at protein levels was successfully downregulated by shRACK1 but not by control shNC (). The left band showed RACK1 protein expression in ESCC cells without transfection. The middle band was the negative control group in which cells were treated by nonsense shRNA. The right band showed RACK1 protein level in shRACK1 transfected cells. We found that the expression of RACK1 protein was down-regulated after shRACK1 transfection. We established a stable downregulated cell strain by G418 selection.
Figure 3. RACK1 regulated cell proliferation in vitro. Activation of protein kinase C promoted cell growth while PKC suppression showed the opposite effect. (A) Expression of RACK1 protein in Eca109 and EC9706 was reduced after shRNA transfection. (B) Left, colonies formed by Eca109 cells; right, colonies formed by EC9706 cells. (C) Downregulation of RACK1 inhibited cell proliferation of Eca109 and EC9706 in vitro. Stable knockdown cell strains formed fewer colonies than the negative control group in Eca109 (P = 0.0044, upper) and EC9706 (P = 0.0008, lower). (D) Cell proliferation was significantly increased by PMA, and decreased by staurosporine (SP). Compared with the control group, PMA treated cells formed more colonies in Eca109 (P = 0.0012, upper) and EC9706 (P = 0.0015, lower), while SP treated cells formed less colonies in Eca109 (P = 0.0016, upper) and EC9706 (P = 0.0059, lower).
Down-regulation of RACK1 inhibited cell proliferation of ESCC cells in vitro
To identity whether RACK1 affect the ability of cell proliferation in Eca109 and EC9706 cells, we performed colony formation assay. Compared with the negative control group, we found that cell proliferation was significantly decreased by the downregulation of RACK1 after shRACK1 transfection(Eca109:521 ± 20 vs. 291 ± 9, P = 0.0044; EC9706:562 ± 20 vs. 296 ± 8, P = 0.0008). However, no difference was found between the blank control cells(475 ± 11;487 ± 9) and those of the negative control(Eca109:P = 0.1030; EC9706:P = 0.1089). The results were showed in .
Down-regulation of RACK1 inhibited tumor growth in vivo
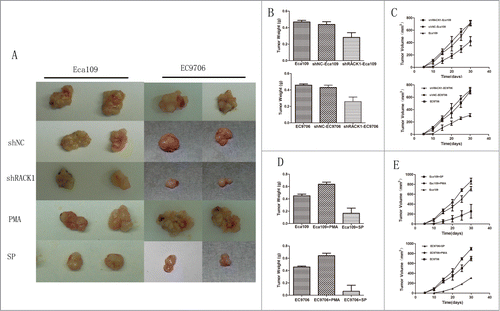
Stable down-regulated cells and control cells were injected subcutaneously into the left flank of male nude mice as we described in “Material and Methods.” The mice were sacrificed after 4 weeks and the tumors were removed. Tumor weight and volume were measured and analyzed. The results were showed in . Tumor weight from the downregulated cells and negative control cells were 0.285 ± 0.0212 g, 0.455 ± 0.00707 g in Eca109, and 0.275 ± 0.0282 g, 0.420 ± 0.00828 g in EC9706 respectively. The difference was statistically significant between these 2 groups (Eca109:P = 0.0003; EC9706:P = 0.0029, ). Tumor volume from the down-regulated cells and control cells were 485 ± 126 mm3, 740 ± 317 mm3 in Eca109 and 310 ± 25 mm3, 714 ± 45 mm3 in EC9706 respectively. Nude mice implanted with downregulated cells developed significantly smaller tumors than the negative control groups(Eca109:P = 0.0119; EC9706:P = 0.0192, ). We did not find significant difference between the blank control cells and those of the negative control. It was worth mentioning that tumors from the down-regulated cells group came out several days later than the control groups.
Figure 4. RACK1 regulated cell proliferation in vivo. Activation of protein kinase C promoted tumor growth in vivo while SP suppressed. (A), Left, representative tumors from Eca109 cells; right, representative tumors from EC9706 cells. (B and C) Down-regulation of RACK1 inhibited tumor growth in vivo. Upper: Eca109; lower: EC9706. B, tumor weight from the down-regulated cells were significantly lower than the negative control group (P = 0.0003, upper; P = 0.0029, lower). (C) Tumor volume from the downregulated cells were significantly lower than the negative control group (P = 0.0119, upper; P = 0.0192, lower). (D and E) PMA promoted tumor growth in vivo while SP suppressed. Upper: Eca109; lower: EC9706. (D) Tumor weight from the PKC activated cells were significantly higher than the negative control group (both P<0.0001); tumor weight from the PKC suppressed cells were significantly lower than the negative control group (P = 0.0001, upper; P = 0.0002, lower). (E) Tumor volume from the activated group were significantly higher than the control group (P = 0.0288, upper; P = 0.0405, lower), while the suppressed group showed reverse effect (P = 0.0308, upper; P = 0.0103, lower).
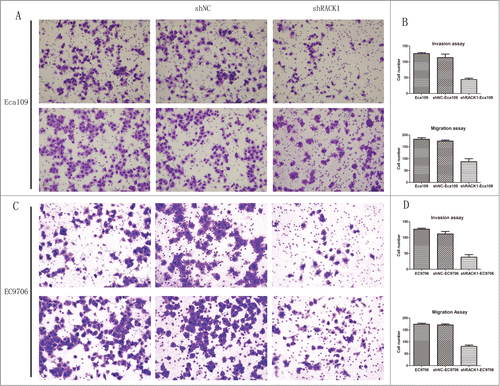
Downregulation of RACK1 inhibited the invasion and migration of ESCC cells
Transwell invasion and migration assay were used to detect the effect of RACK1 down-regulation on biological behaviors of Eca109 and EC9706 cells. The number of invaded cells and migrated cells from the RACK1 knockdown group was 46.5 ± 4.36 and 88.0 ± 12.0 in Eca109, and 38.0 ± 8.02 and 78.0 ± 5.57 in EC9706 respectively, which were significantly less than those of the negative control (Eca109:126.5 ± 3.61, P = 0.0014;184.5 ± 6.65, P = 0.0108, ; EC9706:115.0 ± 8.08, P = 0.0133;171.3 ± 4.16, P = 0.0037, ). However, no difference was found between the cell number from parental cells and those of the control. The results demonstrated that downregulation of RACK1 suppressed the invasion and migration of ESCC cell line.
Figure 5. RACK1 regulated cell invasion and migration in vitro. (A and C) Representative invaded cells and migrated cells stained by crystal violet. Left, blank control cells; middle, shNC transfected cells; right, shRACK1 transfected cells. Upper: Eca109; lower: EC9706. (B and D), results from cell invasion and migration assay were showed in diagrams. The number of invaded cells and migrated cells from the RACK1 knockdown group were significantly smaller than those of the negative control (P = 0.0014, P = 0.0108, upper; P = 0.0133, P = 0.0037, lower).
Effects of activation/suppression of protein kinase C on the behavior of ESCC cells
As binding protein for activated protein kinase(PKC), RACK1 plays a major role through its receptor. In order to confirm the function of RACK1 better, we also investigated the effects of PKC on the behavior of Eca109 and EC9706. PMA/TPA was chosen as an activator while staurosporine as an inhibitor.
The result of colony formation assay showed that cell proliferation was significantly increased by PMA(Eca109:791 ± 11 vs. 475 ± 11, P = 0.0012; EC9706:773 ± 11 vs. 487 ± 9, P = 0.0015), and decreased by staurosporine(Eca109:188 ± 14 vs. 475 ± 11, P = 0.0016; EC9706:224 ± 27 vs. 487 ± 9, P = 0.0059), compared with control group. The results were showed in .
Tumors from nude mouse model were measured and analyzed also. The results were showed in . Tumor weight from the PKC activated group, PKC suppressed group, and control group were 0.605 ± 0.00707 g, 0.167 ± 0.141 g, 0.425 ± 0.0354 g in Eca109 and 0.635 ± 0.0354 g, 0.090 ± 0.127 g, 0.445 ± 0.00707 g in EC9706. We found significant difference between the control cells and those of the drug-treated groups(activated group P < 0.0001 in Eca109 and EC9706; suppressed group P = 0.0001 in Eca109 and P = 0.0002 in EC9706, ). Tumor volume from the PKC activated cells and suppressed cells were 890 ± 60.7 mm3, 199 ± 139 mm3 in Eca109 and 890 ± 64.4 mm3, 149 ± 159 mm3 in EC9706 respectively. Compared with the control group, nude mouse implanted with PKC activated cells developed significantly bigger tumors (Eca109:P = 0.0288; EC9706:P = 0.0405, ), and PKC suppressed cells developed smaller tumors (Eca109:P = 0.0308; EC9706:P = 0.0103, ).
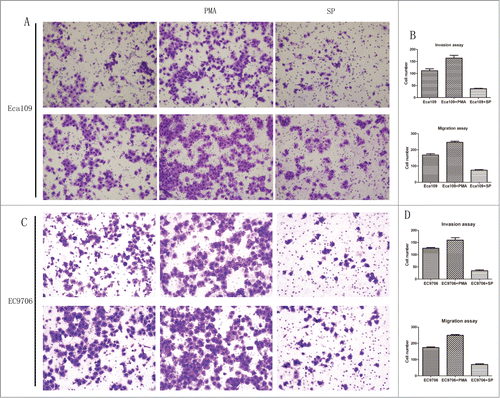
The results of transwell invasion and migration assay demonstrated that PMA increased the invasion and migration of ESCC cells while staurosporine showed the opposite effect. The number of invaded cells and migrated cells from the PKC activated group was 159.5 ± 11.9 and 242.5 ± 7.09 in Eca109, 160.3 ± 9.61 and 249.6 ± 4.51 in EC9706, respectively, which were significantly larger than those of the control group (Eca109: P = 0.0448, P = 0.0040, ; EC9706: P = 0.0138, P = 0.0015, ). The number of invaded cells and migrated cells from the PKC suppressed group was 36.5 ± 1.53 and 73.0 ± 3.79 in Eca109, 34.3 ± 3.79 and 70.3 ± 3.51 in EC9706 respectively, which were significantly smaller than those of the control group(Eca109: P = 0.0049, P = 0.0012; EC9706: P = 0.0007, P = 0.0006).
Figure 6. Activation of protein kinase C promoted invasion and migration of Eca109 while PKC suppression showed the opposite effect. (A and C), representative invaded cells and migrated cells stained by crystal violet. Left, blank control cells; middle, PMA treated cells; right, SP treated cells. Upper: Eca109; lower: EC9706. (B and D), results from cell invasion and migration assay were showed in diagrams. The number of invaded cells and migrated cells from the activated group were significantly larger than those of the control (P = 0.0448, P = 0.0040, upper; P = 0.0138, P = 0.0015, lower), and these number of suppressed group were smaller (P = 0.0049, P = 0.0012, upper; P = 0.0007, P = 0.0006, lower).
All of the results demonstrated that activation of protein kinase C promoted cell growth, invasion and migration of Eca109 and EC9706 while PKC suppression showed the opposite effect. Since RACK1 plays its role through binding with activated PKC, these results also indicate, viewing from another angle, that RACK1 affects the ability of cell proliferation, invasion and migration.
Association of RACK with EMT markers in ESCC
To explore how RACK1 regulated the invasion and migration of ESCC cell lines, we use Western Blotting to detect EMT-related proteins levels after shRACK1 transfected into cells. And the result was shown in . We can find that the levels of vimentin was reduced after RACK1 was downregulated, meanwhile E-cadherin was increased. The WB results showed that RACK1 could regulate EMT-related proteins in ESCC cells.
Figure 7. RACK1 was associated with the expression of EMT markers in ESCC cell lines and ESCC cases. (A) upper, expression of vimentin protein in Eca109 and EC9706 was reduced after shRNA transfection; lower, expression of E-cadherin protein in Eca109 and EC9706 was increased after shRNA transfection. (B and C) Representative immunostainings for EMT markers in a RACK1 positive sample (upper) and a negative sample (lower). Left, RACK1 expression; middle, E-cadherin expression; right, vimentin expression (×200). Vimentin was densely stained in the case of positive RACK1 staining and weakly stained in the case of negative RACK1 staining, while E-cadherin showed an inverse correlation. (D) Association of IHC scores of E-cadherin and vimentin with RACK1 expression. A significant positive correlation was found between RACK1 expression and vimentin, and an inverse correlation between RACK1 expression and E-cadherin (P = 0.0190, P = 0.0047).
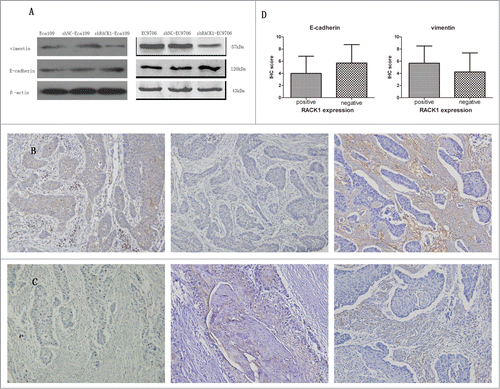
To further study the relationship between RACK1 and EMT-associated proteins, we use immunohistochemistry staining to detect the expression of vimentin and E-cadherin in 100 cases of ESCC patients. Representative samples of E-cadherin and vimentin protein expression in ESCC were shown in . We found there was a significant positive correlation between RACK1 expression and vimentin, and an inverse correlation between RACK1 expression and E-cadherin (, P = 0.0190, P = 0.0047).
Discussion
To reveal the predictive value of RACK1, we collected 100 patients with ESCC and analyzed RACK1 level of them after immunohistochemical staining. Research on clinicopathological characteristics of these patients suggested that RACK1 expression was significantly related with tumor size, T stage, and lymph node metastasis. Survival analyses suggested RACK1 expression in patients with ESCC was significantly related with their DFS and OS (). In a word, RACK1 was associated with larger tumor size, advanced T stage, lymph node metastasis, and poor prognosis for ESCC patients. Results from ESCC cell lines and Heterotopic nude mouse model confirmed that RACK1 promoted cell proliferation, invasion and migration (). To further study how RACK1 promoted migration in ESCC, EMT-related proteins E-cadherin and vimentin were introduced. We found that RACK1 was involved in EMT during cancer migration in ESCC cells and patients ().
The prognostic value of RACK1 has been verified in many malignant tumors. Wang et al.Citation10 found elevated RACK1 alone was an excellent predictor of OSCC recurrence compared with Ki67. Cao et al.Citation11 first identified RACK1 as a superior independent biomarker for diagnosis and prognosis comparing with currently widely used diagnostic index in breast carcinoma. Zhong et al.Citation12 found expression of RACK1 was associated with tumor invasiveness in pulmonary adenocarcinoma and the co-expression of RACK1 and CD147 could be an important prognostic biomarker for stage T1 pulmonary adenocarcinoma. But, to date, there is no report regarding the role of RACK1 level in prognosis of ESCC. Our observation revealed for the first time that the expression of RACK1 was highly related with survival rates of patients with ESCC. We found the survival of patients with RACK1 expression was significantly shorter than that of patients without RACK1 expression (). Multivariate analyses showed that the HR of RACK1 negativity for OS and DFS was 0.532 and 0.523 respectively (). In other words, RACK1 positivity was a risk factor for the survival of ESCC patients. Previous study found that the aim of multiplexed biomarkers discovery was to determine which treatment provides the greatest benefit for individuals and select the appropriate preventive measures for each individual at high risk or with poor prognosis to improve outcomes.Citation18 Since RACK1 positivity is associated with a poorer prognosis, patients with different level of RACK1 expression may receive different treatment regimens to make sure that each individual get maximum benefit from their regimens, and receive the right preventive intervention. Patients can get improved survival and save lives in this way.
As to RACK1s role in tumor growth, data from clinical analysis revealed that RACK1 expression was significantly associated with tumor size (, length: P = 0.012; Diameter: P = 0.047). This result was consistent with the effect of RACK1 on cell proliferation in vitro() and vivo(). RACK1 was reported to affect cell proliferation in various cancers. Through activating Sonic Hedgehog signaling pathway, RACK1 promoted non-small-cell lung cancer tumorigenicity.Citation19 RACK1 promoted hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase Kinase 7 activity.Citation20 However, it does not always act as promoter in cancer proliferation, sometimes RACK1 is functioned as a suppressor. RACK1 suppressed gastric tumorigenesis through Wnt/β-catenin signaling pathway by stabilizing the β-Catenin destruction complex.Citation21 RACK1 inhibited growth of colon cells by suppressing Src activity at G1 and mitotic checkpoints, and consequently delaying cell cycle progression.Citation22
Consistent with previous findings,Citation23 our study showed that RACK1 promoted cell invasion and migration(; ). Although many carcinogenesis activities are covered when researching how RACK1 promotes migration in ESCC, the correlation between RACK1 and EMT is not reported before. Previous study showed that RACK1 linked integrin engagement with focal adhesion disassembly and cell motility, reacted with IGF-1 to regulate tumor cell proliferation and migration.Citation24 RACK1 controls cancer cell polarity by linking FAK to the recruitment of PDE4D5.Citation25 In our study, we found RACK1 promoted tumor invasion and migration through EMT both in ESCC cells and clinical samples().
With regard to the mechanism of RACK1 as an independent predictor in ESCC, EMT is proved to play a critical role. RACK1 is significantly associated with the depth of tumor invasion and lymph node metastasis in ESCC, which directly lead to poor prognosis. It is EMT by which cells acquiring enhanced migration and invasive abilities, which was confirmed in the present study (). Therefore, RACK1 may participate in primary tumor invasion to deeper layer of human tissue and lymphatic development in ESCC by metastasizing cells who undergo RACK1-mediated EMT. It is a pity that no further studies about how RACK1 regulates lymphatic metastasis and invasion to deeper layer of human tissue are found.
In conclusion, our results clearly suggested that RACK1 was an excellent prognostic factor in ESCC, promoted cell proliferation and migration, and was involved in EMT of ESCC. As a novel biomarker, RACK1 can provide a more accurate prediction of the clinical outcome for patients with ESCC. But how RACK1 is involved in the tumorigenesis and EMT of ESCC and which mechanism lies behind its involvement still remains unknown to us. To address this issue, further study is required.
Material and Methods
Patients and tissue samples
To investigate the expression of RACK1 protein in cancerous tissue, 100 patients were included, who underwent esophagectomy at Qilu Hospital of Shandong University in Jinan (China) from January 2007 to December 2007. The data were procured from surgical pathology files maintained in the Pathology Department. None of these patients had received preoperative adjunctive therapy or suffered from severe postoperative complications. The selected cases involved 79 men and 21 women, aged 42 to 78 y (median, 60 years). After esophagectomy, these patients were closely followed up for 3.0–71.0 months until in January 2013 (median, 49.5 months).
Immunohistochemistry staining
All the specimens had been routinely formalin-fixed, paraffin-embedded, and serially sectioned at 5 μm in thickness. Tissue sections were deparaffinized in xylene and hydrated in graded ethanol. Then the sections were incubated in 3% hydrogen peroxide at room temperature for 5 to 10 minutes to eliminate endogenous peroxidase activity. After that they were heated in 10 mM citrate buffer (pH 6.0) for antigen retrieval. Goat serum was used to block the nonspecific binding. The sections were incubated with primary antibody (RACK1, 1:150, BD Biosciences; vimentin and E-cadherin, 1:100, DAKO) overnight at 4°C. After that the sections were washed and stained with secondary antibodies for 30 min at 37°C. The sections were incubated with avidin–biotin complex for 60 min at 37° and peroxidase activity was developed with diaminobenzidine (DAB, ZLI-9032, ZSGB; Beijing, China). All sections were counterstained with hematoxylin. Images were obtained under a light microscope. For the negative controls, PBS was used to substitute primary antibody.
Evaluation of immunostaining intensity
Immunohistochemical signals were scored by 2 independent observers. Five spots of each stained section were calculated and the results were averaged. Two-way scoring system was used for the analysis of RACK1 results in the present studies. The final score was achieved by multiplying the staining intensity and the percentage of staining cells. Staining intensity was scored as follows: 0, no staining; 1, weak; 2, moderate; 3, strong. Percentage of staining cells was scored as follows: 0, 0%; 1, 1%–24%; 2, 25%–49%; 3, 50%–74%; 4, 75%–100%. The samples were judged as follows: negative (final scores, ≤4 ) and positive (final score, >4).Citation21
Cell culture and transfection
Human esophageal squamous carcinoma Eca109 and EC9706 cell lines were cultured in DMEM (Hyclone) supplemented with 10% fetal bovine serum and incubated at 37°Cin a humidified atmosphere containing 5% CO2. Cells were transfected with Lipofectamine 2000 reagent (Invitrogen). Plasmid with either short hairpin (shRNA) targeting RACK1 gene or nonsense shRNA were transfected into Eca109 and EC9706 cell lines. Stably transfected cells were selected with 600 μg/mL of G418.
Drug treatment
Eca109 and EC9706 cell lines were treated with 100 nM PMA/TPA for 20 h, which was considered as PKC activated group. Some other Eca109 cells and EC9706 were treated with 100 nM staurosporine for 20 h, which was considered as PKC suppressed group.
Western blotting
Cells were lysed and proteins were extracted. The extracts were separated by SDS-PAGE gel electrophoresis. Then the separated bands were blotted onto polyvinylidene difluoride membrane. The membranes were blocked with 5% non-fat dry milk in Tris buffered.
saline (TBS, pH 7.4) and incubated with primary antibody(RACK1, 1:1000, BD Biosciences; vimentin and E-cadherin, 1:1000, DAKO) overnight at 4°C. Then peroxidase-conjugated IgG was used as a secondary antibody. Immunoreactivity was detected with an enhanced chemiluminescence reaction kit (Thermo Scientific Pierce). Actin (1:1000, Santa Cruz Biotechnology) was used as a loading control.
RNA interference of RACK1
A plasmid-mediated shRNA was used for down-regulation of RACK1 expression in Eca109 and EC9706. pGPU6/GFP/Neo plasmid targeting RACK1(shRACK1) was generated by Shanghai JIMA Biologic Company China. The RACK1-specific shRNA sequence was as follows: sense 5-CACCGCATGTATGCATGTGACTTATTTCAAGAGAATAAGTCACAT
GCATACATGCTTTTTG-3; antisense 5-GATCCAAAAAAGCATGTATGCATGTGACTTATT
CTCTTGAAATAAGTCACATGCATACTGC-3. Nonsense shRNA was served as negative control.
Colony formation assay
Transfected cells were treated by Trypsin into single cell suspension. The cell suspension was diluted and cells were plated in dishes. 1 × 103 cells per dish were cultured at 37°C, in 5% CO2 and saturated humidity environment for 2 weeks. Nonsense shRNA transfected cells were used as controls. The colonies were fixed, stained, and counted.
Heterotopic nude mouse model
Transfected cells were treated by Trypsin into single cell suspension. After centrifugation and resuspension the cells were transferred into 1.0 × 107/ml. The cells were injected subcutaneously into the left flank of male nude mice aged 4–6 weeks. Nonsense shRNA transfected cells were used as controls. Each group contained 6 mice. Tumor growth was measured once a week. The mice were sacrificed after 4 weeks and the tumors were removed, weighted and measured. After weighing and measuring they were fixed in 10% formalin for histopathological analyses. Tumor volume was calculated using the formula: tumor volume (mm3) = a × b2 × 0.5, where a represents the longest diameter, b is the shortest diameter.
Transwell invasion and migration assay
We coated a transwell membrane (8-μm pore size, 6.5-mm diameter, Corning Costar) with Matrigel (BD Biosciences). Then we resuspended ESCC cell lines in serum-free medium and added the cells to the upper chamber of precoated transwells at a density of 2.0 × 105/ml. The lower chamber contained normal medium with 10% FBS. After incubation for 24 h, a cotton-tipped swab was used to swab the cells on the upper chamber. The invasive cells, which were attached to the lower surface of the membrane, were fixed with methanol and stained with 0.1% Crystal violet (Sigma). We counted the number of invasive cells (5 fields per filter) under an inverted microscope and calculated the mean numbers of invasive cells. These experiments were performed in triplicate and repeated 3 times. The experimental procedures of transwell migration assay were similar to the transwell invasion assay except that the membrane was not coated with matrigel and the time of incubation was shortened to 12 h.
Ethical standards
All human and animal studies have been approved by the ethics committee of Qilu Hospital of Shandong University. All patients gave their informed consent prior to their inclusion in the study.
Statistical analysis
All statistical analyses were performed using the SPSS 19.0 software (SPSS, Chicago, IL). To test the relationship between the expression of RACK1 protein and clinicopathologic characteristics, Student t test was used for continuous variables and chi-square test or Fisher's exact test for categorical variables. Cox's proportional hazards model was used to assess the prognostic value of RACK1 protein expression associated with overall survival and disease-free survival. Univariable Cox regression analyses were performed using disease recurrence or death as the outcomes with a significance level of P < 0.05. The Cox proportional hazards model with a stepwise procedure was used for multivariate analysis. Survival analyses were performed by Kaplan–Meier curves with log rank tests for significance. For the experiments related to cells, the data are presented as the mean ± SD and 3 individual experiments were performed in triplicate. Two-tailed t test analysis was used to compare the data from different groups. Two-sided P values <0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the Academics Independent Innovation Project of Jinan City (201401252).
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90; PMID:21296855
- Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci U S A 1991; 88:3997–4000; PMID:1850844; http://dx.doi.org/10.1073/pnas.88.9.3997
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci U S A 1994; 91:839–43; PMID:8302854; http://dx.doi.org/10.1073/pnas.91.3.839
- Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun Signal 2011; 9:22; PMID:21978545; http://dx.doi.org/10.1186/1478-811X-9-22
- Vomastek T, Iwanicki MP, Schaeffer HJ, Tarcsafalvi A, Parsons JT, Weber MJ. RACK1 targets the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway to link integrin engagement with focal adhesion disassembly and cell motility. Mol Cell Biol 2007; 27:8296–305; PMID:17908799; http://dx.doi.org/10.1128/MCB.00598-07
- Mamidipudi V, Zhang J, Lee KC, Cartwright CA. RACK1 regulates G1/S progression by suppressing Src kinase activity. Mol Cell Biol 2004; 24:6788–98; PMID:15254245; http://dx.doi.org/10.1128/MCB.24.15.6788-6798.2004
- Mamidipudi V, Chang BY, Harte RA, Lee KC, Cartwright CA. RACK1 inhibits the serum- and anchorage-independent growth of v-Src transformed cells. FEBS Lett 2004; 567:321–6; PMID:15178345; http://dx.doi.org/10.1016/j.febslet.2004.03.125
- Steele MR, McCahill A, Thompson DS, MacKenzie C, Isaacs NW, Houslay MD, Bolger GB. Identification of a surface on the beta-propeller protein RACK1 that interacts with the cAMP-specific phosphodiesterase PDE4D5. Cell Signal 2001; 13:507–13; PMID:11516626; http://dx.doi.org/10.1016/S0898-6568(01)00167-X
- Bolger GB, McCahill A, Yarwood SJ, Steele MR, Warwicker J, Houslay MD. Delineation of RAID1, the RACK1 interaction domain located within the unique N-terminal region of the cAMP-specific phosphodiesterase, PDE4D5. BMC Biochem 2002; 3:24; PMID:12193273; http://dx.doi.org/10.1186/1471-2091-3-24
- Wang Z, Zhang B, Jiang L, Zeng X, Chen Y, Feng X, Guo Y, Chen Q. RACK1, an excellent predictor for poor clinical outcome in oral squamous carcinoma, similar to Ki67. Eur J Cancer 2009; 45:490–6; PMID:19087901; http://dx.doi.org/10.1016/j.ejca.2008.11.012
- Cao XX, Xu JD, Liu XL, Xu JW, Wang WJ, Li QQ, Chen Q, Xu ZD, Liu XP. RACK1: a superior independent predictor for poor clinical outcome in breast cancer. Int J Cancer 2010; 127:1172–9; PMID:20020495; http://dx.doi.org/10.1002/ijc.25120
- Zhong X, Li M, Nie B, Wu F, Zhang L, Wang E, Han Y. Overexpressions of RACK1 and CD147 associated with poor prognosis in stage T1 pulmonary adenocarcinoma. Ann Surg Oncol 2013; 20:1044–52; PMID:22592183; http://dx.doi.org/10.1245/s10434-012-2377-4
- Wu J, Meng J, Du Y, Huang Y, Jin Y, Zhang J, Wang B, Zhang Y, Sun M, Tang J. RACK1 promotes the proliferation, migration and invasion capacity of mouse hepatocellular carcinoma cell line in vitro probably by PI3K/Rac1 signaling pathway. Biomed Pharmacother 2013; 67:313–9; PMID:23582786; http://dx.doi.org/10.1016/j.biopha.2013.01.011
- Ruan Y, Sun L, Hao Y, Wang L, Xu J, Zhang W, Xie J, Guo L, Zhou L, Yun X, et al. Ribosomal RACK1 promotes chemoresistance and growth in human hepatocellular carcinoma. J Clin Invest 2012; 122:2554–66; PMID:22653060; http://dx.doi.org/10.1172/JCI58488
- Cao XX, Xu JD, Xu JW, Liu XL, Cheng YY, Wang WJ, Li QQ, Chen Q, Xu ZD, Liu XP. RACK1 promotes breast carcinoma proliferation and invasion/metastasis in vitro and in vivo. Breast Cancer Res Treat 2010; 123:375–86; PMID:19946739; http://dx.doi.org/10.1007/s10549-009-0657-x
- Shen F, Yan C, Liu M, Feng Y, Chen Y. RACK1 promotes prostate cancer cell proliferation, invasion and metastasis. Mol Med Rep 2013; 8:999–1004; PMID:23912224
- Hu F, Tao Z, Wang M, Li G, Zhang Y, Zhong H, Xiao H, Xie X, Ju M. RACK1 promoted the growth and migration of the cancer cells in the progression of esophageal squamous cell carcinoma. Tumour Biol 2013; 34(6):3893–9; PMID:23893384
- Johann DJ Jr., McGuigan MD, Patel AR, Tomov S, Ross S, Conrads TP, Veenstra TD, Fishman DA, Whiteley GR, Petricoin EF 3rd, et al. Clinical proteomics and biomarker discovery. Ann N Y Acad Sci 2004; 1022:295–305; PMID:15251975; http://dx.doi.org/10.1196/annals.1318.045
- Shi S, Deng YZ, Zhao JS, Ji XD, Shi J, Feng YX, Li G, Li JJ, Zhu D, Koeffler HP, et al. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J Biol Chem 2012; 287:7845–58; PMID:22262830; http://dx.doi.org/10.1074/jbc.M111.315416
- Guo Y, Wang W, Wang J, Feng J, Wang Q, Jin J, Lv M, Li X, Li Y, Ma Y, et al. Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology 2013; 57:140–51; PMID:22903704; http://dx.doi.org/10.1002/hep.25978
- Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long LY, Li G, Ji XD, Shi S, Guan DX, et al. RACK1 suppresses gastric tumorigenesis by stabilizing the beta-catenin destruction complex. Gastroenterology 2012; 142:812-23 e15; PMID:22240482; http://dx.doi.org/10.1053/j.gastro.2011.12.046
- Mamidipudi V, Dhillon NK, Parman T, Miller LD, Lee KC, Cartwright CA. RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. Oncogene 2007; 26:2914–24; PMID:17072338; http://dx.doi.org/10.1038/sj.onc.1210091
- Cao XX, Xu JD, Xu JW, Liu XL, Cheng YY, Li QQ, Xu ZD, Liu XP. RACK1 promotes breast carcinoma migration/metastasis via activation of the RhoA/Rho kinase pathway. Breast Cancer Res Treat 2011; 126:555–63; PMID:20499158; http://dx.doi.org/10.1007/s10549-010-0955-3
- Kiely PA, Baillie GS, Lynch MJ, Houslay MD, O'Connor R. Tyrosine 302 in RACK1 is essential for insulin-like growth factor-I-mediated competitive binding of PP2A and beta1 integrin and for tumor cell proliferation and migration. The J Biol Chem 2008; 283:22952–61; PMID:18567578; http://dx.doi.org/10.1074/jbc.M800802200
- Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, Canel M, Machesky LM, Anderson KI, Frame MC. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol 2010; 20:1086–92; PMID:20493699 10.1016/j.cub.2010.04.042
