Abstract
Glioma is the most common form of primary malignant brain cancers. Tumor cell invasiveness is a critical challenge in the clinical management of glioma patients. The invasive biological feature of glioma cell is stimulated by both autocrine and paracrine factors including chemokine IL-8. In this study, we report that the production of IL-8 is higher in glioma tissues and cells than adjacent nontumor tissues (ANT) and normal glial cells. Autocrine IL-8 can increase the invasive ability of glioma cells by binding to CXCR1. In addition, high expression of IL-8 indicates poor prognosis of glioma patients. Furthermore, IL-8 is capable of modulating cell migration and invasion by regulating the activation of RAC1 which resulted in cytoskeletal reorganisation in an ELMO1 dependent manner. Finally, we found that IL-8 could enhance mesenchymal transition(MT) of glioma cells by activating ELMO1-NF-κB-Snail signaling. Our data indicate that IL-8 autocrine is responsible for the invasive phenotype of glioma and IL-8 may be a useful prognostic marker for glioma and novel therapeutic target for glioma invasion intervention.
Abbreviations
| ANT | = | adjacent nontumor tissues; CM, conditioned medium; CXCR1, chemokine (C-X-C motif) receptor 1; CXCR2, chemokine (C-X-C motif) receptor 2; Dock180, dedicator of cytokinesis; ELMO1, engulfment and cell motility 1; EMT, epithelial-mesenchymal transition; GBM, glioblastoma; GEFs, guanine nucleotide exchange factors; GPCR, G protein coupled receptor; IDH1, isocitrate dehydrogenase 1; IHS, immunohistochemical score; IL-8, interleukin-8; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; MT, mesenchymal transition; NHA, normal human astrocytes; PM, plain medium; TNM, Tumor-Node-Metastasis |
Introduction
Several studies indicate that many tumor types can produce and secrete various factors both in vitro and in vivo.Citation1-3 In in vitro studies, the autocrine cytokines or chemokines from tumor cells in the conditioned medium (CM) showed profound effects on progression of tumor cells.Citation4,5 Glioma is the most common form of primary malignant brain cancers and tumor cell invasiveness is a critical challenge in the management of glioma. The invasive biological feature of glioma cells has a complex mechanism and involves several well orchestrated signaling pathways stimulated by both autocrine and paracrine factors that act on various cell surface-bound receptors including G-protein coupled receptor (GPCR).Citation6 Autocrine of IL-8 in the progression of glioblastoma has been studied extensively.Citation7–11 IL-8 is originally known as a leucocyte chemo-attractant and its secretion is tightly controlled in normal cells.Citation12 The biological effects of IL-8 are mediated by CXCR1 and CXCR2, which are highly related receptors of the 7 transmembrane GPCR super family. Under pathological conditions, IL-8 is detectable and involves in the development and progression of autoimmune diseasesCitation13 and even tumorigenesis.Citation7,14,15 Evidence sustains that IL-8 is high expressed in glioblastoma and is partly responsible for glioma cell invasion.
In receptor-initiated signalings, Rho family GTPases, including Rac, play key roles in the regulation of cell morphology and actin dynamics for cell migration and invasion.Citation8,16 Activation of Rac requires guanine nucleotide exchange factors (GEFs) and it has been reported that engulfment and cell motility 1 (ELMO1) and dedicator of cytokinesis 1(Dock180)(ELMO1/Dock180) complex has a key role in promoting glioma cell invasion by serving as a GEF for Rac1.Citation17 Upon activation of an ELMO/Dock180 complex, the Dock180 protein exposes its Docker domain, which in turn binds to and activates Rac.Citation18,19 Nevertheless, we still do not know whether the ELMO/Dock180 complex plays a role in IL-8-mediated invasion in glioma cells and the mechanisms downstream of IL-8-receptor interaction of glioma cells remain poorly understood.
Mesenchymal transition(MT) of glioma cells leads to an increased invasive or metastatic phenotype leading to tumor progression.Citation20 At the molecular level, MT is interpreted by the down-regulation of glial markers and up-regulation of mesenchymal markers. It has recently been suggested that IL-8 could promote cancer cell metastasis via autocrine and paracrine means which is associated with enhanced epithelial-mesenchymal transition (EMT).Citation21-23 However, the effect of IL-8 on the MT of glioma remains unclear.
In the current study, we demonstrated that IL-8-CXCR1 interacts with ELMO1/Dock180 complex to activate Rac proteins contributing to actin polymerization and to enhance Mesenchymal Transition (MT) involving in migration and invasion in glioma cells.
Materials and Methods
Patients and tissue specimens
Paraffin-embedded specimens from 198 patients who had undergone surgical treatment for primary glioma with pathologic identification in the Affiliated Hospital of Weifang Medical University and the Weifang People's Hospital from 2002 to 2009, guided by a protocol approved by the Institutional Review Board (IRB). Patients gave consent for the use of their tissue specimens in this study. None of them had received chemotherapy or radiotherapy before surgery. The histological characterization and clinicopathological staging of the samples were determined according to the current International Union Against Cancer (UICC) Tumor-Node-Metastasis (TNM) classification. A total of 132 males and 66 females were included in the study, ranging in age from 34 to 78 y (median age 53 years). Clinical information of the samples is described in detail in . For Western blot, 20 pairs of randomly selected frozen (liquid nitrogen) glioma tissues (6 grade II, 6 grade III and 7 grades IV respectively) and correspondingly adjacent nontumor tissues (ANT) were evaluated.
Table 1. Clinicopathologic characteristics of studied patients
Cell culture and reagents
Human glioma U87, H4, U251, LN-229 cells, human lung cancer cell A549 and normal human astrocytes (NHA) cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI 1640 (HyClone, SH30809.01B) supplement with 10% fetal bovine serum (HyClone, SH30070.03). Conditioned medium (CM) was collected in sterile conditions followed by centrifugation at 1500 ×g for 10 min, and then stored at −20°C until use. Unless otherwise mentioned, CM in this study is a conditioned medium collected from the culture medium of U87 cells. Complete medium alone without cells was incubated under the same experimental conditions and referred as plain medium (PM) that served as control. The chemotaxis chambers and membranes were from Neuroprobe (Gaithersburg, MD). Boyden chamber assay for glioma cells plated on the upper cell culture inserts, with CM or PM in the lower chambers in the presence or absence of anti-IL-8 antibody at 10 ug/ml or control IgG. Anti-IL-8 antibody(A00044) and control IgG(A01006) and human recombinant IL-8 (Z03138) were purchased from GenScript (Piscataway, NJ, USA). Bovine Serum Albumin (BSA) was purchased from Amresco (Amresco, 0332, USA).
Plasmid construction, siRNA, and plasmid transfection
Cells were plated in a 35 mm dish for 24 h before transfection into the complete medium. The transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Cells were transfected with scrambled siRNA or siRNAs against human CXCR1 and ELMO1 (Santa Cruz Biotechnology) following instructions provided by the manufacturer.. BLOCK-iT Fluorescent Oligo was used to examine the transfection efficiency (Invitrogen). Seventy-two hours after transfections, cells were harvested and used for further experiments. Stable transfectants were selected by using 500 μg/mL G418.
In addition, H4 cells were transfected with pcDNA3.1-ELMO1 plasmid or pcDNA3.1 vector using Lipofectamine 2000 (Invitrogen) following the protocol. Stable transfected cells were obtained by using selection medium (culture medium with 700 μg/mL G418). Stable transfected cell clones were named H4/ELMO1 and H4/CON cells. The cells were maintained and passaged in culture medium with G418 (400 μg/mL).
Immunohistochemistry
Immunohistochemistry was performed as described previously [10,12]. Primary IL-8 polyclonal antibodiy (LifeSpan BioSciences, LS-C104558, 1:100 dilution) and CXCR1(LifeSpan BioSciences, LS-C178869, 1:100 dilution)were used for immunostaining. The bound primary antibody was detected by HRP-linked Anti-rabbit IgG (Cell Signaling,7074, 1:100 dilution) and the chromogenic substrate 3,3-diaminobenzidine tetrahydrochloride (DAB). The specimens were counterstained with hematoxylin, mounted, and examined by light microscopy (Olympus, IX71).
Immunohistochemical evaluation
The degree of immunostaining of the sections was viewed and scored separately by 2 independent investigators who were blinded to the histopathologic features and patient data of the samples. The results were evaluated by an immunohistochemical score (IHS).Citation24 Tumors were considered IL-8 positive if the percentage of positive tumor cells was>1%. The IHS is calculated by combining an estimate of the percentage of immunoreactive cells (quantity score) with an estimate of the staining intensity (staining intensity score), as follows: no staining is scored as 0, 1–10% of cells stained scored as 1, 11–50% as 2, 51–80% as 3, and 81–100% as 4. The percentage of positive cells was calculated by counting more than 1000 cancer cells in randomly selected high-power fields (400 ×). Staining intensity is rated on a scale of 0 to 3, with 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. When there is multifocal immunoreactivity and there are significant differences in staining intensities between foci, the average of the least intense and most intense staining was recorded. The raw data were converted to the IHS by multiplying the quantity and staining intensity scores. Theoretically, the scores could range from 0 to 12. HIS scores of 0–4 were regarded as low expression, 5–12 were regarded as high expression. Glioma patients were divided into 2 groups based on IL-8 expression level, namely, the low IL-8 expression group and the high IL-8 expression group for clinical survival analysis.
Western blotting assay
For Western blot, cells or tissues were directly lysed in 1×SDS sample buffer.Citation24 The intensities of bands in Western blots were quantified by densitometry analysis using AlphaImager HP (Alpha Innotech, USA) and NIH Image J software (Rockville, MD, USA). Western blot data shown in the paper are representatives from 3 independent experiments. The following commercial antibodies used at 1:1000 dilution in this study were from LifeSpan BioSciences: IL-8(LS-C104558), CXCR1(LS-C178869), CXCR2 (LS-C171552), ELMO1(LS-C167733), N-cadherin(LS-C172138), T-cadherin(LS-C119406), Vimentin (LS-C92412), phospho-RAC1 (LS-C205263), RAC1(LS-C9853), Snail(LS-C176686) Slug(LS-C179060), Twist1(LS-C205209), Zeb1(LS-C31478), Zeb2(LS-C160768) and Nucleolin(LS-C73822); P-IκBα(9240), IκBα(4814), β-actin(4970) and corresponding HRP-linked Anti-rabbit IgG(7074) or HRP-linked Anti-mouse IgG (7076) were from Cell Signaling.
Matrigel invasion assay
The invasion of cells in vitro was measured through Matrigel-coated transwell inserts (Costar, Cambridge, MA, USA) as described previously[16]. CM or PM was added to the lower well with or without anti-IL-8 antibody(10ug/ml) or control IgG. After 24 h of incubation (37°C, 5% CO2), the non-invading cells were removed by wiping the upper side of the membrane, and the invading cells were fixed and stained. The number of invading cells was counted under a microscope (Olympus, IX71) in 5 predetermined fields (CellSens Standard, total magnification, 400×). All assays were repeated at least 3 times independently.
Scratch wound assay
The control scr/U251 and siELMO1/U251 cells were plated in 35 mm dishes in RPMI 1640 supplement with 10% fetal bovine serum for 2 d to grow into a monolayer. Then, it was lined out with an even trace in the middle using a 10ul pipette tip. The cells were then incubated at 37°C in 5% CO2 within an appropriate time and the distance of the wounds was measured under a light microscope. All samples were tested in triplicate, and the data are expressed as the mean ± SD.
Chemotaxis assay
Chemotaxis assay was done as described previously[14]. Briefly, the chemo-attractant IL-8(0, 10, 100, 500, 1000ng/ml) was loaded into the lower chemotaxis chamber and 5 × 105/ml cells suspended in the binding medium (RPMI 1640, 0.1% BSA, and 25 mM HEPES) were added to the upper chambers. The numbers of migrating cells were counted at 400× in 3 separate fields by light microscope (Olympus IX71). Chemotaxis index = the migrating cell number in a chemo-attractant gradient/the migrating cell number in a medium control. All samples were tested in triplicate and the data are expressed as mean ± SD. Statistical analysis was carried out to determine the significance of chemotactic response by using 2-way ANOVA.
Cellular F-actin measurement
The F-actin content was detected as described previously [15]. Scr/U251 cells and SiELMO1/U251 cells were followed with or without the stimulation of IL-8(10 ng/ml) at 37°C. The cells were then fixed, permeablized, and incubated with Oregon Alexa-fluro 568 phalloidin in F-actin buffer (10 mmol/l HEPES, 20 mmol/l KH2PO4, 5 mmol/l EGTA, 2 mmol/l MgCl2, PBS, pH 7.4) at room temperature for 2 h. The cells were washed 5 times. The labeled phalloidin that were bound to F-actin was extracted by using methanol at 4°C for 90 min. The fluorescence was captured at Ex/Em 578/600 nm in each sample (Hitachi, F-7000) and normalized against the total protein content as analyzed by a BCA kit (Pierce, Rockford, IL). The relative F-actin content over different time periods was calculated by the following equations: F-actin Δt/F-actin0 = fluorescence Δt/fluorescence 0. All samples were tested in triplicate, and the data is expressed as mean ± SD.
Enzyme-linked immunosorbent assay(ELISA)
IL-8 protein was measured from the culture supernatant. At predetermined experimental time points (36, 72 h) supernatant from the cells were collected and centrifuged at 1,000 g for 5 min and stored at −80°C. IL-8 was measured by ELISA according to the kit's protocol (R&D, MN, USA). IL-8 concentration in each sample was determined by comparison with a standard curve generated from a provided standard vial. For each sample, the measurement was repeated 3 times and the average concentration of IL-8 was set as the final result.
Gelatin Zymography
Scr/U251 and SiELMO1/U251 cells were stimulated with or without 10 ng/mL IL-8 for 24 h in serum-free medium. Then culture medium was collected and analyzed on 10% SDS–polyacrylamide gel incorporated with 0.1% gelatin.Citation25 The gel was stained with 0.2% Coomasie Brilliant Blue in 40% iso-propanol for 25 min and destained in 7% glacial acetic acid. In our experiments, MMP-9 and MMP-2 were the only gelatinases detected at 92 and 72 kDa, respectively, and were visualized as decolorized bands over blue background.
Immunofluorescent assay
The cells were cultured 1 d before this experiment and then starved in serum-free medium overnight. After the stimulation with 10 ng/mL IL-8 for 24 h, cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. Cells were permeablized with PBS + 0.1% TritonX-100 (PBST). Primary antibody was performed overnight at 4°C.
Antibodies used were: N-cadherin(LifeSpan BioSciences, LS-C172138, 1:100), T-cadherin(LifeSpan BioSciences, LS-C119406, 1:100). Negative control was comprised of normal mouse or rabbit IgG. Cy3-conjugated secondary antibodies and 6-diamidino-2-phenylindole (DAPI) was carried out. The results were analyzed using fluorescence microscopy.
Intracranial brain tumor xenografts, and H&E staining
In the end, to investigate if ELMO1 and CXCR1 could affect glioma progression in vivo, SiELMO1/U251(5 × 105), SiCXCR1/U251(5 × 105) or Scr/U251(5 × 105) cells were stereotactically implanted into the brains of 4-week-old male SCID mice (n = 10), and the morphologies of implanted glioma tumors were examined. Intracranial brain tumor xenografts were performed as described previously.Citation26 All animals received humane care according to the criteria outlined in the Guide (Publication No.85–23, revised 1985) for the care and use of laboratory animals. The study protocol was approved by the Review Committee for the Use of Human or Animal Subjects of Weifang Medical University. The glioma-bearing mice were scarified after 3 weeks of implantation, the whole brains were removed, 5-μm sections were cut and subjected to H&E staining. The number of satellite tumors (tumor foci not connected with the main tumor) were counted to measure invasiveness in vivo. The images were captured using the light microscope system (CellSens Standard, Olympus, IX71). The expression of ELMO1, CXCR1, P-IκBα, IκBα, and Snail proteins in tumor tissues were analyzed ex vivo, using tumor tissues collected at necropsy.Citation27
Statistical analysis
All statistical analyses were carried out using the SPSS 16.0 statistical software package. Data are presented as mean ± SD. Statistical significance for comparisons between groups was determined using Student's paired 2-tailed t test or ANOVA. Chi-square test was used to analyze the relationship between IL-8 expression and the clinicopathologic characteristics. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. All the results were generated from 3 independent experiments. In all cases, P < 0.05 was considered statistically significant.
Results
Upregulation of IL-8 in primary glioma tissues and glioma cell lines
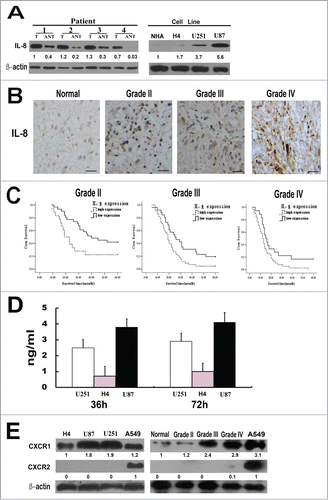
Western blot analyses of IL-8 expression on paired glioma tumor and adjacent nontumor tissues (ANT), with each pair obtained from the same patient, showed that the protein levels of IL-8 were high in glioma samples. In contrast, there was no significant presence or very low expression of IL-8 in any of the corresponding ANT tested (). In addition, analyses on glioma cell lines revealed that the expression of IL-8 was also remarkably high in U87 and U251 cell lines while in NHA and H4 cells the expression of IL-8 was obviously low(). These results showed notable upregulation of IL-8 in both clinical primary glioma tissues and malignant glioma cell lines.
Figure 1. IL-8 expression in glioma tissues and cell lines. (A) Left, Expression of IL-8 protein in paired glioma tissues (T) and adjacent non-tumor tissues (ANT), with each pair obtained from the same patient. Right, Expression of IL-8 protein in cultured glioma cell lines(normal human astrocytes (NHA) cells, H4, U251 and U87). β-actin was used as a loading control. Quantification of relative protein levels is shown below the blots. The results were from a representative of at least 3 repeated experiments. (B) Expression of IL-8 protein in normal brain tissue and glioma tissues (grade II, grade III and grade IV) was examined by immunohistochemical staining, scale bar: 20 um. (C) The statistical significance of the difference between curves of IL-8 high-expressing (dotted line) and low-expressing (bold line) patients was compared within subgroups of WHO grade II, grade III and grades IV. P values were calculated by the log-rank test, P < 0.05 was considered statistically significant. (D) Glioma cells secrete IL-8 in the complete media. Three different types of glioma cell lines were plated in 96 well plates and culture media was collected at the end of 36 and 72 h for ELISA measurement. The results were from a representative of at least 3 repeated experiments. (E) Expression levels of CXCR1 and CXCR2 protein in glioma cell lines (H4, U87, U251) and in normal brain tissue and glioma tissues (grade II, grade III and gradeIV) were detected by Western blot. A549 cell line was used as a positive control, β-actin was used as a loading control. Quantification of relative protein levels is shown below the blots. The results were from a representative of at least 3 repeated experiments.
Increased expression of IL-8 correlates with progression of gliomas
To further investigate whether IL-8 protein is over expressed in clinical samples of glioma, we examined 198 paraffin-embedded, archived glioma tissues, including 22 cases of grade I (pilocytic astrocytoma) (11.1%), 49 cases of grade II (24.7%), 70 cases of grade III (35.4%) and 57 cases of gradeIV gliomas (28.8%) by immunohistochemistry. Positive IL-8 staining was shown in 159 of 198 (80.3%) cases, among all specimens 87 (43.9%) were identified as low-level IL-8 expression and 111 cases (56.1%) as high-level IL-8 expression. However, in corresponding ANTs, low-level IL-8 expression was only in 11 of 40 (27.5%) cases and there was no high-level IL-8 expression. The expression of IL-8 is closely correlated with WHO grading (P = 0.004) () and survival status of glioma patients (P = 0.000), but not to age and gender. In addition, as the IDH1 status of a tumor has profound influence on survival, we analyzed the correlation between IL-8 expression and IDH1 mutation status. The result showed that the expression level of IL-8 was negatively correlated with IDH1 mutation status (P = 0.022) (). Furthermore, the expression of IL-8 is closely related to WHO grading and survival status in both subgroup of patients with IDH1 mutant and that without IDH1 mutant (Tables S1 and S2). Taken together, our results suggested that the expression of IL-8 was significantly correlated with clinicopathologic grades of gliomas.
Table 2. Correlation between clinicopathologic features and expressions of IL-8 in glioma patients
Association between IL-8 expression and patient prognosis
Kaplan-Meier analysis using the log-rank test was performed to calculate the potency of IL-8 expression on glioma patients’ survival. Pilocytic astrocytoma(glioma gradeI) was excluded from this analysis due to its good prognosis. Significant correlation between high IL-8 expression and shorter overall survival time was found in WHO grading subgroups of gliomas. Patients with tumors exhibiting high IL-8 expression had significantly shorter overall survival than those with low expression of IL-8 in grade II subgroup (n = 49; P = 0.011, log-rank; ). The median survival time of grade II subgroup with low IL-8 expression (40 months, 95% confidence interval: 20.367–59.633) was significantly longer than that of patients with high IL-8 expression (19 months, 95% confidence interval: 13.803–24.197). The results indicate that IL-8 might correlate with the prognosis of low grade glioma. Similarly, the same conclusions was obtained in grade III (n =70; P = 0.010, log-rank; ) and grade IV (n = 56; P = 0.023, log-rank; )). Thus IL-8 might be an independent prognostic indicator for glioma patients at all disease stages.
Automatic secretion of IL-8 protein by glioma cells
Secretion of IL-8 protein by U251, H4, and U87cell lines was measured by ELISA at various time points after plating. The result showed that all of the glioma cell lines secreted IL-8 protein although the magnitude of secretion varied (). In accordance with previous report, U87 cells secrete the highest amount of IL-8 without any exogenous stimuli.
Increased expression of IL-8 is relevant to increased expression of CXCR1
As a series of studies have suggested that CXCR1 and CXCR2 receptors contribute to the biological effects of IL-8 and expression of these 2 receptors is closely associated with tumor progression and metastasis.Citation15,27,28 In this study, we sought to determine the presence of these receptors in glioma cells and primary glioma tissues. High expression of CXCR1 in U87 and U251 cell lines and in primary glioma tissues was shown in . For human lung cancer A549 cells have high expression of both CXCR1 and CXCR2, so A549 cells were used as positive controls. Noticeably, in consistance with IL-8, the expression of CXCR1 is closely correlated with WHO grading. In contrast, there was no significant expression of CXCR2 in any of the glioma cell lines and glioma tissues tested. To assess the functional link between IL-8 and CXCR1 expression, we sought to test whether in clinical glioma tissues the upregulated expression of IL-8 was associated with increased level of CXCR1. The correlation between the expression levels of IL-8 and CXCR1 was examined in 198 paraffin-embedded glioma clinical specimens. Spearman correlation analysis showed a strong correlation between IL-8 and CXCR1 expression in the tested tissue samples (r = 0.429; P = 0.000; ). These results suggest that in human gliomas, expression of IL-8 is correlated with expression of CXCR1 but not CXCR2.
Autocrine of IL-8 increases the invasion ability of glioma cells
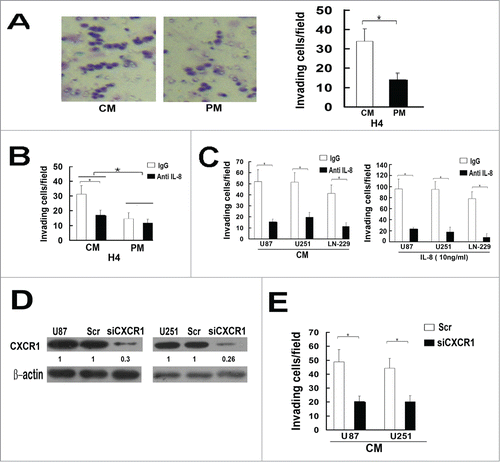
To investigate the role of IL-8 autocrine in glioma invasion, we performed matrigel invasion assay. H4 cells were plated on the upper cell culture inserts, with CM or PM in the lower chambers in the invasion assay. The results showed that CM and recombinant IL-8 (10ng/ml) significantly increased invasion of H4 cells and the invasiveness of H4 cells decreased sharply when treated with anti-IL8 antibody(10ug/ml) (). In addition, We also observed the same effects of anti-IL8 antibody on U87, U251 and LN-229 cells in CM and in medium with recombinant IL-8(10ng/ml) (). These results indicate that autocrine of IL-8 can increase the invasion ability of glioma cells.
Figure 2. Autocrine of IL-8 involves in the invasion of glioma cells. (A) Comparison of the invasiveness of glioma H4 cells in culture medium of CM and PM. Columns, mean of triplicate measurements; Bars, standard deviation. *P < 0.05 (2-way ANOVA). (B) After adding antibody of IL-8(10ug/ml), comparison of the invasiveness of glioma H4 cells in culture medium of CM, PM and in medium with recombinant IL-8(10 ng/ml), IgG was used as a negative control. Columns, mean of triplicate measurements; Bars, standard deviation. *P < 0.05 (2-way ANOVA). (C) After adding antibody of IL-8(10 ug/ml), the invasiveness of glioma cell lines(U87, U251, IN-229) cultured in CM or in medium with recombinant IL-8(10 ng/ml) decreased significantly. IgG was used as a negative control. Columns, mean of triplicate measurements; Bars, standard deviation. *P < 0.05 (2-way ANOVA). (D) Expression of CXCR1 protein in U87 and U251cells transfected with a scrambled siRNA as a control (Scr/U87, Scr/U251) and with stable siRNA-targeting CXCR1 (Si CXCR1/U87, SiCXCR1/U251) was detected by Western blot. β-actin was used as a loading control. Each result is representative from at least 3 independent experiments. Quantification of relative protein levels is shown below the blots. (E) In CM, the invasiveness of SiCXCR1/U87 and SiCXCR1/U251 cells decreased significantly compared with Scr/U87and Scr/U251 cells. In this study, conditioned medium (CM) is a conditioned medium collected from the culture medium of U87 cells.
Knockdown of CXCR1 impaired the IL-8 autocrine induced glioma cells invasion
As we found no CXCR2 expression in the tested glioma cell lines, we predicted that the IL-8 induced invasive response of glioma cells was mediated by CXCR1. To test this hypothesis, we inhibited CXCR1 expression by small interfering RNA (siRNA) and got siCXCR1/U87, siCXCR1/U251 cells and their corresponding control cells Scr/U87 and Scr/U251. The inhibition of protein expression was determined by Western blot analysis (). The control cells and the CXCR1 deficient cells were followed by matrigel invasion assay with CM in lower well of the Boyden chamber with treatment of anti-IL-8 antibody(10ug/ml) or IgG.. Interestingly, in CM, the control cells displayed a higher invasiveness than siCXCR1 cells (). Combining with the above mentioned coherent expression of IL-8 and CXCR1, our results indicated that IL-8 mediates its effects through CXCR1.
Knockdown of ELMO1 impaired glioma cells invasion
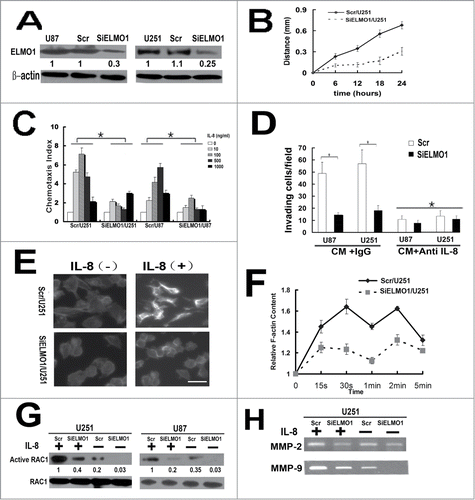
ELMO1/Dock180 complex, a bipartite Rac GEF, has the effect of promoting glioma cell invasion,Citation17 but whether these molecules play a critical role in IL-8-CXCR1 mediating migration and invasion has not been investigated. To disclose the role of ELMO1 in downstream of IL-8-CXCR1, we stably transfected ELMO1 siRNA into U87 and U251 cells to knockdown the expression of ELMO1. SiELMO1/U87, SiELMO1/U251 cells and their corresponding control cells Scr/U87 and Scr/U251 were acquired and used for subsequent experiments (). We detected the rate of cell proliferation of ELMO1 silencing cells and corresponding control cells, the results showed that knockdown of ELMO1 did not significantly influence cell proliferation. Gap filling migration assay was performed to test the directional movement of the control cells and the ELMO1 deficient cells in CM. As shown in , the results showed a significant difference between the directional movement of SiELMO1/U251 and Scr/U251 cells. It took a longer time for SiELMO1/U251 cells to fill the gap. In addition, chemotaxis assay displayed that suppression of ELMO1 inhibited IL-8-induced glioma cell migration significantly (). Furthermore, the invasion of SiELMO1/U87 and SiELMO1/U251 cells in CM with IgG was remarkably attenuated when compared with Scr/U87 and Scr/U251 cells. Interestingly, when anti-IL-8 antibody was added into CM, the invasiveness of Scr/U87 and Scr/U251 cells decreased significantly which indicated that knockdown of ELMO1 impaired invasion of glioma cells ().
Figure 3. IL-8 promoted chemotaxis and invasion of glioma cells in an ELMO1 dependent manner. (A) Expression of ELMO1 protein in U87 and U251cells transfected with a scrambled siRNA as a control (Scr/U87, Scr/U251) and with stable siRNA-targeting ELMO1 (SiELMO1/U87, SiELMO1/U251) was detected by Western blot. β-actin was used as a loading control. Each result is representative from at least 3 independent experiments. Quantification of relative protein levels is shown below the blots. (B) Comparison of cell directional movement between Scr/U251 and SiELMO1/U251 cells by scratch assay. Each data point was an average of triplicate assays. (C) Comparison of chemotactic responses with IL-8 (0, 10, 100, 500, 1000 ng/ml) stimulation in control cells (Scr/U87, Scr/U251) and ELMO1 knockdown cells (SiELMO1/U87, SiELMO1/U251). Columns, mean of triplicate measurements; Bars, standard deviation. *P < 0.05 (2-way ANOVA). (D) Comparison of invasive ability of Scr/U87, SiELMO1/U87, Scr/U251 and SiELMO1/U251 in CM with or without treating with of IL-8 antibody(10ug/ml). Columns, mean of triplicate measurements; Bars, standard deviation. *P < 0.05 (2-way ANOVA). (E) Cytoskeleton rearrangement in Scr/U251 and SiELMO1/U251 cells with or without IL-8(10ng/ml) stimulation was imaged by fluorescence assay. Figures showed representative images from 3 repeated experiments. Scale bars: 5 um. (F) Time course of relative F-actin content in SCR/U251 and siELMO1/U251 cells following 10 ng/ml of IL-8 stimulation. Data from 3 independent experiments (n = 3). (G) Expression of active RAC1 protein in Scr/U251, SiELMO1/U251, Scr/U87 and SiELMO1/U87cells with or without stimulation of IL-8 was detected by Western blot. RAC1 was used as a loading control. Each result is representative from at least 3 independent experiments. Quantification of relative protein levels is shown below the blots. (H) Gelatin zymography analysis showed the activity of MMP-2 and MMP-9 in Scr/U251 and SiELMO1/U251 cells with or without IL-8 treatment.
Reduction of ELMO1 impaired the IL-8-induced F-actin polymerization in U87 cells
Chemokine binding to receptors activates downstream signal pathways which ultimately regulate intracellular actin polymerization and transient F-actin assembly to drive cell migration.Citation18,29 To examine how IL-8 affects cell migration, we performed the F-actin polymerization assay. The F-actin polymerization assay showed that IL-8 elicited a transient actin polymerization at 30s and 120s in the Scr/U251 cells while in siELMO1/U251 cells F-actin polymerization was significantly inhibited (). Thus, the ELMO1 regulates IL-8-induced F-actin polymerization and plays an important role in the cytoskeleton rearrangement.
Knockdown of ELMO1 inhibited the IL-8-induced activatin of RAC1
Activation of a small G-protein, Rac, induces growth of actin filaments and initiates the formation of new actin branches from existing ones, resulting in driving the membrane forward and cell migration.Citation9,30,31 Activation of Rac requires guanine nucleotide exchange factors (GEFs).Citation32 ELMO/Dock180 complexes are evolutionarily conserved and serve as GEFs for Rac proteins controlling actin cytoskeleton. To obtain insights into the molecular mechanisms underlying the IL-8-CXCR1-mediated regulation of cell migration, we detected the IL-8-induced activation of RAC1 in ELMO1 deficient cells. As shown in , reduction of ELMO1 inhibited IL-8-induced activation of RAC1 in SiELMO1/U251 and SiELMO1/U87 cells, consistent with the defects of actin polymerization in SiELMO1/U251 cells. In summary, these findings indicate that the reduction of ELMO1 in glioma cells resulted in a major cytoskeletal reorganization inhibition in response to IL-8 by regulating the activation of RAC1.
Knockdown of ELMO1 is relevant to decreased activation of MMP-2 and MMP-9
We have proved that knockdown of ELMO1 can reduce the invasive ability of glioma cells. Since matrix metalloproteases (MMPs) are known to be involved in glioma invasion, we focused to detect the activation of MMP-2 and MMP-9 in siELMO1/U251 and control cells with or without IL-8 stimulation by gelatin zymography. As shown in , with exogenous IL-8 stimulation, the amounts of active MMP-2 and MMP-9 were obviously downregulated in siELMO1/U251 cells. These results strongly suggest that ELMO1 plays vital roles in glioma invasion.
IL-8 enhanced cell mesenchymal properties which is closely associated with ELMO1
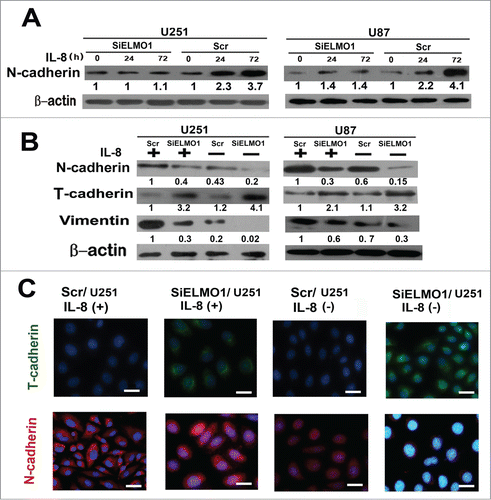
Mesenchymal Transition (MT) plays an important role in the metastatic potential of tumor cells.Citation33,34 To investigate whether IL-8 can promote glioma cell invasion by enhancing cell mesenchymal properties, we detected the expression of mesenchymal marker N-cadherin by Western blot analysis after cells were treated with 10ng/mL IL-8 stimulation at different time points.Citation35 The result showed that expression of N-cadherin increased markedly in Scr/U251 and Scr/U87 glioma cells at various time points whereas no obvious upregulation of N-cadherin in SiELMO1/U251 and SiELMO1/U87 cells(). Furthermore, we compared the expression levels of mesenchymal marker(N-cadherin, vimentin) and glial cell marker (T-cadherin) in ELMO1 silencing cells with those in control cells. Interestingly, with or without IL-8 stimulation, there was relatively high expression of N-cadherin and vimentin and low expression of T-cadherin in control cells. While in ELMO1 silencing cells, the result is just the opposite. At the same time, with exogenous stimulation of IL-8, both control cells and ELMO1 silencing cells had higher expression of N-cadherin and vimentin and lower expression of T-cadherin than corresponding cells without IL-8 stimulation(). Consistent with these results, immunofluorescence staining of cells also showed downregulation of ELMO1 inhibited the MT of glioma cells (). Hence, all these findings indicate that IL-8 enhanced the mesenchymal properties of cells and induced MT via ELMO1 signaling transduction.
Figure 4. IL-8 enhanced cell mesenchymal properties and induced MT via ELMO1. (A) The expression of N-cadherin in Scr/U251, SiELMO1/U251, Scr/U87 and SiELMO1/U87 cells with 10 ng/mL IL-8 at different time points. β-actin was used as a loading control. Each result is representative from at least 3 independent experiments. Quantification of relative protein levels is shown below the blots. (B) Expression of glial markers, T-cadherin, as well as mesenchymal markers, N-cadherin and vimentin, were examined by Western blot in Scr/U251, SiELMO1/U251, Scr/U87 and SiELMO1/U87 cells with or without 10 ng/mL IL-8 for 24 h. β-actin was used as a loading control. Each result is representative from at least 3 independent experiments. Quantification of relative protein levels is shown below the blots. (C) Fluorescence microscopic staining of T-cadherin (green) and N-cadherin (red) is indicated in Scr/U251 and SiELMO1/U251 cells with or without IL-8 for 24 h. Nuclear DNA was stained with DAPI (blue). Scale bar: 10 um. Data were collected in this set of figures from a representative of at least 3 independent experiments.
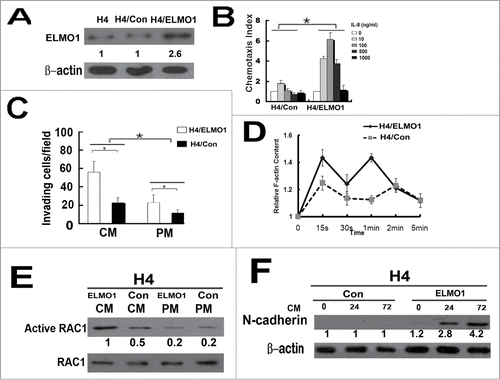
Increased expression of ELMO1 enhanced the invasion of H4 cells in vitro
To further prove the role of ELMO1 in CM-induced motility and invasion, H4 cells were overexpressed with ELMO1 () and then used for chemotaxis assay and invasion assay. Our results showed that the chemotaxis capacity of H4/ELMO1 cells was much higher than that of H4/Con cells with the stimulation of IL-8(). The invasiveness of H4 cells in PM were almost unaffected with ELMO1 overexpression while in CM the invasiveness of H4/ELMO1 increased significantly (). Moreover, F-actin polymerization was significantly increased in H4/ELMO1cells than that of H4/Con cells (). Furthermore, overexpression of ELMO1 promoted CM-induced activation of RAC1 and MT in H4/ELMO1 cells(). These results provide another line of evidence in support of the role of ELMO1 in CM-induced invasiveness of glioma cells.
Figure 5. Overexpression of ELMO1 in H4 cell enhanced cell invasion and mesenchymal properties. (A) Overexpression of ELMO1 protein in H4 cells was detected by Western blot. β-actin was used as a loading control. (B) Comparison of chemotactic responses with IL-8 (0, 10, 100, 500, 1000 ng/ml) stimulation in control cells(H4/Con) and ELMO1 overexpression cells (H4/ELMO1). Columns, mean of triplicate measurements; Bars, standard deviation. *P < 0.05 (2-way ANOVA). (C) Comparison of invasive ability of H4/Con and H4/ELMO1 cells in culture medium of CM or PM. Columns, mean of triplicate measurements; Bars, standard deviation. *P < 0.05 (2-way ANOVA). (D) Time course of relative F-actin content in H4/Con and H4/ELMO1 cells following 10 ng/ml of IL-8 stimulation. Data from 3 independent experiments (n = 3). (E) Expression of active RAC1 protein in H4/Con and H4/ELMO1 cells with culture medium of CM or PM was detected by Western blot. RAC1 was used as a loading control. Each result is representative from at least 3 independent experiments. Quantification of relative protein levels is shown below the blots. (F) Expression of N-cadherin in H4/Con and H4/ELMO1 cells cultured in CM at different time points was detected by Western blot. β-actin was used as a loading control. Quantification of relative protein levels is shown below the blots. Each result is representative from at least 3 independent experiments.
IL-8 stabilised Snail via ELMO1
To identify the mechanism involved in IL-8-modulated MT, the expression levels of several known transcriptional factors in glioma cells were detected. Since it is known that Snail, Twist1, Slug, Zeb1 and Zeb2 involve in the process of MT,Citation36 we examined the protein expression levels of these transcriptional factors by western blotting. After treated with IL-8(10 ng/mL) for 24 h, the expression of Snail protein significantly increased in Scr/U251 cells, but in SiELMO1/U251 cells Snail protein level had no obvious change(). The expression levels of other transcriptional factors (Twist1, Slug, Zeb1 and Zeb2) were stable in both Scr/U251 and SiELMO1/U251 cells with or without IL-8 stimulation for 24 h (Fig.6A). Meanwhile, it is notable that the expression level of Snail mRNA transcripts did not change in response to altered Snail protein levels with IL-8 stimulation (). The Snail protein is known to be destabilized after its translocation out of the nucleus, following its phosphorylation.Citation37,38 We tested whether ELMO1 was involved in the Snail translocation event with IL-8 stimulation. The result showed that Snail protein was up-regulated in nuclear compartments of Scr/U251 but not in that of SiELMO1/U251 cells with IL-8(10 ng/mL) stimulation (), indicating that IL-8 stabilised Snail via ELMO1.
Figure 6. IL-8 binding to CXCR1 stabilizes Snail via the ELMO1-NF-κB signaling pathway. (A) Expression levels of transcription factors Snail, Twist1, Slug, Zeb1 and Zeb2 in Scr/U251 and SiELMO1/U251 cells with or without IL-8 for 24 h were detected by Western blot. β-actin was used as a loading control. (B) Expression levels of Snail mRNA in Scr/U251 and SiELMO1/U251 cells with or without IL-8 for 24 h were detected by Real time PCR. Bars, standard deviation. (C) Nuclear expression level of Snail in Scr/U251 and SiELMO1/U251 cells with or without IL-8 for 24 h was examined by Western blot. Nucleolin was used as a loading control. IL-8, 10 ng/mL. (D) U251 cells were pretreated with various inhibitors(all 20uM), BAY11–7082 (inhibitor of IκBα), SB203580(inhibitor of p38MAPK), U0126(inhibitor of ERK1/2) and LY294002(inhibitor of PI3K)for 1 h, DMSO was used as negative control. The expression level of Snail was detected by Western blot. IL-8, 10 ng/mL. (E) Expression levels of P-IκBα in Scr/U251, SiELMO1/U251, Scr/U87 and SiELMO1/U87 cells with or without 10 ng/mL IL-8 were examined by Western blot. IκBα was used as a loading control. IL-8, 10ng/mL. Quantification of relative protein levels is shown below the blots. Data were collected in this set of figures from a representative of at least 3 independent experiments (A–E).

NF-κB is the upstream regulator of Snail expression in IL-8-induced MT
To further explore the underlying mechanism by which IL-8 mediated Snail stabilization, we then examined pathways downstream of ELMO1. Previous reports showed that PI3K, ERK1/2, p38MAPK and NF-κB pathway could regulate Snail levels,Citation39,40 thereby we treated U251 cells with inhibitors specific to each of these signaling pathways to determine which particular signaling pathway is involved in IL-8 induced Snail stabilization. The results showed that the stabilization of Snail related to ELMO1 was only notably inhibited by NF-κB inhibitor (BAY11–7082) (). In addition, with or without IL-8 stimulation, the expression level of P-IκBα(indicating the activation of NF-κB) significantly decreased in ELMO1 knockdown cells which indicated the key role of ELMO1 in activation of NF-κB (). This finding suggests that the NF-κB pathway is critical for IL-8 induced Snail stabilization and MT.
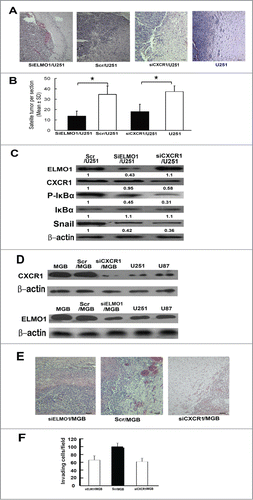
Reduction of ELMO1 and CXCR1 decreased glioma cells invasion in vivo
To evaluate potential contribution of ELMO1 and CXCR1 to the invasive implications of glioma in vivo, we transplanted SiELMO1/U251, SiCXCR1/U251 or matched Scr/U251 cells into brains of male SCID mice through intracranial injection and compared glioma cell dispersal into brain tissue 3 weeks after transplantation. As shown in , we found a considerable high number of satellite tumors infiltrating into surrounding brain tissue in mice implanted with Scr/U251 cells, and notably, these satellite tumors had spread far away from the original injection site. On the other hand, most tumor cells were limited in the injection site and less invasive intracranial tumors in surrounding brain tissue were detected in the mice implanted with SiELMO1/U251 or SiCXCR1/U251 cells. The number of satellite tumors (tumor foci not connected with the main tumor) was counted to measure invasiveness in vivo [14,29] and the SiELMO1/U251 and SiCXCR1/U251 groups had a much less number of satellite tumors (13.9 vs. 34.1 and 16.8 vs. 34.1 satellite tumors per section, both P < 0.05; ). Meanwhile, we also detected the roles of CXCR1 and ELMO1 in glioma invasion in primary human high invasive glioblastoma (GBM) cells and got similar results (Fig. 2S). All these data indicate that reduction of ELMO1 and CXCR1 inhibited glioma cell invasion in vivo. Furthermore, we also detected expression levels of IL-8, P-IκBα, IκBα and Snail in implanted tumor tissues. Consistent with the results in vitro, expression levels of P-IκBα and Snail decreased obviously in SiELMO1/U251 and SiCXCR1/U251 transplanted tissues (), further demonstrating the critical role of CXCR1-ELMO1-NF-κB-Snail pathway in IL-8 induced MT.
Figure 7. Reduction of ELMO1 or CXCR1 inhibited U251 cells invasion in vivo. (A) Representative microscope graphs showing less satellite tumors in SiELMO1/U251 or SiCXCR1/U251 injected mice brain tissues than that of Scr/U251 or U251 injected mice brain tissues, Scale bar: 50 um. (B) The number of satellite tumors (tumor foci not connected to main body of tumor) were counted in sections from nude mice sacrificed at day 21 of the study (n = 10 for all group, *P < 0.05, 2-tailed unpaired t-test). (C) Western blot analysis of expression levels of ELMO1, CXCR1, P-IκBα, IκBα and Snail in SiELMO1/U251, Scr/U251 and SiCXCR1/U251 implanted tumor tissues. β-actin was used as a loading control. Quantification of relative protein levels is shown below the blots. Data were collected from a representative of at least 3 independent experiments. (D) Western blot analysis of CXCR1 expression levels in GBM, Scr/GBM, siCXCR1/GBM, U251 and U87 cells(above). Western blot analysis of ELMO1 expression levels in GBM, Scr/GBM, siELMO1/GBM, U251 and U87 cells (under). β-actin was used as a loading control. Quantification of relative protein levels is shown below the blots. Data were collected from a representative of at least 3 independent experiments. (E) Representative microscope graphs showing less satellite tumors in siELMO1/GBM or siCXCR1/GBM injected mice brain tissues than that of Scr/GBM injected mice brain tissues, Scale bar: 50 um. (F) The number of satellite tumors (tumor foci not connected to main body of tumor) were counted in sections from nude mice sacrificed at day 21 of the study (n = 10 for all group, *P < 0.05, 2-tailed unpaired t-test).
Discussion
Various autocrine motility factors expressed by invasive glioma cells are drivers of glioma invasion which signal through receptors on tumor cells.Citation3 In the present study, we report 4 major chemokine/receptor-induced alteration in glioma cells: First, the production of IL-8 is higher in glioma tissues and cells than ANT and normal glial cells. Autocrine IL-8 can increase the invasive ability of glioma cells and high expression of IL-8 indicates poor prognosis of glioma patients. Second, glioma cells express CXCR1 but not CXCR2 and IL-8 is clinically relevant to increased expression of CXCR1 which indicated that IL-8 mediates its effects through CXCR1, this is in accordance with previous report.Citation8 Third, IL-8 is capable of modulating cell migration and invasion by regulating the activation of RAC1 which resulted in cytoskeletal reorganisation in an ELMO1 dependent manner. Fourth, IL-8 could enhance MT of glioma cells by activating ELMO1-NF-κB-Snail signaling.
In an autocrine manner, CM significantly potentiated the invasive ability of glioma cells than PM. At the same time, the effects of CM was inhibited by neutralizing antibody against IL-8 and knockdown of CXCR1. In accordance with previous reports,Citation41,42 highly expressed IL-8 and CXCR1 in glioma and autocrine activity of IL-8 have been clearly demonstrated in this study. By binding to CXCR1, the IL-8 produced by the glioma cells themselves is responsible for the invasive and mesenchymal features of glioma.
The activation of Rac, a small G-protein, mediates polymerization of actin filaments. Serving as a GEF for Rac1, ELMO1/Dock180 complex has an important role in promoting glioma cell invasion.Citation17 It has been shown that activation of G-protein coupled receptor (GPCR) promotes an interaction between the ELMO/Dock complex and heterotrimeric G-protein subunits which controls chemotaxis.Citation43 We therefore wondered whether the disruption of ELMO1 expression by siRNA would be sufficient to affect IL-8-mediated migration and invasion of glioma cells. In agreement with previous reports, our results proved knockdown of ELMO1 abolished IL-8-induced RAC1 activation, then resulting in a significant cytoskeletal reorganization reduction, indicating the functional importance of ELMO1/Dock-RAC1 signaling pathway in IL-8 induced chemotaxis and invasion in glioma cells both in vitro and in vivo.
Mesenchymal transition (MT) is considered a critical event in cancer cell invasion. Induction of MT is driven through complex interacting mechanisms between tumor environment and cancer cells. In this report, we show that exogenous administration of IL-8 resulted in significantly increased expression of mesenchymal marker N-cadherin, vimentin and decreased expression of glial cell marker T-cadherin. At the same time, similar to previous reports, the cells underwent MT which was associated with increased NF-κB activity and stabilization of Snail. Furthermore, the MT was dramatically deactivated by knockdown of ELMO1, indicating the functional importance of ELMO1 in IL-8 induced NF-κB-Snail-MT signaling.
In this report, we present extensive in vitro and in vivo work to show that IL-8 autocrine plays important roles in glioma progression by binding to CXCR1. Moreover, our findings provide molecular insight into the regulation and function of IL-8 in bridging 2 major pathways, i.e., ELMO/Dock180/RAC1 and ELMO/Dock180/NF-κB/Snail, for the regulation of glioma cell migration and invasion. Thus, IL-8 may be a useful prognostic marker for glioma and novel therapeutic target for glioma invasion intervention.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Statement of Author Contributions
Conception and design: Baogang Zhang.
Designed research: Baogang Zhang.
Performed research: analyzed data: Baogang Zhang, Lihong Shi, Shijun Lu Xiuning Sun, Yuqing Liu, Xuejian Wang, Chunzhen Zhao, Hongli Li, Heng Zhang, Ying Wang.
All authors were involved in writing the paper and had final approval of the submitted and published versions.
Supplementary Tables
Download MS Word (32.5 KB)Additional information
Funding
References
- Burke F, Relf M, Negus R, Balkwill F. A cytokine profile of normal and malignant ovary. Cytokine 1996; 8:578–85; PMID:8891439; http://dx.doi.org/10.1006/cyto.1996.0077
- Auf G, Jabouille A, Delugin M, Guerit S, Pineau R, North S, Platonova N, Maitre M, Favereaux A, Vajkoczy P, et al. High epiregulin expression in human U87 glioma cells relies on IRE1alpha and promotes autocrine growth through EGF receptor. BMC Cancer 2013; 13:597; PMID:24330607; http://dx.doi.org/10.1186/1471-2407-13-597
- Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 2007; 99:1583–93; PMID:17971532; http://dx.doi.org/10.1093/jnci/djm187
- Tsareva SA, Wagner S, Muller A, Corvinus F, Friedrich K. Cell-cell contacts induce STAT3 activity in colon carcinoma cells through an autocrine stimulation loop. J Cancer Res Clin Oncol 2011; 137:857–63; PMID:20830487; http://dx.doi.org/10.1007/s00432-010-0943-3
- Jennings MT, Maciunas RJ, Carver R, Bascom CC, Juneau P, Misulis K, Moses HL. TGF beta 1 and TGF beta 2 are potential growth regulators for low-grade and malignant gliomas in vitro: evidence in support of an autocrine hypothesis. Int J Cancer 1991; 49:129–39; PMID:1874566; http://dx.doi.org/10.1002/ijc.2910490124
- Wittekind C, Neid M. Cancer invasion and metastasis. Oncology 2005; 69 (Suppl 1):14–6; PMID:16210871; http://dx.doi.org/10.1159/000086626
- Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol 2005; 7:122–33; PMID:15831231; http://dx.doi.org/10.1215/S1152851704001061
- Raychaudhuri B, Vogelbaum MA. IL-8 is a mediator of NF-kappaB induced invasion by gliomas. J Neurooncol 2011; 101:227–35; PMID:20577780; http://dx.doi.org/10.1007/s11060-010-0261-2
- Sun CX, Magalhaes MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol 2007; 179:239–45; PMID:17954607; http://dx.doi.org/10.1083/jcb.200705122
- Desbaillets I, Diserens AC, de Tribolet N, Hamou MF, Van Meir EG. Regulation of interleukin-8 expression by reduced oxygen pressure in human glioblastoma. Oncogene 1999; 18:1447–56; PMID:10050881; http://dx.doi.org/10.1038/sj.onc.1202424
- Salmaggi A, Eoli M, Frigerio S, Silvani A, Gelati M, Corsini E, Broggi G, Boiardi A. Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol 2003; 62:297–303; PMID:12777082; http://dx.doi.org/10.1023/A:1023367223575
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol 2002; 72:847–55; PMID:12429706
- Smyth MJ, Zachariae CO, Norihisa Y, Ortaldo JR, Hishinuma A, Matsushima K. IL-8 gene expression and production in human peripheral blood lymphocyte subsets. J Immunol 1991; 146:3815–23; PMID:1827816
- Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev 2001; 12:375–91; PMID:11544106; http://dx.doi.org/10.1016/S1359-6101(01)00016-8
- Bates RC, DeLeo MJ, 3rd, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res 2004; 299:315–24; PMID:15350531; http://dx.doi.org/10.1016/j.yexcr.2004.05.033
- Slettenaar VI, Wilson JL. The chemokine network: a target in cancer biology? Adv Drug Deliv Rev 2006; 58:962–74; PMID:16996642; http://dx.doi.org/10.1016/j.addr.2006.03.012
- Jarzynka MJ, Hu B, Hui KM, Bar-Joseph I, Gu W, Hirose T, Haney LB, Ravichandran KS, Nishikawa R, Cheng SY. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res 2007; 67:7203–11; PMID:17671188; http://dx.doi.org/10.1158/0008-5472.CAN-07-0473
- Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 2007; 17:383–93; PMID:17765544; http://dx.doi.org/10.1016/j.tcb.2007.05.001
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 2002; 4:574–82; PMID:12134158
- Velpula KK, Dasari VR, Tsung AJ, Dinh DH, Rao JS. Cord blood stem cells revert glioma stem cell EMT by down regulating transcriptional activation of Sox2 and Twist1. Oncotarget 2011; 2:1028–42; PMID:22184289
- Yu J, Ren X, Chen Y, Liu P, Wei X, Li H, Ying G, Chen K, Winkler H, Hao X. Dysfunctional activation of neurotensin/IL-8 pathway in hepatocellular carcinoma is associated with increased inflammatory response in microenvironment, more epithelial mesenchymal transition in cancer and worse prognosis in patients. PLoS One 2013; 8:e56069; PMID:23418512; http://dx.doi.org/10.1371/journal.pone.0056069
- Palena C, Hamilton DH, Fernando RI. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol 2012; 8:713–22; PMID:22764769; http://dx.doi.org/10.2217/fon.12.59
- Li XJ, Peng LX, Shao JY, Lu WH, Zhang JX, Chen S, Chen ZY, Xiang YQ, Bao YN, Zheng FJ, et al. As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial-mesenchymal transition and activation of AKT signaling. Carcinogenesis 2012; 33:1302–9; PMID:22610073; http://dx.doi.org/10.1093/carcin/bgs181
- Zhang B, Yin C, Li H, Shi L, Liu N, Sun Y, Lu S, Liu Y, Sun L, Li X, et al. Nir1 promotes invasion of breast cancer cells by binding to chemokine (C-C motif) ligand 18 through the PI3K/Akt/GSK3beta/Snail signalling pathway. Eur J Cancer 2013; 49 (18):3900–13; PMID:24001613; http://dx.doi.org/10.1016/j.ejca.2013.07.146
- Das G, Shiras A, Shanmuganandam K, Shastry P. Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. Mol Carcinog 2011; 50:412–23; PMID:21557327; http://dx.doi.org/10.1002/mc.20723
- Takino T, Nakada M, Miyamori H, Yamashita J, Yamada KM, Sato H. CrkI adapter protein modulates cell migration and invasion in glioblastoma. Cancer Res 2003; 63:2335–7; PMID:12727859
- Shamaladevi N, Lyn DA, Escudero DO, Lokeshwar BL. CXC receptor-1 silencing inhibits androgen-independent prostate cancer. Cancer Res 2009; 69:8265–74; PMID:19861539; http://dx.doi.org/10.1158/0008-5472.CAN-09-0374
- Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology 1999; 67:12–8; PMID:9873223; http://dx.doi.org/10.1159/000028045
- Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol 2004; 5:626–34; PMID:15366706; http://dx.doi.org/10.1038/nrm1435
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112:453–65; PMID:12600310; http://dx.doi.org/10.1016/S0092-8674(03)00120-X
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 2009; 17:310–22; PMID:19758556; http://dx.doi.org/10.1016/j.devcel.2009.08.012
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol 2004; 265:23–32; PMID:14697350; http://dx.doi.org/10.1016/j.ydbio.2003.06.003
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871–90; PMID:19945376; http://dx.doi.org/10.1016/j.cell.2009.11.007
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420–8; PMID:19487818; http://dx.doi.org/10.1172/JCI39104
- Huang ZY, Wu Y, Hedrick N, Gutmann DH. T-cadherin-mediated cell growth regulation involves G2 phase arrest and requires p21(CIP1/WAF1) expression. Mol Cell Biol 2003; 23:566–78; PMID:12509455; http://dx.doi.org/10.1128/MCB.23.2.566-578.2003
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 2005; 17:548–58; PMID:16098727; http://dx.doi.org/10.1016/j.ceb.2005.08.001
- Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell 2009; 15:416–28; PMID:19411070; http://dx.doi.org/10.1016/j.ccr.2009.03.016
- Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem 2005; 280:11740–8; PMID:15647282; http://dx.doi.org/10.1074/jbc.M413878200
- Pilot-Storck F, Chopin E, Rual JF, Baudot A, Dobrokhotov P, Robinson-Rechavi M, Brun C, Cusick ME, Hill DE, Schaeffer L, et al. Interactome mapping of the phosphatidylinositol 3-kinase-mammalian target of rapamycin pathway identifies deformed epidermal autoregulatory factor-1 as a new glycogen synthase kinase-3 interactor. Mol Cell Proteomics 2010; 9:1578–93; PMID:20368287; http://dx.doi.org/10.1074/mcp.M900568-MCP200
- Pardo R, Andreolotti AG, Ramos B, Picatoste F, Claro E. Opposed effects of lithium on the MEK-ERK pathway in neural cells: inhibition in astrocytes and stimulation in neurons by GSK3 independent mechanisms. J Neurochem 2003; 87:417–26; PMID:14511119; http://dx.doi.org/10.1046/j.1471-4159.2003.02015.x
- Bonavia R, Inda MM, Vandenberg S, Cheng SY, Nagane M, Hadwiger P, Tan P, Sah DW, Cavenee WK, Furnari FB. EGFRvIII promotes glioma angiogenesis and growth through the NF-kappaB, interleukin-8 pathway. Oncogene 2012; 31:4054–66; PMID:22139077; http://dx.doi.org/10.1038/onc.2011.563
- Kim DS, Kim JH, Lee JK, Choi SJ, Kim JS, Jeun SS, Oh W, Yang YS, Chang JW. Overexpression of CXC chemokine receptors is required for the superior glioma-tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev 2009; 18:511–9; PMID:18624673; http://dx.doi.org/10.1089/scd.2008.0050
- Yan J, Mihaylov V, Xu X, Brzostowski JA, Li H, Liu L, Veenstra TD, Parent CA, Jin T. A Gbetagamma effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev Cell 2012; 22:92–103; PMID:22264729; http://dx.doi.org/10.1016/j.devcel.2011.11.007
