Abstract
Withaferin A (WA), a withanolide from the plant, Ashwagandha (Withania somnifera) used in Ayurvedic medicine, has been found to be valuable in the treatment of several medical ailments. WA has been found to have anticancer activity against various solid tumors, but its effects on hematological malignancies have not been studied in detail. WA strongly inhibited the survival of several human and murine B cell lymphoma cell lines. Additionally, in vivo studies with syngeneic-graft lymphoma cells suggest that WA inhibits the growth of tumor but does not affect other proliferative tissues. We demonstrate that WA inhibits the efficiency of NF-κB nuclear translocation in diffuse large B cell lymphomas and found that WA treatment resulted in a significant decrease in protein levels involved in B cell receptor signaling and cell cycle regulation. WA inhibited the activity of heat shock protein (Hsp) 90 as reflected by a sharp increase in Hsp70 expression levels. Hence, we propose that the anti-cancer effects of WA in lymphomas are likely due to its ability to inhibit Hsp90 function and subsequent reduction of critical kinases and cell cycle regulators that are clients of Hsp90.
Abbreviations
| WA | = | Withaferin A |
| DLBCL | = | Diffuse large B cell lymphoma |
Introduction
Non-Hodgkin Lymphoma (NHL) is the seventh most common cancer in the United States and represents 4.3% of new cancer cases.Citation1 The majority of NHLs are derived from B cells and the most common and aggressive forms are diffuse large B cell lymphomas (DLBCL) contributing to 30% of newly diagnosed cases.Citation2 B cell lymphomas can be classified based on cellular origin pertaining to pre- or post-germinal center, mantle, follicular, or marginal zone B cells. DLBCLs can be classified into germinal center B cell (GCB) like and activated B cell (ABC) like which carries a worse prognosis and lower patient survival.Citation3,4 Activated B cell like DLBCLs have been found to have high NF-κB activation, known to promote transcription of cell survival and proliferation genes.Citation5 Tonic BCR signaling can lead to constitutive NF-κB activation by signaling through key kinases like SFKs, Syk, Akt and Btk.Citation6,7 We and others have shown that similar to normal B cell survival, B cell lymphomas require B cell receptor (BCR) signaling for growth and survival.Citation2,8,9 The increased expression and activity of these kinases and NF-κB are known to promote B cell lymphoma survival and are significant targets in cancer therapies.Citation10
Current therapies for DLBCL involve a range of chemotherapeutics and immunotherapies (R-CHOP) with potential beneficial outcomes, yet result in many adverse side effects, warranting the need for novel therapies for the treatment of this disease.Citation11 Interest has sparked in examining the use of natural products for cancer prevention and treatment.Citation12 Withaferin A (WA) is a steroidal lactone isolated from the Ayruvedic medicinal plant, Ashwagandha (Withania Somnifera), that has been studied for its biological activities which include anti-inflammatory, anti-angiogenic, and anti-tumor effects.Citation13 WA, the active compound found in the plant extract, shows promise to be a key anti-cancer therapeutic.Citation14 WA has been reported to inhibit proliferation and induce cell death in a variety of tumor models such as pancreatic,Citation15 breast,Citation16 lung,Citation17 cervical,Citation18 and prostate,Citation19 but its effects on DLBCL are not yet known.
Reports have suggested multiple mechanisms are involved in the anticancer activity of WA indicating the promiscuous nature of its effects on cancer cell survival.Citation14 Several studies found that WA stimulates apoptosis through restoring p53 levels and thus activating Bax and other pro-apoptotic Bcl-2 family proteins to induce apoptosis of cervical cancer cells,Citation18 or by enhancing levels of the proapoptotic factor, Par-4, and downstream caspase activation in prostate cancer cells.Citation19 In most cancer cell lines WA inhibits the proliferation of tumor cells through a G2/M phase cell cycle arrest,Citation20 and inhibits the activation of NF-κB via interaction with the IKKγ subunit preventing IκB phosphorylation.Citation21,22 In pancreatic cancer cells, Yu et. al. demonstrated that WA treatment leads to degradation of a variety of client proteins of the Hsp90 chaperone, suggesting that WA works by inhibiting heat shock proteins.Citation15 This mechanism of action has not yet been described in any other cancer cells. The Hsp90/Cdc37 chaperone complex has been described to have functional roles in promoting the maturation of client proteins such as Src, Akt, IKK, Cdc2, and CDK4.Citation23 Several of these client proteins are dysregulated in many cancers including DLBCL and are current or potential therapeutic targets.
In this study we show that WA has anti-proliferative activity on multiple types of B cell lymphomas including those with the most aggressive phenotype, in both in vitro and in vivo models. Our mechanistic studies suggest that Hsp90 is an important target in the anti-lymphoma activity of WA.
Results
WA inhibits proliferation of B cell lymphoma cells
Treatment with WA induced a dose dependent inhibition of the growth of a variety of human and mouse B lymphoma cell lines when measured by the MTT assay (). WA was effective against the human DLBCL cell lines LY-3, LY-10, SudHL-6, a Burkitt's lymphoma, Raji, and a mantle cell lymphoma, MINO with an EC50 in the range of 1.92–3.6 μM (). The Burkitt's lymphoma, Ramos was the most sensitive with an EC50 of 0.45 µM whereas the mantle cell lymphoma, JEKO was most resistant. We are currently investigating the basis of apparent resistance of JEKO cells to WA mediated growth inhibition. Growth of the murine immature B-cell lymphoma, BKS-2 and the germinal center lymphoma, A20-luc/YFP was also strongly inhibited by WA.
Figure 1. Effect of WA on the survival of human diffuse large B cell lymphoma, Burkitt's lymphoma, mantle Cell lymphoma, and murine DLBCL cell lines. B cell lymphoma cells were treated with different concentrations of Withaferin A for 48hr and then proliferation and viability was measured by MTT assay. Data points indicate percentage of viable cells of triplicate cultures and an average of at least 2 experiments. The curves are plotted on a log scale with 0.01 µM drug concentration representing no drug added. Bars indicate standard error of the mean.
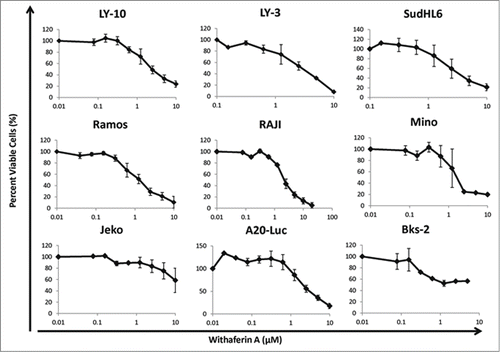
Table 1. Effective Concentrations of Withaferin A on various NHL cell lines
WA induces a cell cycle arrest
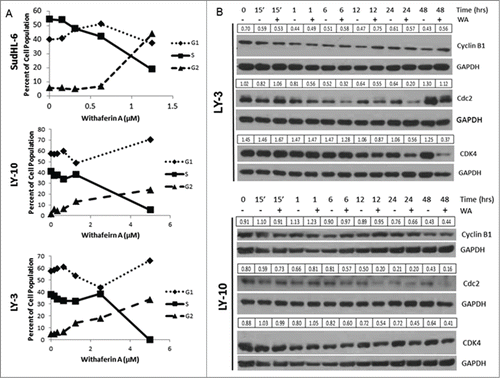
Cell cycle analysis using SudHL-6 cells showed that increasing doses of WA progressively reduced cells in G1 and S phase but increased cells in G2/M phase indicating a cell cycle arrest at the G2/M checkpoint (). The EC50 based on the cell cycle analysis was 1.25µM which is in the same range as that calculated by the MTT assay. A similar decrease in S phase cells and an increase in G2/M was also demonstrated with LY-3 and LY-10 cells (). There was a slight increase in G1 phase cells which could be due to an incomplete G2/M arrest in these cells. Because we saw a halt in the cell cycle, we also examined the expression of cell cycle regulators after drug treatment. shows that there is a decrease in expression of CDK4, which is a kinase required for G1-S progression. Similarly cdc2, a kinase required for G2/M progression, was also reduced in WA treated cells (). Interestingly, cyclin B which is required for cdc2 activation, () as well as cyclin A and Cdk2 (Fig. S1) were not affected by WA treatment of LY-10 and LY-3 cells. These data collectively suggest that WA has a negative effect on cell cycle progression, preventing B cell lymphoma proliferation.
Figure 2. WA induced a G2/M cell cycle arrest in B cell lymphoma accompanied by a decrease in expression of cell cycle regulators. (A) Cultures of human SudHL-6, LY-10, and LY-3 cells (of 0.75 × 106 cells/ml) were treated with different concentrations of WA for 48hr. The cells were then collected and stained for cell cycle analysis. (B) LY-10 and LY-3 cells were treated with 2.5 μM WA and collected at multiple time points. Cells were harvested and total protein was isolated. Immunoblots were probed for cell cycle regulators including Cyclin B1, cdc2, and CDK4. Blots were then stripped and probed for GAPDH. Expression values for each band were normalized to the corresponding GAPDH band.
WA induces apoptosis in B cell lymphoma lines
To determine if WA induced growth inhibition of lymphoma cells is due to apoptosis, Annexin V expression was measured in LY-3 and LY-10 cell lines treated with increasing doses of WA for 24hrs. shows a dose dependent response of increasing Annexin V positive cells with increasing concentrations of drug. The EC50 values calculated with Annexin V data for LY-10 and LY-3 are 2.5 and 1.25 µM respectively, which is in agreement with the MTT data in . Similar results were obtained with Ramos and SudHL-6 cell lines, once again Ramos showing increased sensitivity.
Figure 3. Withaferin A treatment results in apoptosis of diffuse Large B cell lymphoma lines. (A) LY-10 and LY-3 cells were treated with 2.5 µM WA for 48hrs. Early apoptotic cells were detected by flow cytometry with Annexin-V staining. (B) Bcl-2 and Mcl1 protein expression in LY-10 and LY-3 lymphoma cells were determined by western blot analysis. (C) Cleaved Caspase 3 and total Caspase 3 levels were measured in WA treated LY-3 lymphoma cells. Band intensity values are normalized to the control GAPDH.
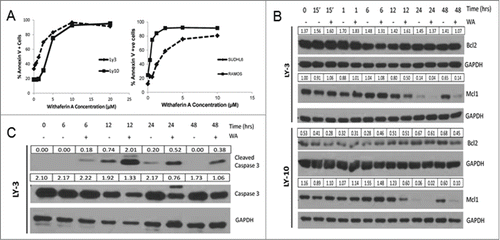
WA has been suggested to induce apoptosis in a variety of ways in different tumor models.Citation24,25 Srinivasan et. al studied the effects of WA in prostate cancer cells and reported that WA induces apoptosis by enhancing the pro-apoptotic protein, Prostate apoptosis response-4 (Par-4).Citation19 We have confirmed that DLBCL cells constitutively express functional Par-4 (data not shown) and hypothesized that WA may induce apoptosis of B cell lymphoma through a Par-4 dependent pathway. However, we found that total levels of Par-4 protein decreased in WA treated LY-3 and LY-10 cells. This was contradictory to previous studies.Citation19 Hence we considered the possibility that B cell lymphoma cells may secrete more Par-4 after WA treatment, which can then induce apoptosis extracellularly.Citation26 We quantified secreted and intracellular Par-4 (as described in Methods) in SudHL-6 and LY-10 lymphoma cells treated with WA. WA treatment decreased the levels of secreted as well as intracellular Par-4 in both cell lines (). These results suggested that B cell lymphomas do not rely on Par-4 mediated apoptosis after WA treatment and led us to investigate other mechanisms of apoptosis. We measured the levels of anti-apoptotic proteins Bcl-2 and Mcl1, which are involved in blocking the intrinsic apoptosis pathway. Immunoblot analysis in showed that there was a slight decrease in expression of Bcl-2 protein but a greater decrease in Mcl1 protein, which is likely to prevent the stabilization of the mitochondrial membrane leading to caspase activation and apoptosis. Indeed, drug treatment led to increased cleavage of Caspase 3 in LY-3 cells () and reduced levels of intact caspase-3.
WA inhibits NF-κB nuclear translocation
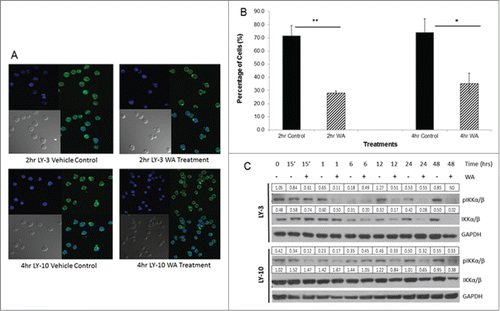
The results indicating that WA reduces B cell lymphoma survival and proliferation in conjunction with previous findings in other tumor models led us to examine the effect of drug treatment on NF-κB activity. shows that in LY-3 and LY-10 lymphoma cells, the p65 subunit of NF-κB is found within the cytoplasm and the nucleus indicating that NF-κB is constitutively activated in untreated cells as noted by previous studies.Citation5 The right panels of show that in WA treated LY-3 and LY-10 cells, there is less positive staining for p65 within the nucleus compared to the control, untreated cells, suggesting that WA inhibits the efficiency of nuclear translocation of the p65 and hence NF-κB activation. Quantitative analysis of these data presented in shows that there was a significant difference in nuclear staining between the control, untreated cells and WA treatment after 2 and 4 hours (). We confirmed the decrease in efficiency of NF-κB nuclear translocation by showing a decrease in total levels of IKKα/β (kinases required for IκB degradation and nuclear translocation of p65) in LY-10 and LY-3 cell lysates ().
Figure 4. Withaferin A inhibits NF-κB nuclear translocation. (A) Cultures of 0.75 ×106 cells/ml human LY-10 and LY-3 lymphoma cells were treated with 2.5 µM WA for 2 and 4 hours. Cells were then removed from drug and fixed to be stained for the p-65 subunit of NF-kB. Images were taken through fluorescent microscopy to observe the cytoplasmic and nuclear location of NF-kB. (B) Sixteen fields were counted and data represents mean ± standard deviation of triplicate samples. Statistical significance of difference between groups was determined by ANOVA. * P<0.02 and ** P < 0.001. (C) LY-10 and LY-3 lymphoma cells were treated with 2.5 μM WA and collected at multiple time points. Cells were harvested and total protein was isolated. Western blots of lysates were probed for pIKKα/β and total IKK α/β. Blots were then stripped and probed for GAPDH. Expression values for IKK α/β band were normalized to the corresponding GAPDH band and the pIKKα/β values were normalized to the total IKKα/β. ND = not determined due to total IKKα/β band being undetectable.
WA reduces expression of pro-survival signals in B cell lymphomas
Observation of increased pro-apoptotic signals in B cell lymphomas led us to examine the effect of WA on BCR signaling pathway that involves activation of both tyrosine and serine/threonine protein kinases like Akt, Lyn, Syk, and Btk. Levels of the activated form of Akt (pS473) were decreased after 12, 24, and 48 hrs of WA treatment along with total levels of Akt at 24 and 48hrs (). This led us to conclude that loss of the reduction in the activated form of Akt may be dependent on the total loss of the protein with WA treatment.
Figure 5. Effect of WA treatment on the pro-survival factors of B cell lymphomas; all client proteins of Hsp90. (A) LY-10 and LY-3 lymphoma cells were treated with 2.5 µM WA for varying time points or the appropriate volume of DMSO vehicle. Cells were harvested and total protein was isolated. Western blots of lysates were first probed for pAkt (Ser473), stripped and then for total Akt. (B) LY-10 and LY-3 cells treated with 2.5 µM WA were first probed for phosphorylated-SFK with pSrc (Y416) antibody and then probed for total Lyn, an SFK. (C) LY-10 and LY-3 cell lysates were probed for total protein levels of Syk and Btk. (D) LY-10 and LY-3 cells treated with 2.5 µM WA were probed for total expression of Hsp90β and Hsp70. Densitometry values for total protein were normalized to corresponding GAPDH band, whereas the phosphoprotein values were normalized to the corresponding total protein.
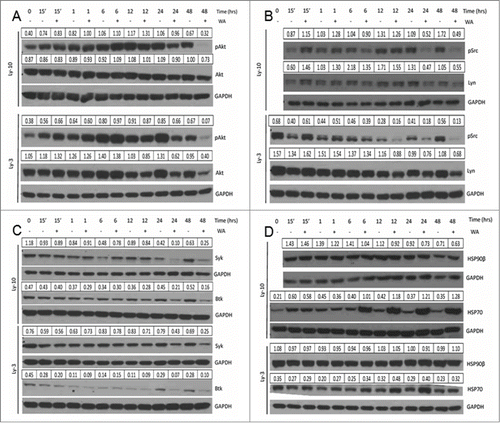
Previously we have shown that SFKs are constitutively activated in B cell lymphoma lines and are necessary for B cell lymphoma growth.Citation8 Therefore, we investigated the effect of WA treatment on the protein activity and expression of Lyn, a known SFK needed for the phosphorylation of the BCR co-receptors.Citation27 As shown in , there is a dramatic loss of expression of pSrc at late time point treatments with WA. There is also decreased expression of total Lyn protein, indicating that WA treatment results in degradation of the kinase critical for BCR induced survival signals. The non-receptor tyrosine kinases, Syk and Btk, which are the downstream components of the BCR signaling pathway, were also inhibited by WA treatment (). Down regulation of multiple protein kinases in BCR signaling and cell cycle progression suggested that WA may be affecting a key target that affects the stability of these proteins. Yu et. al. showed that the WA treatment led to inhibition of Hsp90 chaperone activity in pancreatic cancer cells.Citation15 Decrease in total protein levels of several kinases (Akt, Lyn, CDK4, IKKα/β), which have been demonstrated to be Hsp90 substrates led us to postulate that WA may also inhibit Hsp90 function in lymphoma cells. Best indication of decrease in Hsp90 function is rapid induction of Hsp70 which could compensate for Hsp90 partially. In agreement with this concept, we found that Hsp90 levels were unaltered in WA treated lymphoma cells but there was a robust increase in Hsp70 expression levels as early as after 6 hours of drug treatment ().
In vivo tumor growth inhibition after WA treatment
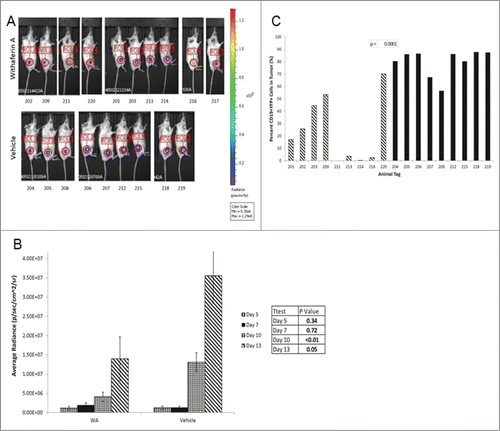
Here we examined the effect of WA in vivo on a murine DLBCL line, A20-Luc. Results indicate that tumor size was reduced after treatment with WA as shown by the bioluminescence images () and by the average tumor volume per treatment group () and per individual mouse (). Measurements for days 5–13 are represented on the histogram as tumor volumes were minimal before day 5. There was a significant difference between the tumor size of vehicle and WA treated groups on day 10 and day 13 with p-values <0.05 determined by student t-test. We also collected the tumor after 15 days and determined the percentage of CD19+YFP+ tumor cells within the tissues by flow cytometry. There was a significant reduction in the percentage of tumor cells within the focal area of the animals treated with WA (). We wondered if the drug may be targeting other actively proliferating cells in vivo and therefore examined the histology of colon sections as it contains epithelial cells that undergo basal proliferation. Histological examination of the colons from both the vehicle and WA treated groups, showed that the villus structure of the epithelial layer was intact, suggesting that WA did not have an effect on other proliferating cells besides the A20-luc lymphoma cells ().
Figure 6. Withaferin A inhibits B cell lymphoma growth in vivo. (A) WA significantly inhibited the subcutaneous tumor growth of A20-Luc, a murine B cell lymphoma. Tumor size was measured through bioluminescence imaging. Images show growth of tumor at Day 13 comparing the vehicle treated animals (bottom) and WA treated animals (top). The numbers below the images refer to mouse tags. (B) Histogram depicts the average growth of tumor over time comparing the vehicle and drug treatments. Size of tumor is measured in photons/sec/cm2. Error bars represent standard error of the mean per group at each day of measurement. (C) Tumors were excised on Day 15 of the study and the percentage of CD19+YFP+ cells were measured by flow cytometry and compared between vehicle and WA treated groups.
Discussion
Previously a few studies have examined the effect of WA, a naturally occurring steroid lactone on lymphoblastic and myeloid leukemia,Citation28,29 but none had studied its ability to modulate the growth of DLBCL which are aggressive tumors. Here we showed that WA has a significant anti-cancer role in the growth and survival of this aggressive tumor using both in vitro as well as an in vivo lymphoma model. Our data suggests that WA targets the functional activity of the Hsp90 chaperone complex resulting in degradation of client proteins that include critical pro-survival kinases, cell cycle regulators, pro-survival factors, and B cell receptor signaling components.
Many studies have reported that WA treatment mediates a G2/M phase arrest resulting from decreased expression of CDK1 (cdc2), a kinase required for G2/M transition and accumulation of cyclin B1 which is suggestive of an M phase arrest.Citation14,18,20,30 Accordingly we also found that WA treatment resulted in decreased expression of CDK1 but not cyclin B1 in both LY-10 and LY-3 cells lines, which may lead to G2/M phase arrest. Results of WA treatment on SudHL-6 cells were consistent with the G2/M phase arrest seen in melanoma, glioblastoma, and cervical cancer cells. Such G2/M arrest was seen in LY-10 and LY-3 lymphoma cells but interestingly with an addition of a G1/S arrest () which could be due to a decrease in CDK4, a kinase required for G1 progression.Citation18,31,32 These results support the hypothesis that WA targets the activity of Hsp90 in DLBCL, resulting in decreased expression of its client proteins that include CDK1 and CDK4,Citation33,34 which affect different phases of the cell cycle, but does not have a significant effect on non-client proteins of Hsp90 including CDK2 or cyclin A1 (). The many client proteins of Hsp90 involved in different stages of the cell cycle may be unique to different tumor models treated with WA, and why we observe blocks at multiple phases of the cell cycle in DLBCLs.
Many hematologic cancers are characterized by constitutive activation of NF-κB, making it a key target of therapeutics.Citation6 WA has been shown to inhibit TNF-α induced nuclear translocation of the p65 subunit of NF-κB in human lung epithelial cells.Citation35 Kaileh et. al. suggested that WA inhibits NF-κB by inhibiting the IKKβ kinase activity to phosphorylate IκB, the inhibitor of NF-κB, and thus preventing the translocation of the p65 subunit to the nucleus.Citation22 This idea was further expanded by Heyninck et. al., suggesting WA targets the Cys179 residue of IKKβ and inhibits the IKK complex directly.Citation36 Conversely, it has been suggested in lymphoma cells that WA inhibits TNF-α induced ubiquitin dependent organization of the IKKγ (NEMO) rather than targeting IKKβ directly.Citation37 Although we cannot rule out the direct effects of WA on IKKβ, our data is consistent with the inhibitory effect of WA on Hsp90 chaperone activity which can also lead to down regulation of total IKKα/β levels () resulting in reduced translocation of p65 as shown by our fluorescence imaging studies ().
One study examining the mechanism of apoptosis in human leukemia U937 cells suggested WA induced a decrease in Bcl-2, Bcl-XL, and Mcl1 expression with activation of caspase-3.Citation38 Bcl-2 is known to be a client protein of Hsp90.Citation39 Surprisingly, WA treatment did not reduce Bcl-2 levels in all of our DLBCL cell lines except SudHL-6 cells (, ). One potential reason for the difference could due to the subtypes of DLBCL. Many GCB-like DLBCL cells are associated with a translocation between the short arm of chromosomes 14 and 18 allowing for the Bcl-2 gene to be under the Ig heavy chain enhancer allowing for overexpression of Bcl-2.Citation40 While Bcl-2 is also expressed in ABC-like DLBCL, it may be regulated by a different mechanism such as NF-κB activation.Citation5 There was a clear reduction in Mcl1 expression after WA treatment in all DLBCL lines () which is consistent with other studies suggesting that one mechanism by which DLBCL cells are induced to undergo apoptosis by WA is through destabilization of Mcl1.
Several studies have suggested that WA may induce apoptosis of cancer cells through Par-4 dependent apoptotic mechanisms.Citation19,24,25 Par-4 is ubiquitously expressed, but is found to be elevated in cancer cells, and requires both endogenous and secreted forms to induce apoptosis. Surprisingly WA treatment of DLBCL cell lines, SudHL-6 and LY-10, resulted in decreased total and secreted protein expression levels of Par-4 (). Par-4 is also known to be cleaved by caspase-3 and decreased protein levels were thought to be attributed to loss of the full protein.Citation41 Surprisingly there was no increase in levels of cleaved Par-4 during treatment with WA (data not shown) but a significant decrease, which was not altered by inclusion of Z-VAD FMK, a pan-caspase inhibitor (, and data not shown). These results suggest that the B-lymphoma cells studied here do not undergo Par-4 dependent apoptosis after WA treatment and the mechanism of apoptosis is very likely dependent on decreased NF-κB activity, loss of Mcl1 stability, and caspase activation.
We have observed that WA treatment results in the decrease of activated and total Akt, a pro survival kinase (). Other groups have published similar findings on the inhibition of Akt and have suggested that it may be a result of WA targeting the intermediate filament, Vimentin.Citation13,14 To determine if inhibition of vimentin function is important in DLBCL, we examined the effect of another inhibitor of vimentin, Arylquin 1, which is thought to bind vimentin and induce Par-4 dependent apoptosis.Citation42 We found that cytotoxicity required 10 fold higher concentration of Arylquin 1 in DLBCL than shown in mouse embryonic fibroblasts and PC-3 cells (data not shown).Citation42 These results suggest that WA inhibition of vimentin is not likely to be the primary reason for decrease in Akt and apoptosis of DLBCL. We suggest that the reduced protein levels of Akt are a result of decreased Hsp90 function as Akt is a confirmed client protein of the Hsp90/cdc37 chaperone complex.Citation43,44 Some therapeutics have targeted the PIP3/AKT cascade due to the constitutive activation of the pathway in a variety of cancers.Citation45,46 Hence, WA which affects this pathway may also be a key small molecule for cancers that depend on enhanced Akt activity.
B cell lymphomas require BCR signaling for survival and growth.Citation9 SFKs, such as Lyn and Btk, and Syk involved in BCR signaling were reduced after WA treatment and are also known to be client proteins of Hsp90.Citation47–49 These results are novel findings as we are the first to show that WA inhibits key survival kinases that are required for B cell growth.Citation2,8
In conclusion, we have proposed a mechanism of action by WA that encompasses the broad range of effects shown in DLBCL. It is clear that WA has anti-cancer activities not only in hematological malignancies but also in many solid tumor models previously described. Based on the results presented here and the findings of Yu et. al., we conclude that WA inhibits the growth and survival of DLBCL by interacting and inhibiting the activity of the chaperone Hsp90, resulting in reduced expression of its client proteins.Citation15 Studies also indicate that WA has no noticeable toxicity on normal lymphocytesCitation28, and our results illustrate that WA does not appear to affect proliferating colon epithelial cells in vivo. Further analyses on in vivo stability of WA and its effects on normal tissues need to be completed in order evaluate the clinical potential of WA as a therapeutic for DLBCL but current findings are promising.
Material and Methods
Reagents
WA was isolated from extract of Withania somnifera (Sabinsa Corp) using a series of solvent extractions and silica gel-based vacuum liquid column chromatography at the University of Louisville and at the laboratory of Dr. I. P. Singh, National Institute of Pharmaceutical Education Research (NIPER), India. The isolated WA was found to be >94% pure by UPLC. Phospho specific antibodies against Src (Y416) (#2101S), Akt (S473) (#9271L), IKKα/β (S176/180) (#2687P) were obtained from Cell Signaling Technologies. Antibodies against total Akt (#9272S), IKK (#2682), Syk (#2712), Btk (#3533S), Mcl1(#5453P) Hsp70 (#4872S), and GAPDH (#2118S) were also obtained from Cell Signaling. Hsp90β (#GTX101448) antibody was obtained from GeneTex, Inc.. Antibodies against total Lyn (#SC-15), Par-4 (#SC-1807), Col1A (#SC-28657), and Bcl-2 (#SC-7382) were obtained from Santa Cruz Biotechnologies (SCBT). Peroxidase coupled secondary antibodies were also obtained from SCBT (#SC-2004, SC-2005).
Cell Proliferation and Survival Assays
Human and mouse B lymphoma cell lines have been described previously.Citation8,9,50 Luciferase and YFP expressing A20 B lymphoma cells were obtained from Dr. Scott Bryson with permission from Dr. Negrin.Citation51 Cell survival and proliferation was determined by the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) assay.Citation52 Lymphoma cells were cultured (1–1.5×105/well) in 96 well flat-bottom microtiter plates in 200µl media supplemented with 10% fetal bovine serum (FBS) (Atlanta Biological Systems). The cells were treated with 0–10 µM WA dissolved in DMSO for 48hr and then media was changed and incubated with MTT (0.5 mg/ml; Sigma Aldrich, M5655) for 4hr followed by solubilization in acidic isopropanol and spectrophotometric measurements at 560 nm and 690 nm. The OD values (560–690 nm) of cultures without WA were set to 100%. DMSO concentration did not exceed 0.02% of culture medium and had no measurable effect on cell viability.
Cell Apoptosis Analysis
B lymphoma cells (0.75 × 105/ml) were treated with 0–20µM WA for 2 days and then stained with Annexin V (FITC, BD PharMingen, 556420) and propidium iodide (PI, Sigma Aldrich, P4170) (1μg/ml). To determine if cells were undergoing extrinsic Par-4 mediated apoptosis via secreted Par-4, cells (0.75 × 105/ml) were treated with 2.5 µM WA for 6 hours and then media changed to low serum (0.05% FBS). Cells were left to incubate for 12hr and the media containing secreted Par-4 was then collected and concentrated. The cell pellets were also collected to measure total protein content.
Cell Cycle Analysis
B lymphoma cells (0.75 × 105/ml) were treated with 0–20µM WA for 48hrs and then fixed with cold 70% ethanol for 1hr at 4°C and then incubated with a mixture of 1µg/ml PI and 25 µg/ml RNase A (Sigma Aldrich, R6513) at 37°C for 30 min. The level of PI fluorescence was measured with a FACSCaliber flow cytometer. Cell populations in G1, S, G2/M phase were calculated using ModFit Software.
NF-κB Nuclear Translocation Assay
B lymphoma cells (0.75 × 105/ml) were incubated with 2.5µM WA for 2 and 4 hours and then fixed with cold 70% ethanol for 1hr at 4°C. Cells were blocked in 10% Normal goat serum (NGS) for 1hr and then stained with primary antibody to NF-κB p65. Cells were then stained with secondary DyLight 488 conjugated affini pure F(ab')2 goat anti-rabbit antibody (Jackson Immunoresearch, #111–486–046) for 1hr in the dark. Cells were then stained with DAPI (Life Technologies, #D1306) for 15 min. Cells were washed and then resuspended in Prolong Gold Anti-Fade Reagent (Life Technologies, #P36930). Cells were viewed on a FV1000 confocal microscope. At least 16 fields were counted and data represents mean ± SD of triplicate samples. Statistical significance of difference between groups was determined by ANOVA.
Western Blot Analysis
B lymphoma cells (5 × 106 cells) were cultured in a 60mm tissue culture dish at 0.75 × 105/ml for various time points with vehicle or 2.5 µM WA treatment. Cells were lysed with Cell Signaling Lysis buffer (#9803S). Western blot analysis was performed as described previously.Citation50 Intensities of bands were quantified using the Gel Analysis method of the NIH ImageJ program. All blots were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Cell Signaling, #2118S) expression.
In vivo tumor inhibition study
16-20 week old female Balb/c mice (Jackson Labs) were given a sublethal dose of 4 Gy radiation. Mice were injected subcutaneously with 5 ×105 A20-Luc murine B lymphoma cells with 1:1 Matrigel (BD Biosciences, #354234) in 100μl volume 2hr after irradiation. Cell growth was measured utilizing IVIS Spectrum Luminescent imager after injecting 30mg/kg luciferin potassium salt (Luciferin D) (Regis Technologies, Inc. #360222) substrate intraperitoneally to each mouse. Bioluminescence which is proportional to the size of the tumor was measured by radiance quantified as photons/sec/cm2. Once tumors reached 1.5×105 p/sec/cm2 (average of 3 days), mice were randomly divided into vehicle vs. drug treated groups. Animals received 12mg/kg WA [delivered in 10% DMSO, 90% Glyceryl trioctanoate (Sigma Aldrich, #T9126)] or vehicle every other day for 2 weeks.Citation53 Tumor size was measured every 3 days by bioluminescence imaging. Mice were sacrificed after 15 days; spleens and tumors were excised homogenized and stained for CD19 (Biolegend, #115508). A20-Luc Tumor cells were analyzed by flow cytometry by gating on YFP+CD19+ cells. Tumor growth was plotted over time based on the radiance measurements quantified by the Living Image Acquisition/Analysis Software Package. Colon tissues were collected and fixed in 10% Formalin (Fisher Scientific, #SF93–4). Tissue sections were cut and stained for Hematoxylin and Eosin provided by the Imaging and Histology Facility at the University of Kentucky. All animals were housed and maintained by the Department of Laboratory Animal Resources at the University of Kentucky (Lexington, KY). All animal studies were approved by the Institutional Animal Care and Use Committee and were carried out in accordance with the Animal Welfare Act.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Figures
Download MS Word (1.4 MB)Acknowledgments
We thank Dr. Sunil K. Noothi for helpful discussions and for his critical comments on the manuscript. We thank Dr. David Watt for providing us with Arylquin 1. We thank Ms. Cynthia Long and the Imaging Facility at the University of Kentucky for assistance with histology. We thank Dr. Chi Wang for his assistance with statistical analysis. We thank Sabinsa Corp. for providing enriched extract of Withania somnifera. We also thank Dr. I. P. Singh of NIPER, India for further purifying Withaferin A and Dr. Farrukh Aqil for determining its purity by UPLC.
Funding
We thank UK Flow Cytometry and Cell Sorting Core Facility, and the small animal imaging facility which is supported in part by the Office of the Vice President for Research, the Markey Cancer Center and an NCI Center Core Support Grant (P30 CA177558) to the University of Kentucky Markey Cancer Center. This research was funded by NIH Grants R01 CA165469, T32 CA165990, Agnes Brown Duggan Endowment (RG), and the Edwards P Evans Foundation.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/
- Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discovery 2013; 12:229-43; PMID:23449308; http://dx.doi.org/10.1038/nrd3937
- Blenk S, Engelmann J, Weniger M, Schultz J, Dittrich M, Rosenwald A, Muller-Hermelink HK, Muller T, Dandekar T. Germinal center B cell-like (GCB) and activated B cell-like (ABC) type of diffuse large B cell lymphoma (DLBCL): analysis of molecular predictors, signatures, cell cycle state and patient survival. Cancer Informat 2007; 3:399-420; PMID:19455257
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403:503-11; PMID:10676951; http://dx.doi.org/10.1038/35000501
- Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2001; 194:1861-74; PMID:11748286; http://dx.doi.org/10.1084/jem.194.12.1861
- Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harbor Perspect Biol 2010; 2:a000109; http://dx.doi.org/10.1101/cshperspect.a000109
- Lim KH, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF-kappaB in lymphoid malignancies. Immunol Rev 2012; 246:359-78; PMID:22435566; http://dx.doi.org/10.1111/j.1600-065X.2012.01105.x
- Ke J, Chelvarajan RL, Sindhava V, Robertson DA, Lekakis L, Jennings CD, Bondada S. Anomalous constitutive Src kinase activity promotes B lymphoma survival and growth. Mol Cancer 2009; 8:132; PMID:20043832; http://dx.doi.org/10.1186/1476-4598-8-132
- Gururajan M, Jennings CD, Bondada S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol 2006; 176:5715-9; PMID:16670274; http://dx.doi.org/10.4049/jimmunol.176.10.5715
- Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol 2013; 13:578-91; PMID:23883968; http://dx.doi.org/10.1038/nri3487
- Cultrera JL, Dalia SM. Diffuse large B-cell lymphoma: current strategies and future directions. Cancer Control 2012; 19:204-13; PMID:22710896
- Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacol Res 2009; 59:365-78; PMID:19429468; http://dx.doi.org/10.1016/j.phrs.2009.01.017
- Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol 2012; 84:1282-91; PMID:22981382; http://dx.doi.org/10.1016/j.bcp.2012.08.027
- Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone. AAPS J 2014; 16:1-10; PMID:24046237; http://dx.doi.org/10.1208/s12248-013-9531-1
- Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, Li Y, Gunatilaka AA, Zhan CG, Sun D. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol 2010; 79:542-51; PMID:19769945; http://dx.doi.org/10.1016/j.bcp.2009.09.017
- Nagalingam A, Kuppusamy P, Singh SV, Sharma D, Saxena NK. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res 2014; 74:2617-29; PMID:24732433; http://dx.doi.org/10.1158/0008-5472.CAN-13-2081
- Cai Y, Sheng ZY, Chen Y, Bai C. Effect of Withaferin A on A549 cellular proliferation and apoptosis in non-small cell lung cancer. Asian Pacific J Cancer Prevention 2014; 15:1711-4; PMID:24641396; http://dx.doi.org/10.7314/APJCP.2014.15.4.1711
- Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 2011; 32:1697-705; PMID:21859835; http://dx.doi.org/10.1093/carcin/bgr192
- Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res 2007; 67:246-53; PMID:17185378; http://dx.doi.org/10.1158/0008-5472.CAN-06-2430
- Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutrition Cancer 2008; 60 1:51-60; PMID:19003581; http://dx.doi.org/10.1080/01635580802381477
- Grover A, Shandilya A, Punetha A, Bisaria VS, Sundar D. Inhibition of the NEMO/IKKbeta association complex formation, a novel mechanism associated with the NF-kappaB activation suppression by withania somnifera's key metabolite withaferin A. BMC Genomics 2010; 11 4:S25; PMID:21143809; http://dx.doi.org/10.1186/1471-2164-11-S4-S25
- Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, Libert C, De Keukeleire D, Essawi T, Haegeman G. Withaferin a strongly elicits IkappaB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem 2007; 282:4253-64; PMID:17150968; http://dx.doi.org/10.1074/jbc.M606728200
- Gray PJ, Jr., Prince T, Cheng J, Stevenson MA, Calderwood SK. Targeting the oncogene and kinome chaperone CDC37. Nat Rev Cancer 2008; 8:491-5; PMID:18511936; http://dx.doi.org/10.1038/nrc2420
- Raina A, Kaul D. LXR-α genomics programmes neuronal death observed in Alzheimer's disease. Apoptosis 2010; 15:1461-9; PMID:20927647; http://dx.doi.org/10.1007/s10495-010-0541-5
- Franchitto A, Torrice A, Semeraro R, Napoli C, Nuzzo G, Giuliante F, Alpini G, Carpino G, Berloco PB, Izzo L, et al. Prostate apoptosis response-4 is expressed in normal cholangiocytes, is down-regulated in human cholangiocarcinoma, and promotes apoptosis of neoplastic cholangiocytes when induced pharmacologically. Am J Pathol 2010; 177:1779-90; PMID:20724592; http://dx.doi.org/10.2353/ajpath.2010.091171
- Burikhanov R, Shrestha-Bhattarai T, Qiu S, Shukla N, Hebbar N, Lele SM, Horbinski C, Rangnekar VM. Novel mechanism of apoptosis resistance in cancer mediated by extracellular PAR-4. Cancer Res 2013; 73:1011-9; PMID:23204231; http://dx.doi.org/10.1158/0008-5472.CAN-12-3212
- Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene 2004; 23:8001-6; PMID:15489917; http://dx.doi.org/10.1038/sj.onc.1208075
- Mandal C, Dutta A, Mallick A, Chandra S, Misra L, Sangwan RS. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis 2008; 13:1450-64; PMID:18987975; http://dx.doi.org/10.1007/s10495-008-0271-0
- Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, Suri KA, Qazi GN, Singh J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 2007; 12:2115-33; PMID:17874299; http://dx.doi.org/10.1007/s10495-007-0129-x
- Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Proliferation 2003; 36:131-49; PMID:12814430; http://dx.doi.org/10.1046/j.1365-2184.2003.00266.x
- Samadi AK, Cohen SM, Mukerji R, Chaguturu V, Zhang X, Timmermann BN, Cohen MS, Person EA. Natural withanolide withaferin A induces apoptosis in uveal melanoma cells by suppression of Akt and c-MET activation. Tumour Biol 2012; 33:1179-89; PMID:22477711; http://dx.doi.org/10.1007/s13277-012-0363-x
- Grogan PT, Sleder KD, Samadi AK, Zhang H, Timmermann BN, Cohen MS. Cytotoxicity of withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Invest New Drugs 2013; 31:545-57; PMID:23129310; http://dx.doi.org/10.1007/s10637-012-9888-5
- Wang SA, Li HY, Hsu TI, Chen SH, Wu CJ, Chang WC, Hung JJ. Heat shock protein 90 stabilizes nucleolin to increase mRNA stability in mitosis. J Biol Chem 2011; 286:43816-29; PMID:21998300; http://dx.doi.org/10.1074/jbc.M111.310979
- Vaughan CK, Gohlke U, Sobott F, Good VM, Ali MM, Prodromou C, Robinson CV, Saibil HR, Pearl LH. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell 2006; 23:697-707; PMID:16949366; http://dx.doi.org/10.1016/j.molcel.2006.07.016
- Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-α-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Intl Immunopharmacol 2009; 9:614-9; PMID:19236958; http://dx.doi.org/10.1016/j.intimp.2009.02.002
- Heyninck K, Lahtela-Kakkonen M, Van der Veken P, Haegeman G, Vanden Berghe W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKbeta. Biochem Pharmacol 2014; 91:501-9; PMID:25159986; http://dx.doi.org/10.1016/j.bcp.2014.08.004
- Jackson SS, Oberley C, Hooper CP, Grindle K, Wuerzberger-Davis S, Wolff J, McCool K, Rui L, Miyamoto S. Withaferin A disrupts ubiquitin-based NEMO reorganization induced by canonical NF-kappaB signaling. Exp Cell Res 2015; 331:58-72; PMID:25304104; http://dx.doi.org/10.1016/j.yexcr.2014.09.034
- Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH, Lee JM, Kim YH, Park JW, Kwon TK. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis 2008; 13:1494-504; PMID:19002588; http://dx.doi.org/10.1007/s10495-008-0273-y
- Dias S, Shmelkov SV, Lam G, Rafii S. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood 2002; 99:2532-40; PMID:11895790; http://dx.doi.org/10.1182/blood.V99.7.2532
- Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, Dave S, Xiao L, Cao K, Zhu Q, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol 2004; 165:159-66; PMID:15215171; http://dx.doi.org/10.1016/S0002-9440(10)63284-1
- Chaudhry P, Singh M, Parent S, Asselin E. Prostate apoptosis response 4 (Par-4), a novel substrate of caspase-3 during apoptosis activation. Mol Cell Biol 2012; 32:826-39; PMID:22184067; http://dx.doi.org/10.1128/MCB.06321-11
- Burikhanov R, Sviripa VM, Hebbar N, Zhang W, Layton WJ, Hamza A, Zhan CG, Watt DS, Liu C, Rangnekar VM. Arylquins target vimentin to trigger Par-4 secretion for tumor cell apoptosis. Nat Chem Biol 2014; 10:924-6; PMID:25218743; http://dx.doi.org/10.1038/nchembio.1631
- Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 2002; 277:39858-66; PMID:12176997; http://dx.doi.org/10.1074/jbc.M206322200
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A 2000; 97:10832-7; PMID:10995457; http://dx.doi.org/10.1073/pnas.170276797
- LoPiccolo J, Granville CA, Gills JJ, Dennis PA. Targeting Akt in cancer therapy. Anti-Cancer Drugs 2007; 18:861-74; PMID:17667591
- LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resistance Updates 2008; 11:32-50; PMID:18166498; http://dx.doi.org/10.1016/j.drup.2007.11.003
- Trentin L, Frasson M, Donella-Deana A, Frezzato F, Pagano MA, Tibaldi E, Gattazzo C, Zambello R, Semenzato G, Brunati AM. Geldanamycin-induced Lyn dissociation from aberrant Hsp90-stabilized cytosolic complex is an early event in apoptotic mechanisms in B-chronic lymphocytic leukemia. Blood 2008; 112:4665-74; PMID:18768392; http://dx.doi.org/10.1182/blood-2008-02-139139
- Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, Hatzi K, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol 2011; 7:818-26; PMID:21946277; http://dx.doi.org/10.1038/nchembio.670
- Castro JE, Prada CE, Loria O, Kamal A, Chen L, Burrows FJ, Kipps TJ. ZAP-70 is a novel conditional heat shock protein 90 (Hsp90) client: inhibition of Hsp90 leads to ZAP-70 degradation, apoptosis, and impaired signaling in chronic lymphocytic leukemia. Blood 2005; 106:2506-12; PMID:15972449; http://dx.doi.org/10.1182/blood-2005-03-1099
- Gururajan M, Chui R, Karuppannan AK, Ke J, Jennings CD, Bondada S. c-Jun N-terminal kinase (JNK) is required for survival and proliferation of B-lymphoma cells. Blood 2005; 106:1382-91; PMID:15890690; http://dx.doi.org/10.1182/blood-2004-10-3819
- Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood 2003; 101:640-8; PMID:12393519; http://dx.doi.org/10.1182/blood-2002-06-1751
- Plumb JA. Cell sensitivity assays: the MTT assay. Method Mol Med 2004; 88:165-9; PMID:14634227
- Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PloS one 2012; 7:e42265; PMID:22860102; http://dx.doi.org/10.1371/journal.pone.0042265
