Abstract
Bevacizumab and Tamoxifen are valid therapeutic options for metastatic breast cancer (mBC) patients. In this report, we describe a 47 year old woman with mBC successfully treated with a maintenance therapy with Bevacizumab+Tamoxifen. A maintenance approach using 2 different drugs with different targets and mechanism of action, such as anti-angiogenic and anti-hormonal treatment is particularly intriguing because they affect different pathways involved in mBC progression. Further studies including a large number of patients are needed, in order to select women who could benefit from this maintenance approach.
Introduction
The range of treatment options for metastatic breast cancer (mBC) continues to grow; many of these advances have been achieved through the development of targeted therapies. The increasing number of agents leads to increasingly complex treatment decisions, which are accompanied by better outcomes for patients.
Bevacizumab (Bev) is a human monoclonal antibody targeted against soluble VEGF-A and is currently approved for the treatment of many solid tumors, including colon, renal, brain (glioblastomas), non-squamous non-small cell lung and ovarian cancers. Three randomized clinical trialsCitation1-3 have demonstrated the efficacy and safety of first-line Bev, including chemotherapy in HER2-negative metastatic breast cancer (mBC) patients. The addition of Bev to chemotherapy significantly prolonged median progression-free survival (PFS) and nearly doubled the objective response rate in the absence of any improvement in overall survival (OS). Recently, IMELDA and TANIACitation4-5 randomized phase III clinical trials in HER2-negative mBC showed the efficacy and safety of maintenance Bev.
Tamoxifen (hormonal therapy HT) is an antagonist of the estrogen receptor in breast tissue via its active metabolite 4-hydroxytamoxifen. In other tissues, such as the endometrium, it behaves as an agonist and thus may be characterized as a mixed agonist/antagonist. Tamoxifen is the usual endocrine (anti-estrogen) therapy for hormone receptor-positive breast cancer in pre-menopausal women and is also a standard drug used in post-menopausal females, although aromatase inhibitors are also frequently used in this setting.
In this report, we describe a patient with mBC who was treated with maintenance therapy using Bev + HT and who demonstrated a prolonged stable disease for 5 y.
Case report
In 2008, a Caucasian 47-year-old postmenopausal woman was admitted to our department with a diagnosis of breast cancer, which had been made in another hospital (mammography and breast ultrasounds examination results were not in our possession).
The patient has 2 children and no important comorbidity with ECOG 0. The family history was positive for breast cancer (mother), and the patient did not consume alcohol, although she is a smoker. She used contraceptives for 7 years and had 3 pregnancies and 2 abortions. A breast examination revealed a lump in the lateral superior quadrant of the left breast that was >3 cm in diameter. Total body computed tomography (CT) showed multiple irregular thickening in the left breast region with polycyclic margins; bilateral pleural effusions were more abundant on the right breast; in both lungs, many nodular lesions were observed, which was consistent with metastasis; pathological lymph nodes at the level of the lodge of Barety and in the bilateral axillary; multiple osteoblastic and osteolytic lesions, which were consistent with secondarity in different skeletal segments. In addition, a bone scan confirmed the presence of multiple bone secondary lesions (spinal column, ribs, left homer and right femur). Subsequently, considering the patient's young age and despite the stage of the disease, the patient underwent a radical mastectomy sx + axillary ipsilateral lymph nodes dissection. Pathological evaluation showed ductal invasive carcinoma, G3, with central fibrosis polycentric; the presence of a focal intraductal component and peritumoural vascular invasion; minimum microcalcifications, fibrocystic disease of type and proliferative metaplasia in adenosis of the breast tissue adjacent apocrynal; the presence of ipsilateral satellite nodule of the skin; and carcinomatous infiltration of muscle bundles and of 7 of the axillary lymph nodes that were removed pT4b N2a M1: Ki67 40%, Her2/neu (score 0), ER 80%, PgR 60%). Detection of BRCA1 and BRCA2 mutations was performed, and the results were negative.
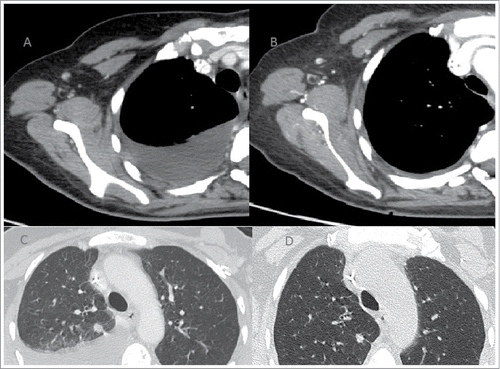
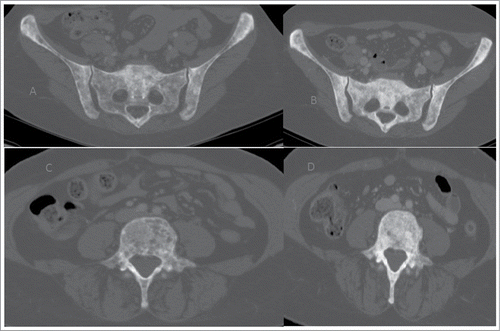
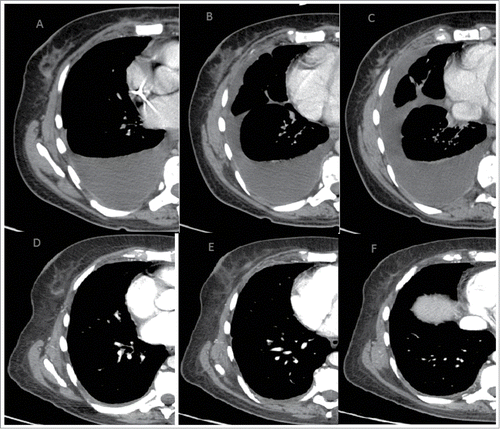
Excluding any other treatment for the systemic dissemination of the tumor, the patient was initially treated with first-line chemotherapy with epirubicin+paclitaxel and zoledronic acid (every 21 days) for 6 cycles. In March 2009, a CT examination documented the disease progression for the appearance of multiple hypervascular nodules in the right breast tissue (). It also reported an improvement in the right pleural effusion, septal thickening in the middle and right lower lobes, and manifestations of carcinomatous lymphangitis. Nevertheless, there was a marked reduction in the size of all secondary lung lesions and mediastinal pathological lymph nodes, and there was also a major osteosclerotic component in the sites of bone metastasis in relationship to the response to therapy (). No encephalic or liver lesions were observed. Thus, a second-line chemotherapy with docetaxel + bevacizumab (every 21 days) + zoledronic acid (March 2009). In July 2009, after 6 cycles, CT examination showed a reduction in the volume and number of all nodules detected in the right breast, secondary lung lesions and right pleural effusion. Septal thickenings, which were previously reported in the middle and right lower lobes, were no longer detectable. Thus, 4 additional cycles of chemotherapy with docetaxel + bevacizumab was administered until October 2009. Subsequently, the patient began maintenance chemotherapy with bevacizumab (every 21 days) + zoledronic acid + tamoxifen. Zoledronic acid was interrupted for treatment completion. Serial CT, which was performed every 4 months, showed stable disease progression until April 2014 (). This last CT examination showed stable disease progression for lung and skeletal localisations with no signs of a loco-regional recurrence. No secondary localisations in the liver or brain were observed. Despite the radiological stability of the disease, there was a progressive increase in CEA and CA15.3. Thus, patients after 5 years of maintenance therapy started a new line of chemotherapy with eribulin.
Figure 1. Stability of disease with minimal reduction of the known breast lesions at the start of therapy (A, B, C) and until in April 2014 (D, E, F)
Discussion
This case report showed a prolonged stable disease of 5 years with maintenance therapy using Bev and HT in an mBC patient.
The continuatiuon of an antiangiogenic agent as maintenance therapy in patients who are responder to first-line Bev-based chemotherapy could be particularly intriguing. Mancuso et al.Citation6 have shown that when tumors were inhibited by VEGF tyrosine kinase receptor the same tumors were completely revascularized within the first week after stopping treatment, suggesting an potentially increase of tumor growth following withdrawal of VEGF inhibitors. Furthermore, preclinical models suggested that the addition of anti-vascular endothelial growth factor therapy could improve the efficacy of anti-estrogens in hormone-sensitive breast cancer,Citation7 on the other hand, endocrine resistance in this tumor model was suggested by a wave of neo-vascularization and tumor re-growth. Some studies reported that endocrine castration initially caused a reduction in VEGF mRNA levels while, at the time of tumor re-growth, VEGF mRNA levels simultaneously rebound.Citation8,9 Therefore, an anti-VEGF therapy may delay or prevent the onset of endocrine therapy resistance in patients with hormone-sensitive breast cancer. Nevertheless, to date, few data exist regarding the efficacy and safety of Bev and HT as maintenance treatment in mBC.
In 2012, a study, which involved 35 patients with HER2-negative mBC, has evaluated the safety and activity of maintenance therapy with Bev alone or combined with HT.Citation10 In this prospective study, 35 patients who experienced a response after first-line taxane- including chemotherapy were given maintenance Bev at a dose of 15 mg/kg every 3 weeks. Among 30 patients with hormonal receptor-positive mBC, 20 patients (66.6%) received HT with Bev. The objective of this study was the outcome and safety of maintenance Bev in 2 groups: patients with and without HT. The authors showed that maintenance therapy with Bev was effective and reported an objective response rate of 48.5%; the median PFS was 6.8 months (95%CI: 0.8-12.7), and the duration of clinical benefit was 17.1 months (95%CI 12.2-21.9). Importantly, the median PFS of patients given Bev and HT was 13 months (1-39+; 95%C.I.: 6.2-27.5), while the median Bev PFS of patients who received only Bev without HT was 4.1 months (P = 0.05). In addition, the median PFS was 10.2 months among patients who received taxane as an adjuvant treatment compared with 6.6 months among patients who did not receive taxane. These data are in line with data which suggest that prior treatment with adjuvant taxanes was predictive of better Bev PFS.Citation11
In our case report, a PFS of 60 months was reported by our patient, which was considerably longer than those of previously reported trials.Citation1-5,10,11 Furthermore, results observed in randomized phase III studies that compared aromatase inhibitors and tamoxifen as first-line therapy for MBC showed a median PFS which ranged from 6 to 8 months in tamoxifen group.Citation12-16 Unfortunately, we do not routinely perform angiogenesis signature in genomic profile of our patients, therefore, it is difficult to address the longer PFS of our case to bev, on the other hand, data of previously reported trials on tamoxifen showed a median PFS considerably shortest than 60 months achieved by our patient. Therefore, although, a maintenance using 2 different drugs with different targets and mechanism of action, such as anti-angiogenic and anti-hormonal treatment appears particularly intriguing because they affect different pathways involved in mBC progression. This alone is insufficient to explain the reasons underlying the longer PFS of our patient. Thus, further studies that include a large number of patients are needed in order to select women who could benefit from this maintenance approach.
Another point to consider is that despite the long exposure to all 2 drugs, our patient did not report important side effects. The adverse event profiles of the 2 drugs have been well described as monotherapy. Bev can cause hypertension, proteinuria, bleeding and thromboembolic events. Tamoxifen can cause endometrial cancer, cardiovascular and metabolic complications. Fortunately, there is little overlap between these toxicity profiles. Thus, it is possible to treat patients for 5 years without significant side effects.
Conclusion
There is a great need for treatments that improve OS in females with mBC. Recently, large trials showed Bev maintenance or secondary treatment in combination with chemotherapy in HER2 negative metastatic breast cancer prolonged PFS and possibly OS. In our report, we describe an exceptional prolonged stable disease of 5 years obtained using a maintenance strategy with Bev+HT. Therefore, the combination with HT in ER positive metastatic breast cancer could be an interesting treatment option that may have some patient potential benefit. Since Bev combined with HT might benefit certain patients with hyper vascular finding like in this patient, further studies are required to validate this approach in all mBC patients.
Ethical Review
Ethical approval was obtained from the ethics committee of the University of Siena. Written informed consent was obtained from the patient for publication of this case report.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007; 357:2666-76; PMID:18160686; http://dx.doi.org/10.1056/NEJMoa072113
- Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010; 28:3239-47; PMID:20498403; http://dx.doi.org/10.1200/JCO.2008.21.6457
- Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 2011; 29:1252-60; PMID:21383283; http://dx.doi.org/10.1200/JCO.2010.28.0982
- Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, Gupta V, Costa R, Srock S, de Ducla S, Freudensprung U, Mustacchi G. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15(12):1351-60; Published online on 28 September; PMID:25273343; http://dx.doi.org/10.1016/S1470-2045(14)70444-9
- Von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, Villanueva C, Romieu G, Lang I, Ciruelos E, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol 2014; (11):1269-78; Published online on 28 September; PMID:25273342; http://dx.doi.org/10.1016/S1470-2045(14)70439-5
- Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 2006; 116:2610-21; PMID:17016557; http://dx.doi.org/10.1172/JCI24612
- Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: Role of vascular endothelial growth factor. Proc Natl Acad Sci U S A 1998; 95:10820-5; PMID:9724788; http://dx.doi.org/10.1073/pnas.95.18.10820
- Manders P, Beex LV, Tjan-Heijnen VC, Span PN, Sweep CG. Vascular endothelial growth factor is associated with the efficacy of endocrine therapy in patients with advanced breast carcinoma. Cancer 2003; 98:2125-32; PMID:14601081; http://dx.doi.org/10.1002/cncr.11764
- Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol 2000; 18:1423-31; PMID:10735889
- Fabi A, Russillo M, Ferretti G, Metro G, Nistic∫ C, Papaldo P, De Vita F, D'Auria G, Vidiri A, Giannarelli D, et al. Maintenance bevacizumab beyond first-line paclitaxel plus bevacizumab in patients with Her2-negative hormone receptor-positive metastatic breast cancer: efficacy in combination with hormonal therapy. BMC Cancer 2012; 12:482; PMID:23083011; http://dx.doi.org/10.1186/1471-2407-12-482
- Cuppone F, Bria E, Vaccaro V, Puglisi F, Fabi A, Sperduti I, Carlini P, Milella M, Nistic∫ C, Russillo M, et al. Magnitude of risks and benefits of the addition of bevacizumab to chemotherapy for advanced breast cancer patients: Meta-regression analysis of randomized trials. J Exp Clin Cancer Res 2011; 12:30-54; PMID:21569417
- Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, Steinberg M, Webster A, von Euler M. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial—arimidex study group. J Clin Oncol 2000; 18:3758-67; PMID:11078488
- Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, Vergote I, Webster A, Steinberg M, von Euler M. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: Results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000; 18:3748-57; PMID:11078487
- Mouridsen H, Gershanovich M, Sun Y, Pérez-Carrión R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jänicke F, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 2001; 19:2596-2606; PMID:11352951
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003; 21:2101-9; PMID:12775735; http://dx.doi.org/10.1200/JCO.2003.04.194
- Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, Piccart MJ, Bogaerts J, Therasse P. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European organisation for research and treatment of cancer breast cancer cooperative group. J Clin Oncol 2008; 26(30):4883-90; PMID:18794551; http://dx.doi.org/10.1200/JCO.2007.14.4659