Abstract
The accumulation of tumor infiltrating lymphocytes (TILs) in ovarian cancer is prognostic for increased survival while increases in immunosuppressive regulatory T-cells (Tregs) are associated with poor outcomes. Approaches that bolster tumor-reactive TILs may limit tumor progression. However, identifying tumor-reactive TILs in ovarian cancer has been challenging, though adoptive TIL therapy in patients has been encouraging. Other forms of TIL immunomodulation remain under investigation including Treg depletion, antibody-based checkpoint modification, activation and amplification using dendritic cells, antigen presenting cells or IL-2 cytokine culture, adjuvant cytokine injections, and gene-engineered T-cells. Many approaches to TIL manipulation inhibit ovarian cancer progression in preclinical or clinical studies as monotherapy. Here, we review the impact of TILs in ovarian cancer and attempts to mobilize TILs to halt tumor progression. We conclude that effective TIL therapy for ovarian cancer is at the brink of translation and optimal TIL activity may require combined methodologies to deliver clinically-relevant treatment.
Abbreviations
| TIL | = | tumor infiltrating lymphocyte |
| Tregs | = | regulatory T-cell |
| IDO | = | indoleamine 2,3-dioxygenase |
| CAR | = | chimeric antigen receptor |
Introduction
Tumor infiltrating lymphocytes (TILs) are present in ovarian cancer and are prognostic for increased survival. Given the impact of immunomodulatory regimens in melanoma, renal cell carcinoma, and lung cancer, and the recent elucidation of bona fide tumor-reactive TILs in ovarian cancer, the development of approaches that mobilize tumor-reactive TILs for successful eradication of ovarian cancer is now a high priority. In spite of strong rationale, attempts at administration of adoptive T cell transfer, immune enhancing antibody, and tumor immune environment conditioning in ovarian cancer have been minimal with some positive results reported in patients. In light of the recent development of new immunotherapeutic agents and treatment regimens that bolster host TIL activity, we review the current understanding of the immunobiology of human ovarian cancer including the impact of TIL subset accumulation on ovarian cancer survival and the effect of immune activation in the tumor microenvironment, and conclude that a new line of cancer immunotherapy investigations is warranted in ovarian carcinoma.
Ovarian cancer is the second most common and most lethal gynecologic malignancy in the United States with approximately 22,000 new cases and 14,000 deaths expected in 2013.Citation1 Ovarian cancer is often diagnosed at an advanced stage, with the large majority of new cases spread past the primary site. Since there are no current recommendations for ovarian cancer screenings, most efforts at combating ovarian cancer have been targeted at the discovery of new treatments rather than preventative measures. Currently, surgical staging and cytoreduction followed by chemotherapy is the mainstay of treatment, although in some cases neo-adjuvant chemotherapy is attempted.Citation2 The standard for surgical staging consists of total hysterectomy and bilateral salpingo-oophrectomy with pelvic and para-aortic lymph node dissection.Citation3 Aggressive cytoreductive surgery is the corner stone of therapy providing optimal response to postoperative chemotherapy, reduction in disease related symptoms, and improvement in quality of life, and possibly an improvement in host immune competence by removal of immunosuppressive cytokines.Citation4,5 Finally, adjuvant treatment of ovarian cancer usually consists of platinum-based combination of chemotherapy.Citation2 In spite of this optimal therapy regimen, 5 y survival in Stages III and IV, which represent the bulk of patients, is between 18–47%.Citation3 Despite these grim statistics, there are positive prognostic factors in ovarian cancer. In a large scale analysis of Stage III ovarian cancer patients treated with recommended therapy, age, serous tumor histology, performance status, and low volume of residual disease were independent predictors of positive prognosis.Citation6,7 Recently, it has been shown that the presence of tumor infiltrating lymphocytes (TILs) is also a positive predictor for overall survival, suggesting a probable functional role for host T cell immunity the control of ovarian cancer progression.
Background on Tumor Infiltrating Lymphocytes
By definition, TILs are white blood cells, for example, T-cells, B-cells, macrophages or natural killer cells, which have left the vasculature and have localized in tumor stroma or intraepithelium. TILs are generally segregated into those that penetrate the tumor islet (intraepithelial) and those that reside in the peritumoral space (stromal). The immune system, in particular the intraepithelial TIL, is thought to play an extensive role in the control of tumor growth in virtually all solid tumors,Citation8 and ovarian cancer is no exception (). While it is now well-established that CD8+ or CD4+T-lymphocytes can recognize cancer antigens or over-expressed self-antigens and inhibit the development of cancer, some cancer cells can thwart immune recognition and response.Citation9 If the endogenous immune system is capable of recognizing growing tumor in tissue, it increases inflammatory signals at the site of insult. In this setting, tumor-specific antigen presenting cells trigger the development of a T-cell response and cytokines such as interferon-gamma promote local inflammation, leading to complete destruction of the tumor, in a process termed elimination. Tumors that survive elimination enter an equilibrium period where immune pressure in the form of cytokine and lymphocyte attacks is in balance with tumor outgrowth. This occurs until a time point when immune pressure selects for tumor cells that are rapidly mutating and unstable, which then acquire resistance. Finally, the tumor can enter an escape phase, eluding immune recognition and killing, and become malignant, eventually metastasizing.Citation9
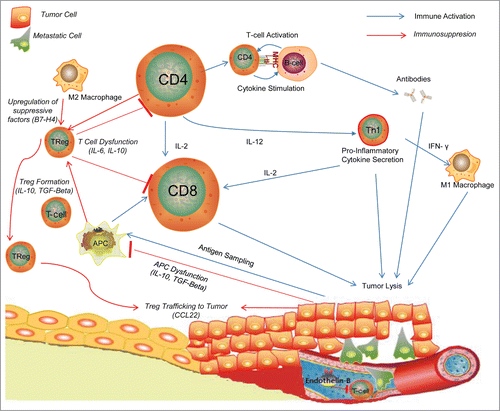
Figure 1. Inflammatory cells in Ovarian Tumor Milieu.Citation54 Tumor cells are sampled by dendritic cells and antigen presenting cells and presented to T-cells, which are then activated to cytotoxic T-cells to directly destroy tumor cells or Helper T-Cells which can propagate the immune response through cytokine secretion and stimulation of other pro-inflammatory cells. To combat this inflammatory response, tumor cells release inhibitory cytokines such as TGF-β and IL-10 to manipulate APCs into transforming T-cells into Treg cells which can downregulate the anti-tumor inflammatory response and CCL22 which recruits Tregs to tumors.Citation46 Endothelin-B and other molecules act on the tumor vasculature to block entry of inflammatory cells to the tumor milieu.Citation80
Tumor Infiltrating Lymphocytes and Survival
CD4+ and CD8+ TILs have long been known to exist in ovarian cancer.Citation10 The presence of TILs has been established as a positive prognostic factor in a number of solid cancers including, but not limited to, melanomaCitation11 and colon cancer.Citation12 The survival benefits of TILs in ovarian cancer has been documented since 1991,Citation13 but most major studies have been in recent years. Coukos and colleagues performed analysis on 186 samples of advanced stage ovarian cancer and found that 55% of patients with detectable intraepithelial CD3+ TILs had a 5 y survival of 38% compared with just 4.5% in patients with no TILs.Citation14 That 5-year survival benefit jumps to 73.9% when considering patients who had a complete clinical response after debulking and platinum based chemotherapy, compared to just 11.9% of patients without TILs.Citation14 Although few studies have elicited a survival benefit in patients with high CD3+ TILs,Citation15,16 a larger number of studies cite CD8+ TILs as being the cell responsible for the survival benefit in ovarian cancer. Most notably, a study in 2005 by Sato et al. showed that intraepithelial CD8+ TILs demonstrated improved survival while no association with CD3+ TILs was observed.Citation17 Similar results have been reported extensively elsewhere.Citation18-31 Three other studies indicate that both CD3 and CD8+ TILs are of good prognosis in ovarian cancer.Citation32-34 While CD8+ TILs appear to be best associated with improved survival, still other reports indicate that CD4+ TILs are the subset of value in prognosticating ovarian cancer.Citation25,35,36 CD4+ TILs can trigger recruitment of dendritic cells which then prime CD8+ TILs to deliver long term cytotoxicity to ovarian tumors in an animal model, perhaps explaining the survival benefit to CD4+ TILs in patients.Citation37 While the positive prognostic index of TILs is not universal, with a few studies showing a negative correlation or no correlation between TILs and survival,Citation38-40 it is generally accepted that presence of TILs is a positive factor in ovarian cancer prognosis.
A recent meta-analysis by Hwang et al. sought to evaluate the prognostic value of TILs in ovarian cancer and investigate other factors on prognosis including tumor histology.Citation41 Using either CD3 or CD8 as identifiable TIL markers, they found 10 suitable studies to include in their meta-analysis which included 1815 subjects.Citation41 They found significant association between intraepithelial TILs and survival, with a hazard ratio of 2.24 for patients without TILs.Citation41 In patients with CD3+ and CD8+ positive TILs, hazard ratios were in the same direction of association, but hazard ratios for the CD3 markers were less significant.Citation41 Therefore intraepithelial CD8+ TILs appear to be the standard for prognostic evaluation of TILs in ovarian cancer. Discrepancies between TIL presence and optimal surgical debulking were also identified, and TILs in patients in Japan and Europe had greater prognostic effects than in North America, leading to the postulation that immune-modifying factors such as genetic or environmental differences or health care access may have an impact on survival.Citation41 Taken together, these independent studies support the proposition that that TILs, particularly CD8+TILs, are a good prognostic factor in ovarian cancer.
Regulatory T-Cells and Survival Impact in Ovarian Cancer
CD8+ T lymphocytes are primarily ascribed the role of cytotoxic killer cell in the context of cancer immunobiology, however the ovarian cancer microenvironment possesses a vast network of immune inhibitory processes to thwart cytotoxic T cell attack, including the recruitment of various leukocytic subsets with potent immunosuppressive activity. Regulatory T-cells (Tregs) play a central role in the maintenance of self tolerance via regulation of immune responses against self-tissue antigens, however numerous studies indicate an increased Treg presence in tumors that evade immune destructionCitation42 and that Tregs limit antitumor immunity.Citation43 Tregs in ovarian cancer are commonly identified by their expression of extracellular CD4 and CD25 and intracellular forkhead box P3 (FOXP3). Regulatory T-Cells mediate their inhibitory activities through cytokines like TGF-β and IL-10 as well as through cell-cell interactions.Citation44 In ovarian cancer, the immunosuppressive activity of Tregs can be induced under hypoxic conditions within the tumor microenvironment. In an in-vitro ovarian cancer model, the hypoxic environment of ovarian tumors tolerizes host immune responses, due in part to the recruitment of Tregs via induction of the expression of the chemokine CCL28.Citation45 Similarly, Curiel et al. described a study of 104 individuals with ovarian cancer showing that the recruitment of Treg cells to tumor is associated with high death hazard and reduced survival, and mediated by the chemokine, CCL22, which attracts Tregs to tumor sites.Citation46 Tregs in the tumor environment and ascites correlate with poor patient outcomes,Citation47-49 are associated with tumors found to secrete TGF-β,Citation5 and correlate with advanced stage and grade.Citation31 Proper primary debulking in ovarian cancer was associated with a decrease in Tregs and an increase in TILsCitation50 and while suboptimal debulking causes the opposite effect.Citation31 In the aforementioned study by Sato et al., TIL subgroups with higher CD8/CD4 ratios showed better prognosis in terms of survival, suggesting an inhibitory role for Tregs.Citation17 Further, Fialova et al. showed a transition from a strong Th17 immune response in early cancer stages to a dominant population of Tregs by late stages in ovarian cancer patient samples, suggesting tumor progression sculpts Treg involvement in the local immune environment.Citation51 Even after treatment with neo-adjuvant chemotherapy, lower FOXP3+Treg infiltration is correlated with increased survival.Citation52 In contrast to above studies, a recent evaluation of tumor specimens from 73 ovarian cancer patients found that Treg frequency was a positive prognostic factor and no association could be made with other TILs.Citation53 Other studies have correlated Treg cells with increased survival benefit.Citation33,35 What accounts for these differences between studies is not known. However the prevailing view is that Tregs in the tumor microenvironment hamper the ability of the immune system to destroy cancer cells. Accordingly, approaches that selectively reduce Treg number, frequency or function should reveal tumor destructive immune responses and aid in eradicating ovarian cancer,Citation54 and may be cornerstone to future combination immunotherapy strategies.
Other Immune Cells in Ovarian Cancer
B cells and NK cells have been studied in ovarian cancer in terms of their impact on survival. The function of B cells in tumor development is still not clear. However, a study of 49 omental specimens from high grade ovarian cancer revealed increased CD19+ B cell infiltration was associated with a poorer survival.Citation55 Along those same lines, a study of 59 patients with metastatic ovarian carcinoma showed that a higher percentage of CD19+ cells and NK cells predicted poor survival.Citation40 Contrary to those reports, in a group of 199 ovarian cancer patients, CD20+ B-cells were correlated with positive survival.Citation33 Nielsen et al. also demonstrated that in a sample of 40 ovarian cancer patients, CD20+ B cells co-localized with activated CD8+ TILs, expressed antigen presentation markers, and correlated with increased patient survival compared to just the CD8+ TILs alone.Citation28 Although B cells may participate indirectly in tumor cell lysis, it is possible that B cells may facilitate the persistence of CD8+ TILs, produce cytokines to induce local lymphoid structures in the tumor, and produce factors that shift T-cells toward functional phenotypes.Citation56 Perhaps there is some unknown difference between the CD19 and CD20 positive B cells in tumor stroma, which may account for the differences seen in these studies. Further investigation into this distinction, as well as the impact that NK cells have on prognosis, should be performed as these cells no doubt have a capacity to mount antitumor responses.
Adoptive TIL Therapy in Patient Practice
Given the favorable prognostic value of TILs in ovarian cancer, various attempts have been made to reinforce this biomarker of improved survival. One approach, referred to as adoptive immunotherapy, relies upon the isolation of TILs from fresh tumor resections, selection of tumor-reactive subpopulation of TILs when possible, activation and expansion of TILs to large numbers ex-vivo and subsequent autologous administration of the expanded TIL product to the patient (). Adoptive TIL therapy has been at the forefront in new clinical trials in cancer, most promisingly in melanoma. Besser et al.Citation57 and Dudley et al.Citation58-60 evaluated a total of 81 patients with metastatic melanoma and demonstrated 50% objective clinical response to TIL therapy after lymphodepleting preconditioning. Infused TILs, predominantly CD8+, were capable of trafficking, infiltrating and destroying tumor cells, resulting in the majority of patients having regression of their metastatic cancerCitation58 and a generation of memory T-cells with tumor antigen specificity that persisted for 2 months or greater after transfer in patients responding to therapy.Citation61 With response rates ranging up to 72% in metastatic melanoma, adoptive TIL transfer therapy is among the best treatment options available for metastatic disease.Citation62 Although melanoma trials have shown the highest response to TIL therapy, there may be no difference in the susceptibility of ovarian cancer to TILs.Citation62
Figure 2. Schema of Adoptive T-Cell Therapy in ovarian cancer in past clinical trials. T-cells from surgical specimens are extracted, selected for their tumor attacking ability, grown up in lab cultures using IL-2 as a stimulatory cytokine, and then re-injected into the patient.
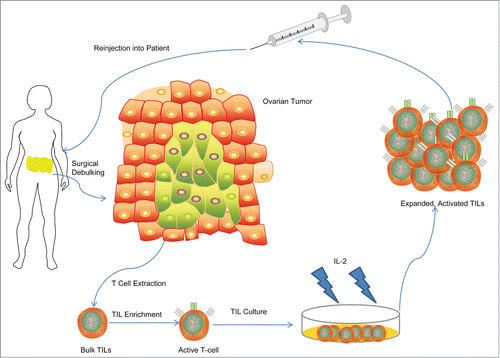
Various methodologies require evaluation for the generation of tumor-reactive TILs for use in ovarian cancer therapy. To date, methodologies have been restricted to TIL cultivation in high concentrations of IL-2 for clinical TIL production. For instance, Freedman et. al. were able to expand TILs on a large-scale basis using a simple 4-step method with high levels of IL-2 and an advanced artificial capillary culture system.Citation63 In melanoma, Dudley et. al. similarly generated TILs for adoptive transfer using high levels of IL-2 and selection of TIL cultures with highest activity followed by rapid expansion which mediated robust clinical response when administeredCitation64 suggesting that enrichment for the tumor-reactive TIL subset may provide clinical efficacy.
Using these lab methods for TIL enrichment and cultivation, early human trials using TIL therapy had mixed results. Aoki et al. from Japan published the first human adoptive TIL trial in advanced or recurrent epithelial ovarian cancer using IL-2 expanded TILs.Citation65 Encouraging results were seen as a response to the TILs were seen in 5/7 patients receiving TILs without chemotherapy and in 9/10 patients receiving TILs with chemotherapy.Citation65 A pilot study from Freedman et al. in 1994 on 11 ovarian cancer patients was performed with intraperitoneal injections of TILs with IL-266. Unfortunately, no significant clinical responses were seen in any of the patients. That same year, another TIL study from Japan from Ikarashi et al. compared 2 sets of patients with previous debulking surgery: 12 patients received TILs and cisplatin and 10 patients who did received chemotherapy alone.Citation67 After a median follow up of 26.5 months, 100% of the patients receiving TILs survived, however all but one of the chemotherapy patients was free of cancer after a similar amount of time.Citation67 In 1995, another study was performed with 13 patients who had tumor debulking and cisplatin treatment followed by TIL therapy.Citation68 These patients were compared to a group of 11 patients who either received tumor debulking and chemotherapy, no TILs, or TIL doses that didn't reach a level of 10ˆ9 cells/ml prior to injection.Citation68 All 13 patients receiving TILs had no evidence of disease after 33.6 months of observation, while the “control” group had a 3 y survival rate of only 67.5%.Citation68 Since T cell accumulation in ovarian cancer is prognostic for survival,Citation14 the use of patients lacking detectable TIL as a control group may have favored the experimental cohort. As a follow-up to their 1994 study, Freedman et al. performed another TIL trial, using IFN-gamma or IL-2 as a tumor aiding cytokine.Citation69 Unfortunately, only 2 of 22 enrolled patients received TIL therapy through intraperitoneal injections, due to a large amount of CD3-CD56+ adherent cells growing in culture precluding active TIL growth.Citation69 Finally, a study involving 7 patients with recurrent, epithelial ovarian cancer confined to peritoneum was performed in 2012 in which they received intraperitoneal injections of cytotoxic T-cells extracted from peripheral blood and not from tumor samples; no correlation between survival and injection of cytotoxic T-cells was seen.Citation70
No further studies have been published on adoptive TIL therapy in human ovarian cancer. Of note, the studies performed in Japan seem to have better outcomes than those performed by Freedman in the United States, echoing the results from the ovarian TIL meta-analysis.Citation41 Although the studies are limited based on sample size and some of the results were encouraging, an exaggerated response to TIL therapy was not seen. There are a number of factors that could have contributed to these mixed results. First, in many of the studies conducted by the Japanese group, only patients who had TILs that could be expanded were treated, representing a significant selection bias given the known association between TIL accumulation and survival.Citation14 Indeed, patients receiving TILs were more likely to survive longer, since having TILs is a positive prognostic factor, and these patients were compared to patients who had no or could not grow TILs, which does not seem to be a reasonable comparison. Further, since the completion of these trials, the base of knowledge surrounding effective TIL therapy has grown drastically, particularly the understanding that prolonged cell culture limits TIL persistence and activity in vivo,Citation61 and that selection of TILs based upon autologous tumor reactivity is important for effective therapy of ovarian cancer.Citation71 Early TIL trials in ovarian cancer seldom addressed either of these issues, with bulk TIL cultures being expanded ex vivo for long durations of time and comprised of NK cells. Secondly, patients in these trials have been in very late stages of ovarian cancer, perhaps too late to overcome the progressive, suppressive tumor microenvironment established in late stages.Citation31 Additionally, the application of host pre-conditioning in the form of lymphodepleting chemotherapy or irradiation provided prior to cell infusion has been shown to be an important factor for enhancing T cell persistence and anti-tumor activity in vivo in both preclinical studiesCitation72 and trials of TILsCitation60 via the reduction in host immunoregulatory elements such as Tregs and the increase in serum levels of endogenous cytokines involved in T cell homeostasis and proliferation, such as IL-7 and IL-15.
Given the potential that TIL therapy holds for ovarian cancer, an improvement in lab techniques in TIL enrichment and activation may improve clinical outcomes in TIL ovarian cancer trials., Identifying methodologies to enrich and expand tumor-reactive TILs, and eliminate non-reactive bystander cell subsets, in clinical ovarian TIL products for infusion may improve their potency. Our group has demonstrated that artificial antigen presenting cells genetically-modified to express co-stimulatory ligands for T-cells, such as CD137L, can help stimulate and expand tumor-reactive ovarian cancer TILs to levels greater than those seen in standard IL-2 culture conditions.Citation73 More importantly, we found that CD137 expression on ovarian cancer TILs identifies the subset of TILs with potent antitumor activity against autologous tumor.Citation71 This tumor-reactive TIL fraction expands in an HLA-dependent manner in presence of IL-7 and IL-15,Citation71 but not IL-2, suggesting that IL-7 and IL-15 may be more optimal cytokines than IL-2 for the activation, survival and expansion of tumor-reactive TILs ex-vivo in clinical cell production, as well as in the ovarian cancer microenvironment. Based on these findings we developed a rapid enrichment strategy that allows for efficient isolation of even rare tumor-reactive TILs from ovarian cancer,Citation71 which will be incorporated in future studies in this and other malignancies. The ex-vivo selection and expansion of these CD137+ TILs may enhance adoptive TIL therapy for ovarian cancer, as demonstrated in our human ovarian cancer allograft models.Citation71 Perhaps when these factors are combined in a future trial, clinical response rates more similar to those observed in melanoma TIL trials might be achieved.
Molecules Influencing Ovarian TIL Function
A number of immune markers and signaling molecules have been elucidated in recent years that may affect that viability of immune therapy relying on endogenous TIL function. The presence of HLA-DMB has been associated with increased numbers of CD8+ TILs and increased survival in a study of 184 ovarian cancer specimens.Citation20 HLA class I expression on ovarian cells is correlated with TIL infiltration and improved ability to expand TILs ex-vivo in a sample of 17 ovarian cancer patients.Citation74 It is notable however, that ovarian cancer cells appear prone to HLA downregulation and frequently have disabled antigen processing that may allow for immune escape and thus limit the utility of TIL therapy.Citation32 The molecule indoleamine 2,3-dioxygenase (IDO), an enzyme with immunosuppressive activity that catalyzes tryptophan, correlates negatively with the number of TILs and is associated with advanced stage and impaired survival.Citation75 Interestingly, IDO suppression has been shown to downregulate tumor growth and peritoneal dissemination in-vivo and promotes the accumulation of NK cells and their increased sensitivity to cancer cells in an in-vitro and in-vivo mouse model.Citation76 Similarly, an immunosuppressive effect of IDO has been reported on CD4+ Th1 cells, CD8+ T cells, and NK cells derived from peripheral blood, ascites, and tumors of ovarian cancer patients.Citation77 Trials are enrolling to test the use of IDO inhibitors in ovarian cancer patients at the time of diagnosis (NCT02042430) or have been proposed as a means to break IDO-induced tolerance, support cancer vaccination and promote anti-tumor immunity on patients with secondary disease (NCT01982487). High levels of COX expression, which has a role in the inflammatory pathway, correlates negatively with prognosis and presence of CD8+ TILs in an analysis of 70 ovarian cancer specimens.Citation30 Letal, a ligand that binds to NKG2D, an immunoreceptor responsible for activating lymphocytes is expressed by ovarian cancer, and has been shown to be a prognostic factor for improved survival in a sample of 43 patients with advanced ovarian cancer and promotes proliferation and survival of CD8+ TILs.Citation78 The presence of ULBP2, another NKG2D ligand, has been shown to correlate negatively with prognosis in ovarian cancer in a Japanese cohort of 82 patients, possibly due to shedding of this ligand in late stages of disease as a means of immune surveillance escape.Citation29 Beyond tumor intrinsic molecules, upregulation of endothelin-B receptor, a protein responsible for the maintenance of the tumor endothelial barrier, in tumors correlates with reduced TIL presence and shorter patient survival time;Citation79 knock-down of endothelin-B enhances targeting of TILs to tumor in mouse models and is being targeted in clinical trials.Citation80 Lastly, in a cohort of 35 patients with high grade ovarian cancer, auto-antibodies to the tumor antigen NY-ESO-1correlated with increased CD8+, CD4+ and FOXP3+ TIL cells.Citation81 In summary, these studies indicate potential targets to enhance adoptive TIL therapy or the possible means to identify candidate patients most sensitive to immunotherapy.
Cytokines involved in TIL suppression and tumor overgrowth have been investigated as potential therapeutic targets or co-therapy with TILs. Expression level of interferon-gamma, a Th1-type pro-inflammatory cytokine produced primarily by CD3+ TILs, in tumor correlates with improved clinical outcome in a cohort of 99 ovarian cancer patients.Citation82 Alternatively, Merogi et al. showed that the presence of Th2 cytokines, IL-10 and TGF-Beta, in tumor correlated with a significant reduction in TILs in 16 ovarian cancer samples using RT-PCR.Citation83 TGF-Beta has been shown to enhance the invasiveness of ovarian cancer cells and may be responsible for transforming active TILs into Tregs.Citation84 An analysis of T-cell lines derived from ovarian cancer showed low IL-2 and IFN-gamma production from TILs, suggesting late-stage T-cell anergy in ovarian cancer TILs.Citation85 In a sample of 13 ovarian tumor and ascites samples, IL-2 receptor α, or CD25, was decreased and IL-2 intracellular production was reduced in TILs, suggesting an acquired dysregulation of the IL-2 pathway in chronically stimulated TILs.Citation86 Poor IL-2 production by ovarian TILs has been reported elsewhereCitation87 and was thought to reflect terminal T-cell differentiation. Together, these cytokine studies implicate a pro-inflammatory Th1 cytokine profile being involved in tumor control, but also reveal the hazards of an immunosuppressed or Th2 environment in tumors which bring up the possibility of cytokine blockade or cytokine therapy to promote pro-inflammatory T-cell responses and enhance adoptive TIL transfer. Further, these studies suggest a functionally anergic or exhausted TIL phenotype in ovarian cancer that may be amenable to rescue or restoration via engagement of agonistic receptors or blockade of immune checkpoint molecules on the T-cell surface.
Immune Checkpoint Inhibitors and Agonists in Ovarian Cancer
In the ovarian cancer microenvironment, where T-cells are exposed to chronic antigen stimulation and various immunosuppressive networks, TILs can express various and multiple inhibitory receptors, many of which are triggered by activation with antigen and for whom cognate ligands are expressed on the ovarian cancer cell surface. B7-H4 is one immune-modulatory ligand that can arrest the cell cycle progression and proliferation of T-cells causing disruption of cytokine secretion and effector function. B7-H4 is thought to be expressed at low levels on normal tissue, but is overexpressed in a large proportion of ovarian cancers on the cancer cells themselves as well as on a subset of tumor associated macrophages.Citation47 The receptor for B7-H4 is expressed on the activated T-cell surface, however, its identity remains poorly defined. The presence of B7-H4-expressing tumor macrophages is correlated with increased Treg presence and poor patient outcome in 103 previously untreated ovarian cancer patientsCitation47 and when B7-H4 is specifically down-regulated, it can restore T-cell stimulation by macrophages and promote tumor regression in a mouse model.Citation88 Alternatively, we found that B7-H4 expressed by human ovarian cancer cells or antigen-pulsed presenting cells can also diminish immune responses by antigen specific T-cells in vitro and in vivo, and this axis of immunosuppression can be disrupted via antibody blockade of B7-H4.Citation89 In addition to its effect on T cells, B7-H4 affects tumor cells themselves. Cheng et al. transfected B7-H4 into an ovarian cancer cell line and found that B7-H4 promoted proliferation and conversion to metastases as well as an increased growth advantage once transplanted into mice.Citation90 In this line, B7-H4 expression increased with tumor staging in a study of 251 ovarian cancer patients, and was elevated in early stages in some cases when CA-125 was not.Citation91 Finally, CD277, a protein that shares many similarities to B7-H4, appears to be upregulated in a subset of ovarian cancer samples and its binding to T-cells inhibits the proliferation and production of Th1 cells,Citation92 yet its protein expression is reportedly associated with increased TIL accumulation, particularly CD4+ T-cells and CD206+ macrophages, and longer overall survival.Citation36
PD-1 is a molecule commonly expressed by TILs that may affect their ability to destroy tumor cells. Although not specific to T-cells, PD-1 is a negative regulator of T-cell activation with structural similarities to CD28.Citation93 PD-1 expression on T-cells blocks entry into the cell cycle and the production of cytokines.Citation94 PD-L1, a common ligand of PD-1, is seldom expressed on human tissues except in cancer, and its upregulation can cause apoptosis of human T-cellsCitation95 and help tumors evade immune destruction.Citation96 In a study of 70 ovarian cancer patient samples, higher expression of PD-L1 was correlated with poor prognosis and the presence of CD8+ TILs was inversely related to PD-L1 expression.Citation19 TILs isolated from ovarian cancer tumors showed increased expression of PD-1 and impaired IFN-gamma and TNF-α production, while PD-1 blockade improved proliferation and cytokine production by TILs, and could be potentiated further by the blockade of a second inhibitory receptor, LAG3, in a study of NY-ESO-1 specific CD8+ T-cells.Citation97 Hirano et al. demonstrated that PD-L1 expression causes TILs to be impaired in tumor cell destruction, and blocking PD-1 or its ligand could potentially reverse this resistance in a mouse model.Citation98 Interestingly, ovarian cancer-infiltrating dendritic cells can express high levels of both PD-1 and PD-L1 and their presence in tumor can both suppress T-cell activity and decrease TIL accumulation.Citation99 Engagement of PD-1 on these tumor-associated DCs inhibits their activation with dampened NF-kB activation, cytokine production and co-stimulatory molecule expression in vitro and blocking PD-1 in mice lowered tumor burden and increased TIL responses.Citation99 Similarly, Curiel et al. found PD-L1 expressed by myeloid derived DCs in ovarian cancer tissue and draining lymph nodes, and further showed that PD-L1 blockade promoted T cell activation and pro-inflammatory cytokine secretion in vitro and improved the DCs ability to instruct T cells to inhibit human cancer outgrowth in vivo.Citation100 In recent large scale human studies, blockade of either PD-1 or PD-L1 has shown clinical benefit in patients with malignancies such as melanoma, renal cell carcinoma and non-small cell lung cancer.Citation101 Ovarian cancer patients were treated in only one of these studies using an anti-PD-L1 antibody with only 1 out of 17 ovarian cancer patients having any response to the antibody treatment.Citation101 More recently, patients with advanced or relapsed, platinum–resistant ovarian cancer were administered anti-PD-1 antibody at 1 or 3 mg/kg doses in a Phase II efficacy study.Citation102 Therapy was well tolerated and partial responses were reported in 20% (2/10) and 33% (1/3) of patients in the 1 and 3 mg/kg dose cohorts, respectively. Still, these are the results of early clinical studies in small numbers of patient and the disruption of the PD-1/PD-L1 axis remains an intriguing target in ovarian cancer.
CTLA-4 is another immune regulatory molecule expressed by T-cells and is a negative regulator of CD28 dependent T-cell responses.Citation103 CTLA-4 acts as a checkpoint blockade, preserving self-tolerance and preventing autoimmunity, but may act as a barrier against immunotherapies.Citation104 Blockade of CTLA-4 offers a mechanism to enhance antitumor TIL responses by reducing activation of weakly reactive cells and removing attenuation of T-cell proliferation.Citation105 Clinical trials conducted with anti-CTLA-4 antibodies have been under clinical investigation for 10 years,Citation106 most notably with melanoma,Citation107 where anti-CTLA-4 antibodies can induce potential cancer remission.Citation106 Infusion of anti-CTLA-4 (Ipilimumab) in 2 ovarian cancer patients showed a stability or reduction in CA-125 levels, although no tumor biopsy was conducted to evaluate impact on TILs.Citation106 The same group treated an additional 9 stage IV ovarian cancer patients and reported that significant antitumor effects were seen in a few patients, one having marked regression of tumor and 3 achieving stable disease after 6 months.Citation108
Building on the promise on monotherapeutic blockade of CTLA-4 and PD-1, antibody therapy against combined targets may sustain the activation and proliferation of TILs, allowing the development of an effective tumor-specific immune response.Citation109 In a mouse melanoma model, combination PD-1 and CTLA-4 blockade in conjunction with whole cancer cell vaccination increases effector T-cell infiltration, resulting in highly advantageous effector T-cell to Treg ratios in the tumor, and tumor rejection.Citation110 Similar effects were recently shown in a mouse ovarian cancer model where dual blockade of CTLA-4 and PD-1 combined with vaccination demonstrated reversal of CD8 TIL dysfunction and led to tumor rejection.Citation111 Mechanistically, dual blockade of CTLA-4 and PD-1 can increase effector T-cell infiltration of melanoma B16, ovarian and colon cancer tumors while eliminating Tregs and myeloid suppressor cells.Citation111,112 In a recent trial involving 53 patients with melanoma, dual blockade of CTLA-4 and PD-1 demonstrated remarkable responses with evidence of clinical response and activity observed in 65% of patients, with a manageable safety profile.Citation113 Rapid and deep tumor regression was demonstrated in a substantial number of patients.Citation113 Together, these positive results rationalize further investigation of immune checkpoint blockade in ovarian cancer and special emphasis should be made on the development of trials that combine the PD-1 and CTLA-4 blockade strategy with whole ovarian cancer cell vaccine approachesCitation114,115 already under clinical investigation.
Future clinical studies are bound to take advantage of newly gained insights in the immmunobiology of human cancer to foster enhanced TIL modulation in vivo. Our finding that the subset of tumor-reactive TILs from patients with ovarian cancer express the co-stimulatory receptor CD137,Citation71 provides the rationale for engagement with agonistic anti-CD137 antibodies,Citation116 alone or in combination with antibodies against PD-1, CTLA-4 or a new array of immunoregulatory molecules. For instance, preclinical studies in a murine model of ovarian cancer indicate a strong potential for coupling antibody engagement of CD137 with simultaneous blockade of the negative immunoregulatory molecule, Tim-3, as an immune intervention with clinical promise.Citation117
Eliminating Immunosuppressor Cells within the Tumor Microenvironment to Potentiate TIL Activity
With a base understanding of factors that contribute to TIL dysfunction, approaches that favorably modify the tumor microenvironment have shed light on new directions in adoptive TIL therapy. For instance, the cornerstone of T-cell priming and activation relies upon the interaction of T-cells with professional antigen presenting cells, such as dendritic cells (DCs), however, during tumor progression, phenotypic and numerical changes can occur in tumor-infiltrating DCs, leading to the transition from an immunostimulatory to an immunosuppressive cell state that promotes T-cell dysfunction.Citation118 Fortunately, TIL dysfunction can be overcome.
Given the poor prognostic value of increased Treg frequencies in ovarian cancer,Citation17,46 the use of preparatory regimens that eliminate Tregs in the tumor environment seems an ideal approach to enhance subsequent adoptive TIL function and therapy. Since Tregs can act as sinks for cytokines that, when otherwise available, can bolster the net cytotoxic activity of TILs,Citation72 eliminating Tregs and other competing host cellular sink elements may improve TIL activity. Administration of low dose, cyclophosphamide leads to preferential apoptosis and decreased function of TregsCitation119 and enhances TIL proliferation, tumor infiltration and induction of positive cytokine expression in a melanoma mouse model.Citation120 Polcher et al. studied the effects of platinum/taxane-based neo-adjuvant chemotherapy on TILs and patient outcome in ovarian cancer and showed that post chemotherapy, low FOXP3+ Treg density in tumor was associated with longer progression free survival.Citation52 In addition, high Granzyme-B+/FOXP3+ratio post-chemotherapy strongly correlated with improved progression free survival compared to low Granzyme B+/Foxp3+ cell ratio,Citation52 rationalizing the further development of therapeutic approaches that foster high effector to Treg ratios. In animal and human studies, anti-CD25 antibodies have been used to deplete Tregs with selective partial depletion reported.Citation121,122 However, as reported by Kreijveld et al. in a study of kidney transplantation,Citation123 Tregs may be as prominent and functional, if not more, after administration of anti-CD25 antibody therapy. In our human clinical trials using targeted immunotoxins against CD25, transient partial reductions of CD25+ Tregs were observed in the blood and tumor of patients with melanoma without significant tumor regression, leading to the postulation that more comprehensive eradication would be necessary to eliminate Treg and achieve clinical benefit.Citation124,125 In an alternative approach to adjust Treg cell levels in vivo, patient leukapheresis samples were selectively depleted of Tregs from using a large-scale immunomagnetic system to tag and removed Tregs based upon their high CD25 expression.Citation126 In this study, transfer of autologous CD25+ Treg-depleted cells and IL-2 to lymphodepleted patients with melanoma was unable to mediate prolonged reductions in Treg frequency and number in vivo,Citation127 perhaps a result of co-administration of exogenous IL-2 cytokine, a known Treg growth factor. One alternative to Treg depletion being explored is to convert Tregs into activated effector cells. Leveque et al. showed that doses of IL-2 converted CD4+ regulatory cells into IL-17 secreting helper T-cells that potentially lost their regulatory activity in ovarian cancer tumor specimens.Citation128
Lymphodepletion prior to TIL based adoptive immunotherapy has greatly improved the success rate in metastatic melanoma treatmentCitation58-60 and extends in-vivo survival of transferred TILs.Citation129 Some authors suggest that profound lymphoablation with stem-cell rescue may further enhance immunotherapies in cancer, and have shown significant results in animal models, although they caution about adopting this method into clinical practice.Citation130 Interestingly, in the previously mentioned first adoptive TIL therapy trial in ovarian cancer, a dose of cyclophosphamide was given in anticipation of TIL injections, which may have actually, as suspected by the investigators at the time, reduced the immunosuppressive tumor immune environmentCitation65 and may explain why the Aoki trial was one of the most successful human ovarian cancer TIL trials. Thus, there are multiple strategies present to effectively reduce the presence of Tregs which should help enhance adoptive TIL therapy in ovarian cancer.
Additional Directions in Immunotherapy
There are a number of immunotherapies besides TIL therapy including vaccines, cytokine injections, targeted antibody based therapy, and engineered T-cells (). TIL therapy and targeted antibody based therapy have been covered in previous sections. Vaccine therapy in ovarian cancer is an intriguing potential treatment and has been thoroughly reviewed elsewhere.Citation131 Therapeutic vaccines for ovarian cancer have shown modest responses in the majority of clinical trials, including the largest phase 3 studies which have been negative.Citation131 Enhancing peripheral T-cells by stimulation with tumor antigen has yielded some clinical evidence of response in ovarian cancer.Citation132 To date, major limitations to these vaccine platforms include weak immunogenicity, as the concern is that the immunosuppressive environment in tumors is largely derailing any immunologic response vaccines might have. Furthermore, it has been recently shown that antitumor vaccination may activate and expand high affinity self-reactive Tregs that may limit immunotherapy.Citation133 Still, authors advocate improving the tumor immune microenvironment in order to use vaccines effectively in ovarian cancer.Citation131
Table 1. Comparison of most recent trials in immunotherapeutic strategies in ovarian cancer
Cytokine injections, particularly IL-2, have been evaluated as a potential future therapy in ovarian cancer. A number of small phase I trials in the 1980s showed that IL-2 injections in ovarian cancer had minimal, but real responses on CA-125 levels,Citation134 the lymphokine cascade,Citation135 stability of cancer progression,Citation136 and lysis of specific tumor targets.Citation137 In a more recent phase II clinical trial, a group of investigators performed intraperitoneal injections of IL-2 into chemotherapy resistant ovarian cancer patients and saw a 25% improvement in response, likely due to increases in T-cell activity.Citation138 We have shown that IL-7 and IL-15 can maximize tumor-reactive TIL expansion ex-vivo. Together, this supports the use of cytokine administration in support of adoptive TIL therapy.Citation71 However, an ovarian cancer trial that used cytokine injections in addition to TILs, showed minimal success.Citation69 We believe that cytokine injections with TILs are an understudied and underutilized method of treatment and, based on recent studies, are a reasonable method of immunotherapy in ovarian cancer.
In the absence of TILs, advances in gene transfer technology and T cell cultivation protocols now provide the opportunity for off-the-shelf targeted T cell therapies for patients with ovarian cancer. T-cells can be genetically modified to recognize tumor antigens by the introduction of genes encoding an exogenous T cell receptor (TCR) or an artificial TCR called a chimeric antigen receptor (CAR). For instance, we recently isolated and characterized an HLA-A2 restricted TCR that can be genetically engineered into human T cells to confer them with the ability to recognize a HER2 epitope that is naturally presented by ovarian cancer cells.Citation139 Similarly, an NY-ESO-1 specific TCR has been identified that has demonstrated safe and effective activity in NY-ESO-1+ synovial sarcoma and melanoma.Citation140 TCRs specific for these and other antigens are in the early stages of clinical testing. In contrast to a TCR, a CAR usually consists of an extracellular antibody targeted to a tumor associated antigen fused to the intracellular signaling domain of the T-cell receptor, allowing it to recognize tumors independent of antigen processing or MHC presentation. Multiple preclinical, but limited clinical, studies in ovarian cancer have been performed using CAR T-cell technology. Parker et al. transduced lymphocytes from patient's peripheral blood with a folate receptor-α specific CAR which enabled the redirection of these cells against ovarian cancer.Citation141 In a Phase I study, the first reported CAR T-cell trial in oncology, Kershaw and colleagues administered such CAR T-cells to 14 patients with ovarian cancer, but did not observe reduction in tumor burden which likely resulted from poor CAR T-cell transduction and persistence after infusion.Citation142 We recently redesigned CAR constructs to incorporate a CD137 co-stimulatory domain within the intracellular portion of the CAR, which has enabled folate receptor-α CAR T cells to persist after infusion and eradicate human cancer in a xenograft model.Citation143 Based upon these results, we are planning a future phase 1 trial using folate receptor-α CAR T-cells that have a CD137 co-stimulatory domain with a pre-conditioning lymphodepletion regimen,Citation144 hoping to achieve improved results over the past CAR trial.Citation142 A similar approach has been used to augment the activity of a mesothelin specific CAR,Citation145 which is the subject of an active clinical study in ovarian cancer. More recently, we have attempted to further optimize CAR activity by adding in new co-stimulatory domains and have found success using CD27,Citation146 which enhanced antigen-activated CAR T cell cytokine secretion and persistence. In addition to these CARs, others have been designed to target different ovarian cancer antigens including NKG2D Ligand, Lewis-Y, MUC1 and MUC16,Citation147 and trials are destined for future investigation. We have found that HER2 is ubiquitously expressed in human ovarian cancer, and at levels that CAR T cells can recognize and respond against, marking an opportunity for CAR targeting of HER2 in ovarian cancer.Citation148 CARs are limited in their ability to target only one antigen, making them more restricted than whole patient-derived TILs, however more recent data suggests that initial CAR T-cell activity may mobilize and activate endogenous CD4+ and CD8+ TILs to broaden the anti-tumor response in a mouse ovarian cancer model.Citation149 The cooperation of TILs with CAR T-cells, and the success of CAR T-cells in such preclinical models, shows promise for emerging immunotherapy trials.
Final Conclusions
Evidence of TIL accumulation in ovarian cancer is a positive prognostic indicator for survival. We propose that mobilization of endogenous TIL activity is likely to have curative potential in a subset of patients in ovarian cancer. We envision the use of a number of adjunctive treatments to reinforce endogenous TIL activity, or that can be combined with adoptive TIL therapy in a human ovarian cancer trial to maximize potency and benefit. We and others in the field advocate the rational design of preclinical studies and trials that combine multiple immune potentiating strategies with the potential to synergize individual benefits of each methodology. When human trials are conducted, safety will be of utmost concern as toxicity may ensue after broad activation of the immune system.
Finally, we further recommend evaluation of the markers, molecules and cytokines discussed in this review be expanded to future immunotherapy trials in ovarian cancer to determine their prognostic value. Although translational research remains to be completed, future elucidation of a number of prognostic markers and proteins may aid patients and physicians alike in the creation of a personalized route to ovarian cancer therapy. Perhaps in the near future, identification of these markers will guide physicians in terms of deciding the aggressiveness of anti-tumor therapy, identify specific patients for which certain immunotherapy may provide greater benefit, and ultimately give the patient a more accurate sense of the potential progressive nature of their disease than can be determine currently.
Ovarian cancer is an immunogenic tumor and the potential benefits of immunotherapy in its treatment are the source of active investigation. By comparison with melanoma, trials of immune therapy in ovarian cancer have not been as numerous to date, nor as successful. However, there is no doubt that the rationale to continue work in this area remains strong, and that the need to develop effective therapy for ovarian cancer is dire. We anticipate that like melanoma, immunotherapy will show clinical activity in subset of patients with ovarian cancer, but require a combination of immunotherapeutic treatments in order to achieve maximal benefit.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30; PMID:23335087
- Chen L, Berek JS. Overview of epithelial carcinoma of the ovary, fallopian tube, and peritoneum. In: Goff B, ed.: UpToDate. Waltham, MA., 2013.
- Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, Beller U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006; 95 Suppl 1:S161–92; PMID:17161157; http://dx.doi.org/10.1016/S0020-7292(06)60033-7
- Mann WJ, Chalas EJ, Valea FA. Epithelial ovarian cancer: Initial surgical management. In: Goff B, ed. In: UpToDate. Waltham, MA., 2013.
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 2001; 61:4766–72; PMID:11406550
- Landrum LM, Java J, Mathews CA, Lanneau GS, Jr., Copeland LJ, Armstrong DK, Walker JL. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecol Oncol 2013; 130:12–8; PMID:23578540; http://dx.doi.org/10.1016/j.ygyno.2013.04.001
- Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007; 25:3621–7; PMID:17704411; http://dx.doi.org/10.1200/JCO.2006.10.2517
- Eggermont A, Robert C, Soria JC, Zitvogel L. Harnessing the immune system to provide long-term survival in patients with melanoma and other solid tumors. Oncoimmunology 2014; 3:e27560.
- Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 2014; 27C:16–25; http://dx.doi.org/10.1016/j.coi.2014.01.004
- Dadmarz RD, Ordoubadi A, Mixon A, Thompson CO, Barracchini KC, Hijazi YM, Steller MA, Rosenberg SA, Schwartzentruber DJ. Tumor-infiltrating lymphocytes from human ovarian cancer patients recognize autologous tumor in an MHC class II-restricted fashion. Cancer J Sci Am 1996; 2:263–72; PMID:9166543
- Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL, Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer research 2012; 72:1070–80; PMID:22266112; http://dx.doi.org/10.1158/0008-5472.CAN-11-3218
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960–4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- Ma D, Gu MJ. Immune effect of tumor-infiltrating lymphocytes and its relation to the survival rate of patients with ovarian malignancies. J Tongji Med Univ 1991; 11:235–9; PMID:1668016
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203–13; PMID:12529460; http://dx.doi.org/10.1056/NEJMoa020177
- Raspollini MR, Castiglione F, Rossi Degl'innocenti D, Amunni G, Villanucci A, Garbini F, Baroni G, Taddei GL. Tumour-infiltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann Oncol 2005; 16:590–6; PMID:15699022; http://dx.doi.org/10.109/3annonc/mdi112
- Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol 2008; 108:415–20; PMID:18037158; http://dx.doi.org/10.1016/j.ygyno.2007.10.016
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102:18538–43; PMID:16344461; http://dx.doi.org/10.1073/pnas.0509182102
- Matsushita N, Ghazizadeh M, Konishi H, Araki T. Association of ovarian tumor epithelium coexpressing HLA-DR and CA-125 antigens with tumor infiltrating cytotoxic T lymphocytes. J Nippon Med Sch 2003; 70:40–4; PMID:12646975; http://dx.doi.org/10.1272/jnms.70.40
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007; 104:3360–5; PMID:17360651; http://dx.doi.org/10.1073/pnas.0611533104
- Callahan MJ, Nagymanyoki Z, Bonome T, Johnson ME, Litkouhi B, Sullivan EH, Hirsch MS, Matulonis UA, Liu J, Birrer MJ, et al. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin Cancer Res 2008; 14:7667–73; PMID:19047092; http://dx.doi.org/10.1158/1078-0432.CCR-08-0479
- Adams SF, Levine DA, Cadungog MG, Hammond R, Facciabene A, Olvera N, Rubin SC, Boyd J, Gimotty PA, Coukos G. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer 2009; 115:2891–902; PMID:19472394; http://dx.doi.org/10.1002/cncr.24317
- Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother 2009; 58:449–59; PMID:18791714; http://dx.doi.org/10.1007/s00262-008-0583-5
- Stumpf M, Hasenburg A, Riener MO, Jutting U, Wang C, Shen Y, Orlowska-Volk M, Fisch P, Wang Z, Gitsch G, et al. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytes. Br J Cancer 2009; 101:1513–21; PMID:19861998; http://dx.doi.org/10.1038/sj.bjc.6605274
- Gooden M, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, Nijman H, van Hall T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8(+) T lymphocytes. Proc Natl Acad Sci U S A 2011; 108:10656–61; PMID:21670276; http://dx.doi.org/10.1073/pnas.1100354108
- Hamanishi J, Mandai M, Abiko K, Matsumura N, Baba T, Yoshioka Y, Kosaka K, Konishi I. The comprehensive assessment of local immune status of ovarian cancer by the clustering of multiple immune factors. Clin Immunol 2011; 141:338–47; PMID:21955569; http://dx.doi.org/10.1016/j.clim.2011.08.013
- Vermeij R, de Bock GH, Leffers N, Ten Hoor KA, Schulze U, Hollema H, van der Burg SH, van der Zee AG, Daemen T, Nijman HW. Tumor-infiltrating cytotoxic T lymphocytes as independent prognostic factor in epithelial ovarian cancer with wilms tumor protein 1 overexpression. J Immunother 2011; 34:516–23; PMID:21654520; http://dx.doi.org/10.1097/CJI.0b013e31821e012f
- Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, Gilks CB, Clarke B, Kobel M, Nelson BH. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med 2012; 10:33; PMID:22369276; http://dx.doi.org/10.1186/1479-5876-10-33
- Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 2012; 18:3281–92; PMID:22553348; http://dx.doi.org/10.1158/1078-0432.CCR-12-0234
- Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi H, Yamaguchi K, Baba T, Fujii S, Konishi I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother 2009; 58:641–52; PMID:18791713; http://dx.doi.org/10.1007/s00262-008-0585-3
- Liu M, Matsumura N, Mandai M, Li K, Yagi H, Baba T, Suzuki A, Hamanishi J, Fukuhara K, Konishi I. Classification using hierarchical clustering of tumor-infiltrating immune cells identifies poor prognostic ovarian cancers with high levels of COX expression. Mod Pathol 2009; 22:373–84; PMID:18997734; http://dx.doi.org/10.1038/modpathol.2008.187
- Barnett JC, Bean SM, Whitaker RS, Kondoh E, Baba T, Fujii S, Marks JR, Dressman HK, Murphy SK, Berchuck A. Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol 2010; 116:556–62; PMID:20006900; http://dx.doi.org/10.1016/j.ygyno.2009.11.020
- Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, et al. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res 2008; 14:3372–9; PMID:18519766; http://dx.doi.org/10.1158/1078-0432.CCR-07-4433
- Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PloS one 2009; 4:e6412; PMID:19641607; http://dx.doi.org/10.1371/journal.pone.0006412
- Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, Kalloger S, Han G, Ceballos K, Cadungog MG, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol 2009; 22:393–402; PMID:19060844; http://dx.doi.org/10.1038/modpathol.2008.191
- Tsiatas ML, Gyftaki R, Liacos C, Politi E, Rodolakis A, Dimopoulos MA, Bamias A. Study of T lymphocytes infiltrating peritoneal metastases in advanced ovarian cancer: associations with vascular endothelial growth factor levels and prognosis in patients receiving platinum-based chemotherapy. Int J Gynecol Cancer 2009; 19:1329–34; PMID:20009885; http://dx.doi.org/10.1111/IGC.0b013e3181b7a40e
- Le Page C, Marineau A, Bonza PK, Rahimi K, Cyr L, Labouba I, Madore J, Delvoye N, Mes-Masson AM, Provencher DM, et al. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PloS One 2012; 7:e38541; PMID:22685580; http://dx.doi.org/10.1371/journal.pone.0038541
- Nesbeth YC, Martinez DG, Toraya S, Scarlett UK, Cubillos-Ruiz JR, Rutkowski MR, Conejo-Garcia JR. CD4+ T cells elicit host immune responses to MHC class II-negative ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol 2010; 184:5654–62; http://dx.doi.org/10.4049/jimmunol.0903247
- Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol 2008; 109:215–9; PMID:18314181; http://dx.doi.org/10.1016/j.ygyno.2008.01.010
- Al-Attar A, Shehata M, Durrant L, Moseley P, Deen S, Chan S. T cell density and location can influence the prognosis of ovarian cancer. Pathol Oncol Res 2010; 16:361–70; PMID:20024633; http://dx.doi.org/10.1007/s12253-009-9230-5
- Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, Davidson B, Risberg B. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol 2006; 125:451–8; PMID:16613351; http://dx.doi.org/10.1309/15B66DQMFYYM78CJ
- Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol 2012; 124:192–8; PMID:22040834; http://dx.doi.org/10.1016/j.ygyno.2011.09.039
- Sehouli J, Loddenkemper C, Cornu T, Schwachula T, Hoffmuller U, Grutzkau A, Lohneis P, Dickhaus T, Grone J, Kruschewski M, et al. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics 2011; 6:236–46; PMID:20962591; http://dx.doi.org/10.4161/epi.6.2.13755
- Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 2012; 72:4351–60; PMID:22798340; http://dx.doi.org/10.1158/0008-5472.CAN-12-0579
- Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 2009; 69:3995–4000; PMID:19383912; http://dx.doi.org/10.1158/0008-5472.CAN-08-3804
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011; 475:226–30; PMID:21753853; http://dx.doi.org/10.1038/nature10169
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942–9; PMID:15322536; http://dx.doi.org/10.1038/nm1093
- Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res 2007; 67:8900–5; PMID:17875732; http://dx.doi.org/10.1158/0008-5472.CAN-07-1866
- Giuntoli RL, 2nd, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res 2009; 29:2875–84; PMID:19661290
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 2005; 11:8326–31; PMID:16322292; http://dx.doi.org/10.1158/1078-0432.CCR-05-1244
- Napoletano C, Bellati F, Landi R, Pauselli S, Marchetti C, Visconti V, Sale P, Liberati M, Rughetti A, Frati L, et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J Cell Mol Med 2010; 14:2748–59; PMID:19780872; http://dx.doi.org/10.1111/j.1582-4934.2009.00911.x
- Fialova A, Partlova S, Sojka L, Hromadkova H, Brtnicky T, Fucikova J, Kocian P, Rob L, Bartunkova J, Spisek R. Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int J Cancer 2013; 132:1070–9; PMID:22865582; http://dx.doi.org/10.1002/ijc.27759
- Polcher M, Braun M, Friedrichs N, Rudlowski C, Bercht E, Fimmers R, Sauerwald A, Keyver-Paik MD, Kubler K, Buttner R, et al. Foxp3(+) cell infiltration and granzyme B(+)/Foxp3(+) cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer Immunol Immunother 2010; 59:909–19; PMID:20087581; http://dx.doi.org/10.1007/s00262-010-0817-1
- Mhawech-Fauceglia P, Wang D, Ali L, Lele S, Huba MA, Liu S, Odunsi K. Intraepithelial T cells and tumor-associated macrophages in ovarian cancer patients. Cancer Immun 2013; 13:1; PMID:23390372
- Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol 2010; 117:366–72; PMID:20144842; http://dx.doi.org/10.1016/j.ygyno.2010.01.019
- Yang C, Lee H, Jove V, Deng J, Zhang W, Liu X, Forman S, Dellinger TH, Wakabayashi M, Yu H, et al. Prognostic significance of B-cells and pSTAT3 in patients with ovarian cancer. PloS One 2013; 8:e54029; PMID:23326565; http://dx.doi.org/10.1371/journal.pone.0054029
- Nielsen JS, Nelson BH. Tumor-infiltrating B cells and T cells: Working together to promote patient survival. Oncoimmunology 2012; 1:1623–5; PMID:23264915; http://dx.doi.org/10.4161/onci.21650
- Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 2010; 16:2646–55; PMID:20406835; http://dx.doi.org/10.1158/1078-0432.CCR-10-0041
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298:850–4; PMID:12242449; http://dx.doi.org/10.1126/science.1076514
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23:2346–57; PMID:15800326; http://dx.doi.org/10.1200/JCO.2005.00.240
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233–9; PMID:18809613; http://dx.doi.org/10.1200/JCO.2008.16.5449
- Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr., Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol 2004; 173:7125–30; http://dx.doi.org/10.4049/jimmunol.173.12.7125
- Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 2009; 21:233–40; PMID:19304471; http://dx.doi.org/10.1016/j.coi.2009.03.002
- Freedman RS, Tomasovic B, Templin S, Atkinson EN, Kudelka A, Edwards CL, Platsoucas CD. Large-scale expansion in interleukin-2 of tumor-infiltrating lymphocytes from patients with ovarian carcinoma for adoptive immunotherapy. J Immunol Methods 1994; 167:145–60; PMID:8308273; http://dx.doi.org/10.1016/0022-1759(94)90084-1
- Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003; 26:332–42; PMID:12843795; http://dx.doi.org/10.1097/00002371-200307000-00005
- Aoki Y, Takakuwa K, Kodama S, Tanaka K, Takahashi M, Tokunaga A, Takahashi T. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res 1991; 51:1934–9; PMID:2004379
- Freedman RS, Edwards CL, Kavanagh JJ, Kudelka AP, Katz RL, Carrasco CH, Atkinson EN, Scott W, Tomasovic B, Templin S, et al. Intraperitoneal adoptive immunotherapy of ovarian carcinoma with tumor-infiltrating lymphocytes and low-dose recombinant interleukin-2: a pilot trial. J Immunother Emphasis Tumor Immunol 1994; 16:198–210; PMID:7834119; http://dx.doi.org/10.1097/00002371-199410000-00004
- Ikarashi H, Fujita K, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K. Immunomodulation in patients with epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Cancer Res 1994; 54:190–6; PMID:8261438
- Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res 1995; 1:501–7; PMID:9816009
- Freedman RS, Kudelka AP, Kavanagh JJ, Verschraegen C, Edwards CL, Nash M, Levy L, Atkinson EN, Zhang HZ, Melichar B, et al. Clinical and biological effects of intraperitoneal injections of recombinant interferon-gamma and recombinant interleukin 2 with or without tumor-infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin Cancer Res 2000; 6:2268–78; PMID:10873077
- Wright SE, Rewers-Felkins KA, Quinlin IS, Phillips CA, Townsend M, Philip R, Dobrzanski MJ, Lockwood-Cooke PR, Robinson W. Cytotoxic T-lymphocyte immunotherapy for ovarian cancer: a pilot study. J Immunother 2012; 35:196–204; PMID:22306908; http://dx.doi.org/10.1097/CJI.0b013e318243f213
- Ye Q, Song D, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ, Jr. CD137 accurately identifies and enriches for naturally-occurring tumor-reactive T cells in tumor. Clin Cancer Res 2013:44-55.
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005; 202:907–12; PMID:16203864; http://dx.doi.org/10.1084/jem.20050732
- Ye Q, Loisiou M, Levine BL, Suhoski MM, Riley JL, June CH, Coukos G, Powell DJ, Jr. Engineered artificial antigen presenting cells facilitate direct and efficient expansion of tumor infiltrating lymphocytes. J Translat Med 2011; 9:131; PMID:21827675; http://dx.doi.org/10.1186/1479-5876-9-131
- Kooi S, Zhang HZ, Patenia R, Edwards CL, Platsoucas CD, Freedman RS. HLA class I expression on human ovarian carcinoma cells correlates with T-cell infiltration in vivo and T-cell expansion in vitro in low concentrations of recombinant interleukin-2. Cell Immunol 1996; 174:116–28; PMID:8954611; http://dx.doi.org/10.1006/cimm.1996.0301
- Ino K. Indoleamine 2,3-dioxygenase and immune tolerance in ovarian cancer. Curr Opin Obstet Gynecol 2011; 23:13–8; PMID:20930628; http://dx.doi.org/10.1097/GCO.0b013e3283409c79
- Wang D, Saga Y, Mizukami H, Sato N, Nonaka H, Fujiwara H, Takei Y, Machida S, Takikawa O, Ozawa K, et al. Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int J Oncol 2012; 40:929–34; PMID:22179492
- Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JD, Jr., Andrews C, Matsuzaki J, Valmori D, Ayyoub M, Frederick PJ, et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res 2009; 69:5498–504; PMID:19491279; http://dx.doi.org/10.1158/0008-5472.CAN-08-2106
- Conejo-Garcia JR, Benencia F, Courreges MC, Gimotty PA, Khang E, Buckanovich RJ, Frauwirth KA, Zhang L, Katsaros D, Thompson CB, et al. Ovarian carcinoma expresses the NKG2D ligand Letal and promotes the survival and expansion of CD28- antitumor T cells. Cancer Res 2004; 64:2175–82; PMID:15026360; http://dx.doi.org/10.1158/0008-5472.CAN-03-2194
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O'Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 2008; 14:28–36; PMID:18157142; http://dx.doi.org/10.1038/nm1699
- Kandalaft LE, Facciabene A, Buckanovich RJ, Coukos G. Endothelin B receptor, a new target in cancer immune therapy. Clin Cancer Res 2009; 15:4521–8; PMID:19567593; http://dx.doi.org/10.1158/1078-0432.CCR-08-0543
- Milne K, Barnes RO, Girardin A, Mawer MA, Nesslinger NJ, Ng A, Nielsen JS, Sahota R, Tran E, Webb JR, et al. Tumor-infiltrating T cells correlate with NY-ESO-1-specific autoantibodies in ovarian cancer. PloS One 2008; 3:e3409; PMID:18923710; http://dx.doi.org/10.1371/journal.pone.0003409
- Marth C, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Doppler W, Daxenbichler G. Interferon-gamma expression is an independent prognostic factor in ovarian cancer. Am J Obstet Gynecol 2004; 191:1598–605; PMID:15547530; http://dx.doi.org/10.1016/j.ajog.2004.05.007
- Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Human Pathol 1997; 28:321–31; PMID:9042797; http://dx.doi.org/10.1016/S0046-8177(97)90131-3
- Rodriguez GC, Haisley C, Hurteau J, Moser TL, Whitaker R, Bast RC, Jr., Stack MS. Regulation of invasion of epithelial ovarian cancer by transforming growth factor-β. Gynecol Oncol 2001; 80:245–53; PMID:11161867; http://dx.doi.org/10.1006/gyno.2000.6042
- Nash MA, Lenzi R, Edwards CL, Kavanagh JJ, Kudelka AP, Verschraegen CF, Platsoucas CD, Freedman RS. Differential expression of cytokine transcripts in human epithelial ovarian carcinoma by solid tumour specimens, peritoneal exudate cells containing tumour, tumour-infiltrating lymphocyte (TIL)-derived T cell lines and established tumour cell lines. Clin Exp Immunol 1998; 112:172–80; PMID:9649178; http://dx.doi.org/10.1046/j.1365-2249.1998.00576.x
- Santin AD, Hermonat PL, Ravaggi A, Bellone S, Roman JJ, Smith CV, Pecorelli S, Radominska-Pandya A, Cannon MJ, Parham GP. Phenotypic and functional analysis of tumor-infiltrating lymphocytes compared with tumor-associated lymphocytes from ascitic fluid and peripheral blood lymphocytes in patients with advanced ovarian cancer. Gynecol Obstet Invest 2001; 51:254–61; PMID:11408737; http://dx.doi.org/10.1159/000058060
- Ye Q, Loisiou M, Levine BL, Suhoski MM, Riley JL, June CH, Coukos G, Powell DJ, Jr. Engineered Artificial Antigen Presenting Cells Facilitate Direct and Efficient Expansion of Tumor Infiltrating Lymphocytes. J Transl Med 2011; 9:131; PMID:21827675; http://dx.doi.org/10.1186/1479-5876-9-131
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 2006; 203:871–81; PMID:16606666; http://dx.doi.org/10.1084/jem.20050930
- Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ, Jr., Scholler N. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res 2013; 73:4820–9; PMID:23722540; http://dx.doi.org/10.1158/0008-5472.CAN-12-3457
- Cheng L, Jiang J, Gao R, Wei S, Nan F, Li S, Kong B. B7-H4 expression promotes tumorigenesis in ovarian cancer. Int J Gynecol Cancer 2009; 19:1481–6; PMID:19955922; http://dx.doi.org/10.1111/IGC.0b013e3181ad0fa2
- Simon I, Katsaros D, Rigault de la Longrais I, Massobrio M, Scorilas A, Kim NW, Sarno MJ, Wolfert RL, Diamandis EP. B7-H4 is overexpressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol Oncol 2007; 106:334–41; PMID:17498784; http://dx.doi.org/10.1016/j.ygyno.2007.03.035
- Cubillos-Ruiz JR, Martinez D, Scarlett UK, Rutkowski MR, Nesbeth YC, Camposeco-Jacobs AL, Conejo-Garcia JR. CD277 is a negative co-stimulatory molecule universally expressed by ovarian cancer microenvironmental cells. Oncotarget 2010; 1:329–38; PMID:21113407
- Riley JL, June CH. The road to recovery: translating PD-1 biology into clinical benefit. Trends Immunol 2007; 28:48–50; PMID:17188572; http://dx.doi.org/10.1016/j.it.2006.12.001
- Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol 2003; 170:711–8; http://dx.doi.org/10.4049/jimmunol.170.2.711
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793–800; PMID:12091876; http://dx.doi.org/10.1038/nm0902-1039c
- Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009; 114:1545–52; PMID:19417208; http://dx.doi.org/10.1182/blood-2009-03-206672
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 2010; 107:7875–80; PMID:20385810; http://dx.doi.org/10.1073/pnas.1003345107
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005; 65:1089–96; PMID:15705911
- Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, Goode EL, Kalli KR, Knutson KL. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol 2011; 186:6905–13; http://dx.doi.org/10.4049/jimmunol.1100274
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9:562–7; PMID:12704383; http://dx.doi.org/10.1038/nm863
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455–65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Noriomi M, Abiko K, Baba T, Yamaguchi K, Ueda A, et al. Efficacy and safety of anti-PD-1 antibody (Nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. J Clin Oncol 2014; 3s;5s. Supplementary Abstract 5511.
- Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol 2006; 18:206–13; PMID:16464564; http://dx.doi.org/10.1016/j.coi.2006.01.011
- Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006; 90:297–339; PMID:16730267; http://dx.doi.org/10.1016/S0065-2776(06)90008-X
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002; 3:611–8; PMID:12087419; http://dx.doi.org/10.1038/ni0702-611
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 2003; 100:4712–7; PMID:12682289; http://dx.doi.org/10.1073/pnas.0830997100
- Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 2013; 13:5; PMID:23390376
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008; 105:3005–10; PMID:18287062; http://dx.doi.org/10.1073/pnas.0712237105
- Kandalaft LE, Powell DJ, Jr., Singh N, Coukos G. Immunotherapy for ovarian cancer: what's next? J Clin Oncol 2011; 29:925–33; PMID:21079136; http://dx.doi.org/10.1200/JCO.2009.27.2369
- Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PloS One 2011; 6:e19499; PMID:21559358; http://dx.doi.org/10.1371/journal.pone.0019499
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors. Cancer Res 2013; 73:3591–603; PMID:23633484; http://dx.doi.org/10.1158/0008-5472.CAN-12-4100
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010; 107:4275–80; PMID:20160101; http://dx.doi.org/10.1073/pnas.0915174107
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122–33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369
- Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, Montone K, Mantia-Smaldone GM, Smith L, Nisenbaum HL, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res 2013; 19:4801–15; PMID:23838316; http://dx.doi.org/10.1158/1078-0432.CCR-13-1185
- Kandalaft LE, Powell DJ, Jr., Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology 2013; 2:e22664; http://dx.doi.org/10.4161/onci.22664
- Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol 2010; 37:508–16; PMID:21074066; http://dx.doi.org/10.1053/j.seminoncol.2010.09.008
- Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, Zhang S. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Translat Med 2013; 11:215; PMID:24044888; http://dx.doi.org/10.1186/1479-5876-11-215
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med 2012; 209:495–506; PMID:22351930; http://dx.doi.org/10.1084/jem.20111413
- Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005; 105:2862–8; PMID:15591121; http://dx.doi.org/10.1182/blood-2004-06-2410
- Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res 2007; 13:644–53; PMID:17255288; http://dx.doi.org/10.1158/1078-0432.CCR-06-1209
- Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol 2005; 174:90–8; http://dx.doi.org/10.4049/jimmunol.174.1.90
- Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci 2009; 1174:99–106; PMID:19769742; http://dx.doi.org/10.1111/j.1749-6632.2009.04939.x
- Kreijveld E, Koenen HJ, Klasen IS, Hilbrands LB, Joosten I. Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients. Am J Transplant 2007; 7:249–55; PMID:17109733; http://dx.doi.org/10.1111/j.1600-6143.2006.01604.x
- Powell DJ, Jr., Attia P, Ghetie V, Schindler J, Vitetta ES, Rosenberg SA. Partial reduction of human FOXP3+ CD4 T cells in vivo after CD25-directed recombinant immunotoxin administration. J Immunother 2008; 31:189–98; PMID:18481388; http://dx.doi.org/10.1097/CJI.0b013e31815dc0e8
- Powell DJ, Jr., Felipe-Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C, White DE, Mavroukakis S, Kreitman RJ, Rosenberg SA, et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol 2007; 179:4919–28; http://dx.doi.org/10.4049/jimmunol.179.7.4919
- Powell DJ, Jr., Parker LL, Rosenberg SA. Large-scale depletion of CD25+ regulatory T cells from patient leukapheresis samples. J Immunother 2005; 28:403–11; PMID:16000960; http://dx.doi.org/10.1097/01.cji.0000170363.22585.5a
- Powell DJ, Jr., de Vries CR, Allen T, Ahmadzadeh M, Rosenberg SA. Inability to mediate prolonged reduction of regulatory T Cells after transfer of autologous CD25-depleted PBMC and interleukin-2 after lymphodepleting chemotherapy. J Immunother 2007; 30:438–47; PMID:17457218; http://dx.doi.org/10.1097/CJI.0b013e3180600ff9
- Leveque L, Deknuydt F, Bioley G, Old LJ, Matsuzaki J, Odunsi K, Ayyoub M, Valmori D. Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. J Immunother 2009; 32:101–8; PMID:19238008; http://dx.doi.org/10.1097/CJI.0b013e318195b59e
- Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PloS One 2009; 4:e4749; PMID:19270751; http://dx.doi.org/10.1371/journal.pone.0004749
- Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol 2006; 3:668–81; PMID:17139318; http://dx.doi.org/10.1038/ncponc0666
- Liao JB, Disis ML. Therapeutic vaccines for ovarian cancer. Gynecol Oncol 2013; 130(3):667–73.
- Canevari S, Stoter G, Arienti F, Bolis G, Colnaghi MI, Di Re EM, Eggermont AM, Goey SH, Gratama JW, Lamers CH, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Institute 1995; 87:1463–9; PMID:7674333; http://dx.doi.org/10.1093/jnci/87.19.1463
- Cannon MJ, O'Brien TJ. Cellular immunotherapy for ovarian cancer. Expert Opin Biol Ther 2009; 9:677–88; PMID:19456205; http://dx.doi.org/10.1517/14712590902932897
- Beller U, Chachoua A, Speyer JL, Sorich J, Dugan M, Liebes L, Hayes R, Beckman EM. Phase IB study of low-dose intraperitoneal recombinant interleukin-2 in patients with refractory advanced ovarian cancer: rationale and preliminary report. Gynecol Oncol 1989; 34:407–12; PMID:2788602; http://dx.doi.org/10.1016/0090-8258(89)90182-0
- Bertoglio S, Melioli G, Baldini E, Catturich A, Sertoli MR, Civalleri D, Percivale P, Meier W, Galazka A, Badellino F. Intraperitoneal infusion of recombinant interleukin-2 in malignant ascites in patients with gastrointestinal and ovarian cancer. Acta Med Austriaca 1989; 16:81–3; PMID:2609918
- Chapman PB, Kolitz JE, Hakes TB, Gabrilove JL, Welte K, Merluzzi VJ, Engert A, Bradley EC, Konrad M, Mertelsmann R. A phase I trial of intraperitoneal recombinant interleukin 2 in patients with ovarian carcinoma. Invest New Drugs 1988; 6:179–88; PMID:3263958; http://dx.doi.org/10.1007/BF00175395
- Lotze MT, Custer MC, Rosenberg SA. Intraperitoneal administration of interleukin-2 in patients with cancer. Arch Surg 1986; 121:1373–9; PMID:3491595; http://dx.doi.org/10.1001/archsurg.1986.01400120019002
- Vlad AM, Budiu RA, Lenzner DE, Wang Y, Thaller JA, Colonello K, Crowley-Nowick PA, Kelley JL, Price FV, Edwards RP. A phase II trial of intraperitoneal interleukin-2 in patients with platinum-resistant or platinum-refractory ovarian cancer. Cancer Immunol Immunother 2010; 59:293–301; PMID:19690855; http://dx.doi.org/10.1007/s00262-009-0750-3
- Lanitis E, Smith JB, Dangaj D, Flingai S, Poussin M, Xu S, Czerniecki BJ, Li YF, Robbins PF, Powell DJ, Jr. A human ErbB2-specific T-cell receptor confers potent antitumor effector functions in genetically engineered primary cytotoxic lymphocytes. Hum Gene Ther 2014; 25:730–9; PMID:25003657; http://dx.doi.org/10.1089/hum.2014.006
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29:917–24; PMID:21282551; http://dx.doi.org/10.1200/JCO.2010.32.2537
- Parker LL, Do MT, Westwood JA, Wunderlich JR, Dudley ME, Rosenberg SA, Hwu P. Expansion and characterization of T cells transduced with a chimeric receptor against ovarian cancer. Hum Gene Ther 2000; 11:2377–87; PMID:11096442; http://dx.doi.org/10.1089/104303400750038480
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006; 12:6106–15; PMID:17062687; http://dx.doi.org/10.1158/1078-0432.CCR-06-1183
- Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, Figini M, June CH, Coukos G, Powell DJ, Jr. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res 2011; 71:4617–27; PMID:21546571; http://dx.doi.org/10.1158/0008-5472.CAN-11-0422
- Kandalaft LE, Powell DJ, Jr., Coukos G. A phase I clinical trial of adoptive transfer of folate receptor-α redirected autologous T cells for recurrent ovarian cancer. J Translat Med 2012; 10:157; PMID:22863016; http://dx.doi.org/10.1186/1479-5876-10-157
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009; 106:3360–5; PMID:19211796; http://dx.doi.org/10.1073/pnas.0813101106
- Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ, Jr. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 2012; 119:696–706; PMID:22117050; http://dx.doi.org/10.1182/blood-2011-03-344275
- Chekmasova AA, Brentjens RJ. Adoptive T cell immunotherapy strategies for the treatment of patients with ovarian cancer. Discov Med 2010; 9:62–70; PMID:20102688
- Lanitis E, Dangaj D, Hagemann IS, Song DG, Best A, Sandaltzopoulos R, Coukos G, Powell DJ, Jr. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PloS One 2012; 7:e49829; PMID:23189165; http://dx.doi.org/10.1371/journal.pone.0049829
- Spear P, Barber A, Sentman CL. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology 2013; 2:e23564; PMID:23734311; http://dx.doi.org/10.4161/onci.23564
