Abstract
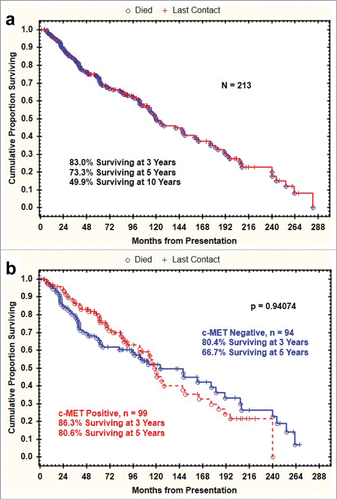
Adenoid cystic carcinoma (ACC), a rare salivary gland malignancy, is a histogenetic, morphologic, and clinical heterogeneous disease. Extensive efforts have been made to characterize molecular events associated with these tumors, including the identification of prognostic and predictive biomarkers. Increased copy number gain and amplification of c-Met, the cell surface receptor for hepatocyte growth factor, has been shown to enhance tumor growth and invasiveness and promote metastasis in certain tumor types. In this study, we evaluated the expression of c-Met by immunohistochemistry (IHC) in a large cohort of salivary gland ACCs and examined its clinicopathologic implications. Archival formalin-fixed paraffin-embedded blocks from 200 ACC patients were used in this study. Pathologic patterns and phenotypic expression of c-Met were recorded and compared with clinical factors including gender, age, disease stage at diagnosis, and clinical outcomes. Correlations between c-MET expression and clinical characteristics were assessed by Pearson's chi-square test or by the 2-tailed Fisher exact test. Curves describing overall survival were generated by Kaplan-Meier product limit method. Strong c-MET expression was seen in inner ductal and outer myoepithelial cells in 53.2% of the cases. There was no correlation between c-Met overexpression and clinicopathologic parameters or patient's overall survival ( p = .94074). In conclusion, c-MET expression is high in a significant subgroup of ACC patients. While c-MET expression is not a prognostic factor in ACC, its role as a predictive marker of benefit from MET inhibitors deserves further investigation.
Introduction
Adenoid cystic carcinoma (ACC), the second most frequent malignancy of the major and minor salivary glands, makes up approximately 15%–23% of all carcinomas at these locations.Citation1,2 Despite undergoing resection with clear margins, up to 60% of patients have locoregional or distant recurrence. The median survival duration of patients with distant metastases is about 3 years, although up to 10% of these patients may survive 10 y or longer with indolent metastases. Extensive efforts have been made to characterize molecular events associated with these tumors, including the identification of biomarkers for prognostication and therapy guidance. Copy number gain and amplification of c- MET, the cell surface receptor for hepatocyte growth factor (HGF), has been shown to enhance tumor growth and invasiveness and promote metastasis in certain tumor types ().Citation3 The relevance of c-MET expression in ACC of salivary gland origin was first described in 2003 in a study published by Suzuki, et al.Citation4 Although the findings of Suzuki, et al. were novel, the study's small number of cases limited its impact. In the present study, we evaluated the expression of c- MET in a large cohort of salivary ACCs and examined its clinicopathologic implications.
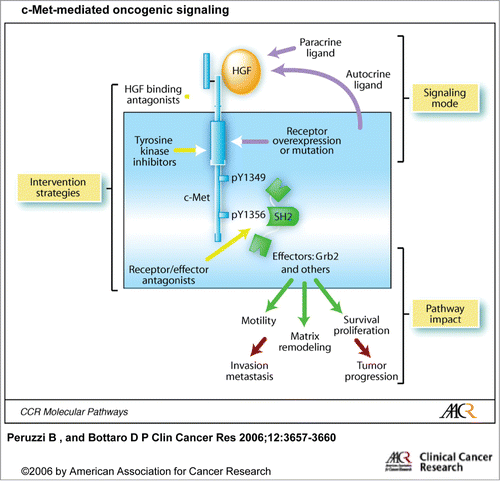
Figure 1. c-Met-mediated oncogenic signaling (from Peruzzi et al). Activation of c-Met-mediated oncogenic signaling occurs most commonly through aberrant paracrine HGF stimulation and consequent receptor overexpression or through autocrine HGF production, receptor mutation, gene amplification or rearrangement. Increased cell proliferation, survival, motility, and extracellular matrix degradation resulting from pathway activation contributes to tumor growth, invasiveness, and metastasis. At least 3 routes of pathway intervention have been followed as selective anticancer drug development strategies: antagonism of ligand binding, inhibition of TK catalytic function, and blockade of interactions between activated receptors and downstream intracellular effectors.© American Association for Cancer Research. Reproduced by permission of American Association for Cancer Research. Permission to reuse must be obtained from the rightsholder.
Materials and Methods
Archival formalin-fixed paraffin-embedded blocks of 200 ACCs patients accessioned at The University of Texas MD Anderson Cancer Center between 1988 and 2006 formed this study cohort. Appropriate written informed consent was obtained after the study was approved by the Institutional Review Board. The material for tissue microarray was created by using 2 1.0-mm-diameter cores of spatially different areas of representative tumor from each paraffin block. Pathologic patterns and phenotypic expression of c- MET were recorded and correlated with patient's clinical characteristics including gender, age, stage, and clinical outcome. Cytospins prepared from a newly validated ACC cell lineCitation5 were also used.
Immunohistochemical and immunoreactivity analyses
Immunohistochemical (IHC) analysis of c-MET was performed with use of the BOND MAX IHC staining protocol by Vision Biosystems (Norwell, MA) on 4-μm paraffin sections of the tissue microarray material. In brief, after the slides were dewaxed, washed, and rehydrated with use of xylene and graded alcohol washes, Tris-EDTA buffer was used for antigen retrieval. Slides were subsequently treated with 3% hydrogen peroxide to block endogenous peroxidase. After incubation with the c- MET primary antibody (Novocastra, 1:50 dilution), the secondary conjugate antibody was applied. Finally, each specimen-containing slide was developed by using the chromogen DAB and counterstained with hematoxylin.
c- MET IHC staining was independently evaluated by 2 pathologists. Only cytoplasmic and membranous staining was considered positive. Lesional tissues with strong staining in >50% of the neoplastic cells were scored as strongly positive. Weak staining or strong staining in <50% of the cells was scored as weakly positive. Less than 5% staining was scored as negative.
Statistical analysis
Descriptive statistics for scaled values and frequencies of study patients within the categories for each of the parameters of interest were enumerated with the assistance of commercial statistical software. Correlations between biomarkers, and between biomarkers and end points, were assessed by Pearson's chi-square test or, when fewer than 10 subjects were in any cell of a 2 × 2 grid, by the 2-tailed Fisher exact test. Curves describing overall survival were generated by the Kaplan-Meier product limit method. The statistical significance of differences between the actuarial curves was tested using log rank test. Follow-up time was calculated from the date of the first appointment at MD Anderson Cancer Center for the primary tumor of concern until the last contact date or death. Calculated p values of <0.05 were considered statistically significant. Statistical tests were performed with use of Statistica (StatSoft, Inc., Tulsa, OK) and SPSS (SPSS for Windows, SPSS Inc., Chicago IL) software applications.
Results
Patient's demographics and tumor staging at diagnosis
The study cohort comprised 98 women and 102 men, who ranged in age from 15 to 86 years, with a median age of 52 y. Primary tumor sites included parotid gland (29), hard palate (28), maxillary sinus (26), submandibular and sublingual glands (20), and minor salivary glands (97). According to AJCC staging criteria, 5 patients had stage I disease, 22 had stage II, 7 had stage III, and 31 had stage IV. Staging information was not available for 135 patients.
Histopathologic findings and clinical outcomes
Histopathologically, tissue cores showed at least 2 distinctive patterns within and between cores of the same case. When more than 50% of a given tumor pattern was present, it was defined as predominant. Among the 194 tumors for which a predominant type could be ascertained, 57 (29.4%) were predominantly tubular, 109 (56.2%) cribriform (composed of epithelial and myoepithelial neoplastic cells), and 28 (14.4%) were solid (mostly devoid of myoepithelial cells). Sixty-seven (43%) patients developed distant metastasis, while 89 patients had no evidence of metastasis at the time of last follow-up. Thirty patients (20.4%) died in less than 3 years, and 117 patients survived more than 3 y. Survival data was not available for 53 patients.
c-Met expression
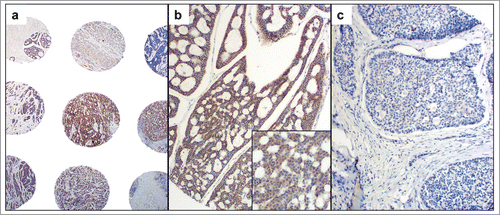
In the normal salivary gland parenchyma, c- MET was negative or faintly expressed in the cytoplasm of salivary ductal cells. In ACCs, c- MET expression was seen in inner ductal cells and outer myoepithelial cells (with a cytoplasmic and membranous pattern of staining) (). Expression of c- MET was strongly positive in 89 (53.2%) of the patients, while 78 (46.8%) had weak or no c- MET IHC staining (several samples did not survive the histopathologic process, and therefore could not be evaluated). The adenoid cystic cell line strongly expressed c-Met in a diffuse cytoplasmic and membranous pattern.
Clinicopathologic correlations
Expression of c-MET was not associated with a particular histologic ACC subtype (cribriform, tubular, solid) (). It also did not correlate with histologic grade, perineural invasion, tumor stage at diagnosis, or distance metastasis.
Table 1 c-MET protein expression by predominant ACC histological subtype
The Kaplan-Meier plot of overall survival for the study cohort is shown in . Epithelial c- MET IHC positivity did not correlate significantly with survival (p = 0.94074) ().
Discussion
The prognostic value of c-MET overexpression seems to be tumor dependent and somehow controversial., While copy number gain and amplification has been shown to enhance tumor growth, invasiveness and promote metastasis in certain tumor types as esophageal, lung adenocarcinoma, and colon cancer,Citation6-8,9 in other tumor types such as breast, lung squamous cell carcinoma, and tonsil squamous cell carcinoma, its overexpression has either no prognostic relevance or is associated with improved patient's outcomes.Citation10-13
To ascertain whether aberrant c-Met expression accompanies human salivary ACC, we used immunohistochemical analysis to examine c-Met protein expression in 200 samples and concluded that 52% of tumors are strongly positive. Overexpression of c-Met was not associated with any clinicopathologic parameter evaluated, including histologic subtype, histologic grade, perineural invasion, tumor stage and presence of metastasis. Survival curves did not differ between the c-MET strong and c-MET weak or null sub-populations, consistent with no prognostic value of c-MET expression in salivary gland ACC.
Suzuki et alCitation4 reported strong c-MET expression in 67% of the 15 ACC samples evaluated. Although their findings were novel, their small number of cases limited the impact of the study and did not allow correlations with clinical outcomes.
Our study describes the largest screening of ACC samples for c-MET expression. The absence of correlation with survival implies that c-MET expression can't be used as a prognostic marker in salivary gland ACC. While a predictive biomarker for c-MET inhibitors benefit has not been fully established, c-MET protein expression is the strongest candidate and is being used to select patients for participation in clinical trials with c-MET inhibitors single agent or in combination.Citation7
Given the high percentage of salivary gland ACC with strong c-MET expression, and the absence of standard of care systemic therapy for this orphan disease, evaluation of c-MET overexpression as a predictive marker of benefit of c-MET inhibitors deserves further investigation in clinical trials.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg 1999; 125:149-52; PMID:10037280; http://dx.doi.org/10.1001/archotol.125.2.149
- Khan AJ, DiGiovanna MP, Ross DA, Sasaki CT, Carter D, Son YH, Haffty BG. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer 2001; 96:149-58; PMID:11410883; http://dx.doi.org/10.1002/ijc.1013
- Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res 2006; 12:3657-60; PMID:16778093; http://dx.doi.org/10.1158/1078-0432.CCR-06-0818
- Suzuki K, Cheng J, Watanabe Y. Hepatocyte growth factor and c-Met (HGF/c-Met) in adenoid cystic carcinoma of the human salivary gland. J Oral Pathol Med 2003; 32:84-9; PMID:12542830; http://dx.doi.org/10.1034/j.1600-0714.2003.00018.x
- Li J, Perlaky L, Rao P, Weber RS, El-Naggar AK. Development and characterization of salivary adenoid cystic carcinoma cell line. Oral Oncol 2014; 50:991-9; PMID:25086988; http://dx.doi.org/10.1016/j.oraloncology.2014.06.012
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12:89-103; PMID:22270953; http://dx.doi.org/10.1038/nrc3205
- Koeppen H, Rost S, Yauch RL. Developing biomarkers to predict benefit from HGF/MET pathway inhibitors. J Pathol 2014; 232:210-8; PMID:24105670; http://dx.doi.org/10.1002/path.4268
- Lacroix L, Post SF, Valent A, Melkane AE, Vielh P, Egile C, Castell C, Larois C, Micallef S, Saulnier P, et al. MET genetic abnormalities unreliable for patient selection for therapeutic intervention in oropharyngeal squamous cell carcinoma. PloS one 2014; 9:e84319; PMID:24465403; http://dx.doi.org/10.1371/journal.pone.0084319
- Gao H, Guan M, Sun Z, Bai C. High c-Met expression is a negative prognostic marker for colorectal cancer: a meta-analysis. Tumour Biol 2015; 36:515-20; PMID:25636446; http://dx.doi.org/10.1007/s13277-014-2659-5
- Baschnagel AM, Mangona VS, Robertson JM, Welsh RJ, Kestin LL, Grills IS. Lung metastases treated with image-guided stereotactic body radiation therapy. Clinical oncology 2013; 25:236-41; PMID:23352916; http://dx.doi.org/10.1016/j.clon.2012.12.005
- Koh YW, Lee HJ, Ahn JH, Lee JW, Gong G. MET expression is associated with disease-specific survival in breast cancer patients in the neoadjuvant setting. Pathol Res Pract 2014; 210:494-500; PMID:24814255; http://dx.doi.org/10.1016/j.prp.2014.04.002
- Kowalczuk O, Kozlowski M, Niklinska W, Kisluk J, Niklinska BJ, Niklinski J. Increased MET gene copy number but not mRNA level predicts postoperative recurrence in patients with non-small cell lung cancer. Translational oncology 2014; 7:605-12; PMID:25389455; http://dx.doi.org/10.1016/j.tranon.2014.08.002
- Kwon MJ, Kim DH, Park HR, Shin HS, Kwon JH, Lee DJ, Kim JH, Cho SJ, Nam ES. Frequent hepatocyte growth factor overexpression and low frequency of c-Met gene amplification in human papillomavirus-negative tonsillar squamous cell carcinoma and their prognostic significances. Hum Pathol 2014; 45:1327-38; PMID:24810547; http://dx.doi.org/10.1016/j.humpath.2014.03.003