Abstract
Phosphoglycerate dehydrogenase (PHGDH) is the key enzyme of de novo serine biosynthesis. Previous reports have demonstrated that PHGDH plays an important role in some malignancies. However, the biological role of PHGDH in human cervical adenocarcinoma has not been explored. We examined the expression of PHGDH in 54 cervical adenocarcinoma samples by immunohistochemistry and evaluated the association with clinicopathological parameters and prognosis. We performed shRNA transfection to knock down PHGDH gene expression in HeLa cells. A cell proliferation test, cisplatin cytotoxicity test and apoptosis test examined the HeLa cell line after PHGDH knockdown in vitro. In vivo tumorigenesis was assessed using a mouse xenograft model. Moreover, we examined the effects on Bcl-2 and cleaved caspase-3 expression after knockdown of PHGDH and treatment of cisplatin for 48h by Western blot. In this study, we demonstrated that elevated PHGDH expression was found in cervical adenocarcinoma and was associated with tumor size and prognosis. Knocking down PHGDH in HeLa cells significantly inhibited cell proliferation and increased cisplatin chemotherapy sensitivity. Silencing PHGDH resulted in inhibition of tumorigenesis in vivo. Furthermore, PHGDH knockdown reduced Bcl-2 and increased cleaved caspase-3 expression. Collectively, our study indicates the novel roles of PHGDH in cervical adenocarcinoma and identifies PHGDH as a new anticancer target.
Abbreviations
| Bcl-2 | = | B cell leukemia/lymphoma-2 |
| Caspase | = | Cysteinyl aspartate specific proteinase |
| CCK-8 | = | cell counting kit-8 |
| DMEM | = | Dulbecco's Modified Eagle Medium |
| FBS | = | fetal calf serum |
| G418 | = | Geneticin |
| GAPDH | = | Glyceraldehyde-3-phosphate dehydrogenase |
| HPV | = | human papilloma virus |
| ICC | = | immuocytochemistry |
| IHC | = | immunohistochemistry |
| PHGDH | = | phosphoglycerate dehydrogenase |
| shRNA | = | short hairpin RNA |
Introduction
Cervical cancer is the third most commonly diagnosed malignant tumor in women worldwide.Citation1 Due to a lack of screening of precancerous lesions and early stage cervical cancer, women in some developing countries have higher incidence rates and are more often diagnosed with advanced stages compared with women in developed countries.Citation2 Moreover, increasing incidence and mortality rates of cervical adenocarcinoma has been reported in many countries among young women.Citation3 Cervical adenocarcinoma is less sensitive to radiotherapy compared to cervical squamous carcinoma. Thus, chemotherapy is a very important adjuvant treatment. Therefore, elevating chemosensitivity of cervical adenocarcinoma and exploring novel treatments are crucial to reducing mortality and promoting prognosis.
A cancerous cell's metabolism differs from a quiescent cell's metabolism. Cancer cells utilize specific alternative pathways derived from glucose for proliferation. It was found that in some cancer cells a relatively large amount of glycolytic carbon flowed into the serine biosynthesis pathway by isotope labeling approach.Citation4 Phosphoglycerate dehydrogenase (PHGDH), which is the key enzyme of de novo serine biosynthesis, oxidizes the glycolytic intermediate 3-phosphoglycerate to generate serine and glycine, providing numerous precursor molecules and other substrates required for cell growth and proliferation.Citation5 It was reported that PHGDH was overexpressed in a subset of breast cancer, melanoma and glioma.Citation4,6,7 Knockdown of PHGDH inhibited the proliferation of amplified breast cancer cell lines. PHGDH could be the new anticancer target. Hyun Min Cho and colleagues found that PHGDH mRNA was markedly expressed in epitheloid carcinoma HeLa S3.Citation8 We previously reported that PHGDH was overexpression in cervical cancer tissues. The expression of PHGDH in cervical cancer was positively related to FIGO stage and tumor size.Citation9 As the number of cases of cervical adenocarcinoma was limited in the prior study, we increased it in this study. Moreover, the effects of PHGDH on cervical adenocarcinoma were not referred to.
To further explore the function of PHGDH in cervical adenocarcinoma, we tested the effects of PHGDH on cell proliferation and cisplatin chemotherapy sensitivity in the HeLa cell line and researched its relevant mechanisms.
Results
PHGDH expression was elevated in cervical adenocarcinoma tissues
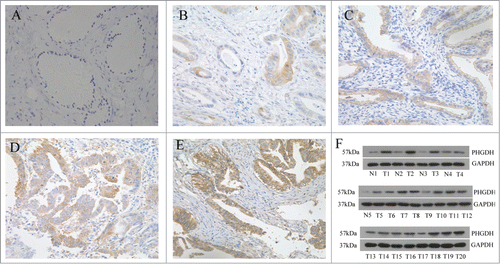
We measured PHGDH expression in 54 cases of cervical adenocarcinoma by immunohistochemistry (IHC). Ten subjects with normal cervical glandular epithelium were used as control. PHGDH was predominantly present in the cytoplasm and membrane of cervical adenocarcinoma cells. IHC staining indicated that moderate/strong positive PHGDH expression was observed in 66.7% (36/54) of cervical adenocarcinoma tissues compared with 20% (2/10) of the normal cervical glandular epithelium (P < 0.05, ). To validate the IHC staining results, we performed Western blot in 20 random cases of cervical adenocarcinoma tissues (T) and 5 cases of normal cervical epithelium (N). We found that PHGDH protein was significantly upregulated in tumor tissues compared with normal tissues ().
Figure 1. PHGDH was upregulated in cervical adenocarcinoma tissues. (A) Negative PHGDH staining in normal cervical glandular epithelium. (B and C) Negative/weak staining of PHGDH in cervical adenocarcinoma tissues. (D and E) Moderate/strong staining of PHGDH in cervical adenocarcinoma tissues. (F) Western blot analysis showed PHGDH expression was higher in cervical adenocarcinoma tissues than that in normal cervical epithelium cells relative to the loading control (Glyceraldehyde 3-phosphate dehydrogenase).
Association of expression of PHGDH with clinicopathological parameters
We then assessed the relationship between PHGDH expression and clinicopathological variables. As shown in , expression of PHGDH was not related to age (P = 0.838), advanced FIGO stage (P = 0.275), lymph node metastasis (P = 0.583), depth of infiltration (P = 0.142) or high-risk human papilloma virus infection (P = 0.428), but positively associated with tumor size (P = 0.027). This provided evidence that PHGDH played a role in cervical adenocarcinoma occurrence and progression.
Table 1. The correlation between expression of PHGDH and clinicopathological parameters in cervical adenocarcinoma
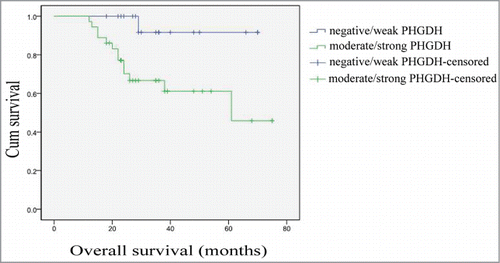
Correlations between PHGDH overexpression and prognosis of cervical adenocarcinoma patients
The median followup time was 29 months (range, 12–75 months). The association between PHGDH expression and prognosis of cervical adenocarcinoma patients was investigated by Kaplan–Meier analysis and log-rank test. Patients with moderate/strong PHGDH expression had a shorter overall survival rate than those with negative/weak PHGDH expression ().
Downregulation of PHGDH inhibited cell proliferation in vitro
To further investigate the functions of PHGDH in cervical adenocarcinoma, we utilized shRNA plasmids to stably silence PHGDH. We assessed efficient knockdown of PHGDH in the HeLa cells transfected with 2 independent shRNA plasmids (shPHGDH-1, shPHGDH-2) by immunocytochemistry (ICC) () and Western blot (). HeLa cells transfected with the empty vector (HeLa-vec) was used as a control.
Figure 3. PHGDH knockdown inhibited cell proliferation in vitro. (A) Downregulation of PHGDH expression by shRNA in HeLa cells was confirmed by ICC. (B) Western blot analysis confirmed PHGDH downexpression in PHGDH-knockdown cells. (C) CCK-8 assays demonstrated that knockdown of PHGDH in HeLa cells induced a remarkable reduction in cell proliferation. Data were expressed as the means ± SD from 3 separate experiments; *P < 0.05, ** P < 0.01.
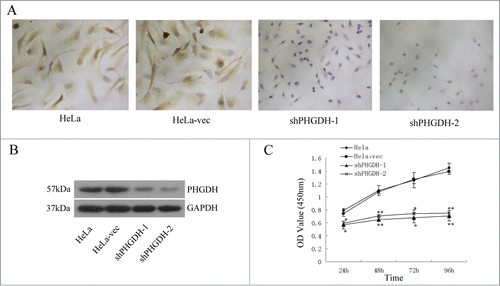
We next tested whether PHGDH knockdown affects the proliferation of HeLa cells using CCK-8 (Cell Counting Kit-8) assays. It was shown that knockdown of PHGDH significantly inhibited the growth of HeLa cells in vitro ().
PHGDH knockdown suppressed tumor growth in vivo
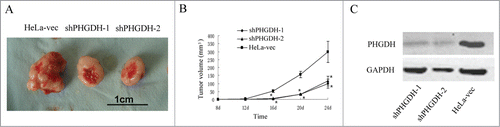
Furthermore, to confirm the effects of PHGDH on cervical adenocarcinoma cell growth in vivo, HeLa cells with PHGDH knockdown and control HeLa-vec cells were injected into the axilla of nude mice to establish a mouse xenograft model. Our results showed that the tumor volumes of the mice injected with shPHGDH-1or shPHGDH-2 cells were significantly smaller compared with those injected with HeLa-vec cells (P < 0.05, respectively, ). Additionally, the results of the Western blot confirmed the downexpression of PHGDH in tumors from shPHGDH mice (). These results indicated that PHGDH knockdown inhibited tumorigenesis of HeLa cells in vivo.
Figure 4. Downregulation of PHGDH suppressed growth of primary cervical adenocarcinoma tumors in a mouse xenograft model. (A) Photograph of a tumor developed in the subcutaneous implanted model. (B) A statistical plot of average tumor volume in the subcutaneous implanted model. Compared with HeLa-vec controls, the average tumor volumes from mice in shPHGDH-1or shPHGDH-2 groups were markedly smaller. The graph shows the mean ± SD; *P < 0.05. (C) Downexpression of PHGDH in tumors from shPHGDH mice was confirmed by Western blot.
Downregulation of PHGDH increased the sensitivity of HeLa cells to cisplatin
Cytotoxicity tests were performed to study the influence of PHGDH on the sensitivity of HeLa cells to cisplatin. First, each group of cells was treated with different concentrations of cisplatin (0.01–10 μg/ml) for 48 h. The CCK-8 assays were used to evaluate the cell growth inhibition rates. In addition, 5 μg/ml cisplatin was treated to different groups of cells for 24 h, 48 h, and 72 h. As shown in , knocking down of PHGDH could enhance the inhibitory effects of cisplatin on HeLa cell growth. The IC50 values of cisplatin were calculated by probit regression analysis. PHGDH knockdown reduced the IC50 values of cisplatin in HeLa cells ().
Figure 5. PHGDH knockdown increased the sensitivity of HeLa cells to cisplatin. (A) CCK-8 assays showed the cell growth inhibition rate in shPHGDH cells was higher compared with HeLa-vec cells under different concentration of cisplatin. (B) The cell growth inhibition rates of shPHGDH cells were significantly higher after treatment with 5 μg/ml cisplatin for 24 h, 48 h, and 72 h. (C) After treatment with 0, 5 or 10 μg/ml cisplatin for 48 h, the apoptosis rate was analyzed with flow cytometry. UR + LR indicated apoptosis. (D) Quantification of the data from Figure 5C. Data were expressed as the means ± SD from 3 separate experiments; *P < 0.05, ** P < 0.01.
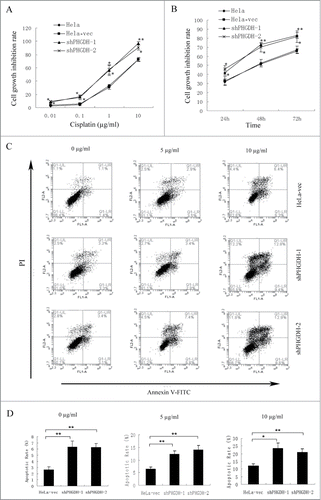
Table 2. Cisplatin IC50 value of each group of HeLa cells
To further assess the effect of PHGDH on HeLa cell apoptosis induced by cisplatin, we utilized flow cytometry to examine the influence of PHGDH knockdown on the cell apoptosis rate of the HeLa cells after treatment with 5 μg/ml or 10 μg/ml cisplatin for 48 h or in the absence of cisplatin. With 0, 5 or 10 μg/ml cisplatin treatment, the apoptosis rate of HeLa cells following PHGDH knockdown was significantly higher than that of HeLa-vec cells ().
Taken together, these results indicated that PHGDH knockdown could enhance the sensitivity of cisplatin on HeLa cells.
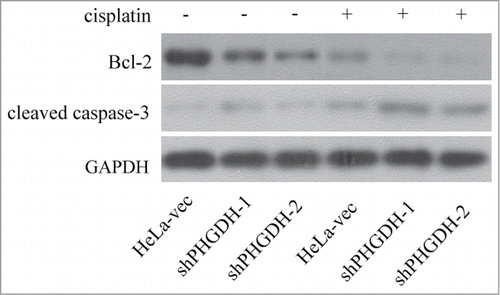
Effect of PHGDH knockdown on the expression of Bcl-2 and caspase-3
To elucidate the mechanisms of PHGDH downexpression inhibiting cell proliferation and increasing sensitivity to cisplatin, we investigated the expression of Bcl-2 and cleaved caspase-3, which are key proteins to regulate apoptosis, in shPHGDH-1, shPHGDH-2 and HeLa-vec cells. No matter whether cells were treated with 5 μg/ml cisplatin for 48 h or not, decreased Bcl-2 and increased cleaved caspase-3 expression were noted in shPHGDH-1or shPHGDH-2 cells compared to HeLa-vec cells (P < 0.05, ). Therefore, we concluded that PHGDH might influence cell proliferation and cisplatin sensitivity through the Bcl−2 and caspase-3 apoptotic pathway.
Discussion
In recent years, interest in cancer metabolism has been renewed. Mutations in tumor suppressors and oncogenes could reprogram cellular metabolisms in order to support their growth and survival.Citation10 Furthermore, some of these metabolic alterations seem to be absolutely required for malignant transformation.Citation11 Altered metabolisms supply 4 major classes of macromolecules, such as carbohydrates, proteins, lipids and nucleic acids, which are beneficial to cell proliferation. It is well known that metabolism reprogramming is an emerging hallmark of the cancer cell. Recent evidence implies that metabolic reprogramming can also occur as the result of genomic modifications of metabolic enzymes and that these alterations independently contribute to tumorigenesis.Citation12 Moreover, metabolites themselves can be oncogenic by altering cell signaling and blocking cellular differentiation.Citation13 It has been concluded that the growing cancer cell is addicted to key enzymes of the metabolic pathway. We could develop novel treatment strategies to improve the response to therapy by researching cell metabolism.
The serine biosynthesis pathway provides many advantages for growing cells. First, it provides precursors for a variety of biosynthetic pathways. Second, conversion of serine to glycine by serine hydroxymethyltransferase is a major source of methyl groups for the one carbon pools required for biosynthesis and DNA methylation. Third, it generates reduced nicotinamide adenine dinucleotide phosphate to maintain appropriate cellular redox status. Furthermore, it generates serine as well as equimolar amounts of α-ketoglutarate. Possemato et al. indicated that α-ketoglutarate production might be the main role of the PHGDH pathway, as it provides 50% of the total anaplerotic flux of glutamine into the citric acid cycle.Citation14 So as the key enzyme of the serine biosynthesis pathway, PHGDH should be crucial for tumor research.
PHGDH is located in chromosome 1p12 and has a predicted 533 amino acid open reading frame, encoding a 56.8 kDa protein.Citation8 Early in 1986, Snell and colleagues reported that the enzyme activity of PHGDH was significantly higher in rat hepatoma, human colon carcinoma and rat sarcoma than in corresponding normal tissue.Citation5,15 Recently, a number of researchers have focused on PHGDH. The overexpression of PHGDH was found in breast cancer, melanoma and glioma. In breast cancer, PHGDH expression was associated with both triple-negative (PR-, ER-, HER2-) and basal sub-types.Citation4,16 In the present study, we found that the expression of PHGDH was elevated in cervical adenocarcinoma tissues and positively correlated with tumor size. Liu et al. suggested that PHGDH was a prognostic marker for patients with glioma with limited samples.Citation7 Kim and colleagues found positive tumoral PHGDH was associated with short overall survival rates (P = 0.019) in breast cancer patients by univariate analysis. However, by multivariate Cox analysis, tumoral PHGDH negativity correlated with short disease-free survival in triple-negative breast cancer.Citation16 Our study indicated overexpression of PHGDH correlated with short overall survival in patients with cervical adenocarcinoma. Therefore, further studies are needed to confirm these findings with larger sample sizes.
Possemato and colleagues found that RNAi-mediated suppression of PHGDH caused a marked decrease in cell number in cell lines with elevated PHGDH expression.Citation14 it was also indicated that PHGDH suppression can adversely affect growth in existing tumors in vivo. It was reported by Locasale et al. that downregulation of PHGDH in amplified melanoma cell lines caused significantly decreased proliferation.Citation4 Additionally, Liu's work demonstrated that PHGDH influenced the proliferation, invasion and tumorigenicity of glioma cells.Citation7 Recently, Chen and colleagues found that PHGDH was dispensable for breast tumor maintenance and growth in vivo.Citation17 The reason for discrepancy might be that prior studies demonstrated the effect of PHGDH on tumor initiation. In our study, both in vitro and in vivo studies demonstrated that knockdown of PHGDH decreased the cell proliferation of the HeLa cell line. To explore the effect of PHGDH on tumor maintenance, further studies are required. The first chemotherapeutic strategy against cervical adenocarcinoma is cisplatin-based chemotherapy. However, cisplatin resistance leads to poor clinical effect.Citation18 For the first time, we observed that knockdown of PHGDH increased cisplatin chemotherapy sensitivity in the HeLa cell line by cytotoxicity test and apoptosis test. Collectively, these results indicated that PHGDH may play an important role in cell proliferation and tumorigenesis. PHGDH could be a potential target for cervical adenocarcinoma therapy.
In general, the exact mechanism of PHGDH function is still unknown. The most prominent action mode of cisplatin involves the generation of DNA lesions followed by the activation of the DNA damage response and the induction of mitochondrial apoptosis.Citation19 Many mechanisms have been related to the resistance of cisplatin, including decreased intracellular concentration, increased reflux or increased inactivation by sulfhydryl molecules, nucleotide excision repair system, DNA mismatch repair system and altered expression of regulatory proteins that control the apoptotic pathway.Citation20 Apoptosis is essential for the maintenance of tissue homeostasis and the elimination of unwanted or damaged cells from multicellular organisms.Citation21 Thus, induction of apoptosis in tumor cells has been considered as a protective mechanism against progression of cancer. Bcl-2 is an important anti-apoptosis factor and is highly expressed in more than half of the human tumors.Citation22-24 Caspases are responsible for crucial aspects of apoptosis-induced cell death.Citation23 It was indicated that the overexpression of Bcl-2 inhibits mitochondrion releasing cytochrome C to cytoplasma and prevents specific caspase-3 cascade activation. So the overexprssion of Bcl-2 and downexpression of cleaved caspase-3 inhibit apoptosis and induce resistance to chemotherapy.Citation25,26 We observed decreasing Bcl-2 and increasing cleaved caspase-3 expression in PHGDH knockdown HeLa cells. Unfortunately, a direct link between PHGDH and Bcl-2 or caspase-3 remains unelucidated. Cisplatin induces apoptosis through multiple mechanisms to suppress tumors. The enhanced cisplatin-induced apoptosis by PHGDH knockdown may be attributed to the regulated response of tumor cells to chemotherapy besides the direct regulation of cell cycle and apoptosis.
In conclusion, we found that PHGDH significantly upregulated in cervical adenocarcinoma tissues. Overexpression of PHGDH correlated with lager tumor size and shorter overall survival. Knockdown of PHGDH could inhibit HeLa cell proliferation both in vitro and in vivo and enhance cisplatin sensitivity in HeLa cells. Moreover, decreased Bcl-2 and increased cleaved caspase-3 expression were noted after knockdown of PHGDH. Therefore, these results suggest that combining PHGDH gene therapy with traditional chemotherapy may be more effective in cervical adenocarcinoma treatment via the enhancement of apoptosis through modulating relevant molecules. PHDGH might serve as a novel target for cancer therapy.
Materials and Methods
Clinical specimens
We retrieved formalinfixed, paraffin-embedded tissue blocks from the archives of the Department of Pathology, China Medical University affiliated Shengjing Hospital (Shenyang, Liaoning province) from July 2008 to June 2013. All patients with cervical cancer were histologically confirmed. 45 patients underwent radical hysterectomy with lymphadenectomy without any neoadjuvant chemotherapy or preoperative radiotherapy and 9 patients underwent biopsy first before treatment. The control cases underwent hysterectomy with uterus myoma. Clinical data and clinicopathological information of each case was obtained from computerized medical record system. For Western blot analysis, fresh cervical cancer tissues were collected from 2012 to 2013 and frozen in liquid nitrogen, then stored at −80°C until use.
Immunohistochemistry staining
IHC staining for PHGDH antigens was performed on 4mm sections obtained from formalin‐fixed, paraffin embedded blocks, using streptavidin-perosidase method. The primary antibody used was PHGDH mouse monoclonal antibody (sc-100317, Santa Cruz Biotechnology, CA). Brown staining of the cytoplasm and plasma membrane indicated positive. The staining results for PHGDH were scored semiquantitatively. Scores representing the percentage of tumor cells stained positive were as follows: zero, no positive tumor cells; 1, 1% to 25% positive tumor cells; 2, 26% to 50% positive tumor cells; 3, 51% to 75% positive tumor cells; and 4, 76% to 100% positive tumor cells. Intensity was scored as follows: zero, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. A final score was calculated by adding the scores for percentage and intensity, resulting in scores of zero and 2 to 7. A score of zero was deemed negative; 2 to 3 was considered weak; 4 to 5 was considered moderate; and 6 to 7 was considered strong.
Cell culture and main reagents
HeLa cell line was purchased from Shanghai institute of cell, Chinese Academy of Sciences and was cultured in DMEM high glucose medium (GIBCO) supplemented with 10% fetal calf serum (FBS; GIBCO). All cells were cultured in a humidified incubator at 37°C and 5% CO2.
Stable transfection
Short hairpin RNA against human PHGDH (pGPU6/GFP/Neo-PHGDH-hemo-1281, pGPU6/GFP/Neo-PHGDH-hemo-1567) and negative control shRNA (pGPU6/GFP/Neo-shNC) were purchased from GenePharma. HeLa cells were seeded in 6-well culture plates one day before transfection, and then were transfected transiently using Lipofectamine LTX (invitrogin) according to the manufacturer's instruction. Forty-eight hours after transfection, a complete medium containing 800 μg/ml G418 was added, and positive clones were obtained by being screened for 2 weeks. Stably expressed strains were acquired by expanded culture.
Immuocytochemistry staining
ICC staining was performed after reparation of cover slips and cells using streptavidin-perosidase method. The primary antibody used was PHGDH mouse monoclonal antibody (sc-100317, Santa Cruz Biotechnology, CA). Brown staining of the cytoplasm and plasma membrane indicated positive.
Cell proliferation assay
Cell proliferation was assessed by the Cell Counting Kit-8 (CCK-8; Dojindo, Japan) according to the manufacturer's protocol. Briefly, cells were seeded into a 96-well plate at a concentration of 5 × 103 cells per well and were incubated at 37°C. At daily intervals (days 1, 2, 3 and 4), the optical density was measured at 450 nm using a microplate reader, and the cell survival rate was expressed as the absorbance relative to that of the control group. The results represent the average of 3 replicates under the same conditions.
Tumor formation in vivo animal model
Four-week-old BALB/c nude mice were purchased from shanghai laboratory animal center. Mice were randomly separated to 2 groups (5 mice per group). Before injection, viability of HeLa shPHGDH cells and HeLa-vec cells was observed by trypan blue dye exclusion and the ability of adherence. Then cells were collected and suspended in 100 μl of PBS at a concentration of 1 × 107 cells/ml, subcutaneously injected into nude mice. Tumor volume was calculated using the formula (W2 × L)/2, where L is the length of the tumor and W is the width of the tumor. Tumor volume was measured every 4 d using calipers. All nude mice were sacrificed and tumors were excised after 24 d, and tumor masses were examined by western blot. All animal experiments comply with the institution's guidelines and animal research laws.
Cytotoxicity tests
Cytotoxicity was determined using CCK-8 method (Dojindo, Japan). Three groups of cells (HeLa shPHGDH cells, HeLa-vec cells and HeLa cells) in logarithmic growth were transferred to 96-well plates (5 × 103 cells/ well). After 24 h of pre-incubation, Cisplatin (1 mg/ml) was added into the medium to achieve different concentration (0.01 μg/ml, 0.1 μg/ml, 1 μg/ml, 10 μg/ml) and incubated for 48 h (37°C,deg; C, 5% CO2). Besides, 3 groups of cells above after 24 h of pre-incubation were incubated with 5 μg/ml cisplatin for 24 h, 48 h or 72 h. Triplicate wells were set for each group. According to the manufacturer's protocol, the absorbance was measured at 450 nm with a microplate reader. The cell viability of each group was calculated by averaging the optical density. Cell growth inhibition rate = [1- (OD value in experimental well- OD value in zero set well)/( OD value in control well- OD value in zero set well)]×100%.
Apoptosis analysis
Cell apoptosis was assessed by flow cytometry (Becton Dickinson, San Jose, CA, USA). Cells were cultured and then treated with cisplatin (5 or 10 μg/ml) for 48 h. Cells were harvested and washed with PBS, and suspended in 500 μl binding buffer. The cells were then stained with 5 μl Annexin V-FITC and 5 μl Propidium Iodide (PI) at room temperature for 15 min in the dark. The stained cells were immediately analyzed by flow cytometry.
Western blot
The cells in each group and fresh tissues were prepared to extract proteins by adding lysis buffer. The protein was quantified using the BCA method, then were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore). The membranes were blocked and then probed with primary antibodies overnight at 4°C. Primary antibodies used in this study were as follows: PHGDH (1:400, sc-100317, Santa Cruz Biotechnology), Bcl−2 (1:200, sc-492, Santa Cruz Biotechnology), cleaved caspase-3 (1:1000, 9664, Cell Signaling Technology). GAPDH (1:1000, BM1985, Boster Biotechnology) was used as a loading control. The membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:5000) and were visualized by ECL chemiluminescence.
Statistical analysis
Statistical analyses were conducted using SPSS 17.0 software. Correlations between PHGDH expression and clinicopathological parameters were analyzed using the Pearson Chi-square (χ2) test. The KaplanMeier analysis and the logrank test were used to evaluate the correlation between PHGDH expression and overall survival. The data are expressed as the mean ± SD. Statistical significance between 2 groups was determined by Student's t-test. IC50 value of cisplatin was calculated by probit regression analysis. A P value of less than 0.05 was considered statistically significant.
Details of ethics approval
The present study was approved by the ethics committee of China Medical University affiliated Sheng Jing Hospital.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (grant #81372776, grant #81202048), the Higher Specialized Research Fund for the Doctoral Program (grant #20122104110014), the Science and Technology Program of Liaoning Province (grant #2011225009), the Peak Medical Construction Special Project of Liaoning Province (grant #2010696), and the Free Researcher Project of Shengjing Hospital (grant #201302).
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 2011; 378:1461–84; PMID:21924486; http://dx.doi.org/10.1016/S0140-6736(11)61351-2
- Mathew A, George PS. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix–worldwide. Asian Pac J Cancer Prev 2009; 10:645–50; PMID:19827887
- Locasale JW1, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet 2011; 43:869–74; PMID:21804546; http://dx.doi.org/10.1038/ng.890
- Snell K, Natsumeda Y, Eble JN, Glover JL, Weber G. Enzymic imbalance in serine metabolism in human colon carcinoma andrat sarcoma. Br J Cancer 1988; 57:87–90; PMID:3126791
- Mullarky E, Mattaini KR, Vander Heiden MG, Cantley LC, Locasale JW. PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res 2011; 24:1112–5; PMID:21981974; http://dx.doi.org/10.1111/j.1755-148X
- Liu J, Guo S, Li Q, Yang L, Xia Z, Zhang L, Huang Z, Zhang N. Phosphoglycerate dehydrogenase induces glioma cells proliferation and invasion by stabilizing forkhead box M1. J Neurooncol 2013; 111:245–55; PMID:23229761; http://dx.doi.org/10.1007/s11060-012-1018-x
- Cho HM, Jun DY, Bae MA, Ahn JD, Kim YH. Nucleotide sequence and differential expression of the human 3-phosphoglycerate dehydrogenase gene. Gene 2000; 245:193–201; PMID:10713460
- Jing Z, Heng W, Aiping D, Yafei Q, Shulan Z. Expression and clinicalsignificance of phosphoglycerate dehydrogenase and squamous cell carcinoma antigen in cervical cancer. Int J Gynecol Cancer 2013; 23:1465–9; PMID:24247658; http://dx.doi.org/10.1097/IGC.0b013e3182a0c068
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11:85–95; PMID:21258394; http://dx.doi.org/10.1038/nrc2981
- DeBerardinis RJ. Serine metabolism: some tumors take the road less traveled. Cell Metab 2011; 14:285–6; PMID:21907134; http://dx.doi.org/10.1016/j.cmet.2011.08.004
- Ward PS, Thompson CB. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012; 21:297–308; PMID:22439925; http://dx.doi.org/10.1016/j.ccr.2012.02.014
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 2011; 476:346–50; PMID:21760589; http://dx.doi.org/10.1038/nature10350
- Snell K, Weber G. Enzymic imbalance in serine metabolism in rat hepatomas. Biochem J 1986; 233:617–20; PMID:3082329
- Kim SK, Jung WH, Koo JS. Differential expression of enzymes associated with serine/glycine metabolism in different breast cancer subtypes. PLoS One 2014; 9:e101004; PMID:24979213; http://dx.doi.org/10.1371/journal.pone
- Chen J, Chung F, Yang G, Pu M, Gao H, Jiang W, Yin H, Capka V, Kasibhatla S, Laffitte B, et al. Phosphoglycerate dehydrogenase is dispensable for breast tumor maintenance and growth. Oncotarget 2013; 4:2502–11; PMID:24318446
- Shen D-W, Pouliot LM, Hall MD, Gottesman MM. Cisplatin Resistance: A Cellular Self-Defense Mechanism Resulting from Multiple Epigenetic and Genetic Changes. Pharmacological Reviews 2012; 64:706–21; PMID:22659329; http://dx.doi.org/10.1124/pr.111.005637.
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene 2012; 31:1869–83; PMID:21892204; http://dx.doi.org/10.1038/onc.2011.384.
- Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res 2001; 478:23–43; PMID:11406167
- Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet 2009; 43:95–118; PMID:19659442; http://dx.doi.org/10.1146/annurev-genet-102108-134850
- Srivastava M, Ahmad N, Gupta S, Mukhtar H. Involvement of Bcl-2 and Bax in Photodynamic Therapy-mediated Apoptosis. J Biol Chem 2001; 276:15481–88; PMID:11278320
- Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985; 228:1440–43; PMID:3874430
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bc1-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993; 74:609–19; PMID:8358790
- Kim KY, Seol JY, Jeon GA, Nam MJ. The combined treatment of aspirin and radiation induces apoptosis by the regulation of bcl-2 and caspase-3 in human cervical cancer cell. Cancer Lett 2003; 189:157–66; PMID:12490308
- Spina A, Sorvillo L, Di Maiolo F, Esposito A, D'Auria R, Di Gesto D, Chiosi E, Naviglio S. Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin via a p53-dependent pathway. J Cell Physiol 2013; 228:198–206; PMID:22674530; http://dx.doi.org/10.1002/jcp.24124