ABSTRACT
Poly (ADP) ribose polymerase (PARP) inhibitors, first evaluated nearly a decade ago, are primarily used in malignancies with known defects in DNA repair genes, such as alterations in breast cancer, early onset 1/2 (BRCA1/2). While no specific mutations in BRCA1/2 have been reported in malignant peripheral nerve sheath tumors (MPNSTs), MPNST cells could be effectively targeted with a PARP inhibitor to drive cells to synthetic lethality due to their complex karyotype and high level of inherent genomic instability. In this study, we assessed the expression levels of PARP1 and PARP2 in MPNST patient tumor samples and correlated these findings with overall survival. We also determined the level of PARP activity in MPNST cell lines. In addition, we evaluated the efficacy of the PARP inhibitor AZD2281 (Olaparib) in MPNST cell lines. We observed decreased MPNST cell proliferation and enhanced apoptosis in vitro at doses similar to, or less than, the doses used in cell lines with established defective DNA repair genes. Furthermore, AZD2281 significantly reduced local growth of MPNST xenografts, decreased the development of macroscopic lung metastases, and increased survival of mice with metastatic disease. Our results suggest that AZD2281 could be an effective therapeutic option in MPNST and should be further investigated for its potential clinical use in this malignancy.
Abbreviations
| ATM | = | ataxia telangiectasia mutated |
| ATR | = | ataxia telangiectasia and Rad3-related protein |
| BRCA1/2 | = | breast cancer 1, early onset 1/2 |
| CDKN1B | = | cyclin dependent kinase inhibitor 1B |
| CHK1/2 | = | checkpoint kinase 1/2 |
| DMEM | = | Dulbecco's modified Eagle's medium |
| DMSO | = | dimethyl sulfoxide |
| FACS | = | fluorescence-activated cell sorting |
| FBS | = | fetal bovine serum |
| FITC | = | fluorescein isothiocyanate |
| H&E | = | hematoxylin-eosin |
| HGF | = | hepatocyte growth factor |
| HR | = | homologous recombination |
| IACUC | = | Institutional Animal Care and Use Committee |
| IHC | = | immunohistochemistry |
| MPNST | = | malignant peripheral nerve sheath tumor |
| MTS | = | 3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt |
| NF1 | = | Neurofibromatosis type 1 |
| NSCs | = | normal Schwann cells |
| PAR | = | poly (ADP) ribose |
| PARP | = | poly (ADP) ribose polymerase |
| PARP-HSA | = | PARP enzyme-high specific activity |
| PBS | = | phosphate buffered saline |
| PDGFRA | = | platelet-derived growth factor receptor, alpha polypeptide |
| PMSF | = | phenylmethylsulfonyl fluoride |
| PI | = | propidium iodide |
| SCID | = | severe combined immunodeficiency |
| SEM | = | standard error of the mean |
| SOLO1/2 | = | study of olaparib in ovarian cancer |
| STS | = | soft tissue sarcoma |
| TP53 | = | tumor protein p53 |
| TMA | = | tissue microarray |
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a relatively rare soft tissue sarcoma, representing only 2% of all sarcomas.Citation1 Fifty percent of MPNST cases are associated with neurofibromatosis type 1 (NF1), an autosomal dominant condition occurring in 1 in 3000 births, making it the most common hereditary cancer predisposition syndrome; 40% of MPNST cases are sporadic; and development of the remaining 10% is associated with prior radiation therapy.Citation1-3 Chemotherapy and radiotherapy have proven to be ineffective in treating MPNST. Therefore, surgical resection is the prime modality of therapy.Citation4-6 In addition, MPNST has a high rate of local recurrence (40%–65%) and a high propensity for metastatic spread, which severely decreases survival outcomes.Citation7,8 All of these factors contribute to a 5-year survival rate of 35%–50% and a dismal 10-year survival rate of only 7.5%.Citation9 Therefore, more efficacious therapeutic options for MPNST patients are urgently needed.
Specific defects in DNA repair pathways can predispose cancer cells to cytotoxic agents, and these defects can be exploited for treatment. Various studies have demonstrated that cancer cells with defective DNA repair proteins such as BRCA1/2, ATR, ATM, and CHK1/2 are profoundly sensitive to poly (ADP) ribose polymerase (PARP) inhibitors.Citation10,11 The PARP family consists of 18 proteins, each identified by their structural homology to PARP1.Citation12
The activity of PARP1 and PARP2 are the most clearly defined of the PARP family. Once activated by DNA damage, they produce chains of poly (ADP) ribose (PAR) to post-translationally modify acceptor proteins. PARP activity recruits single strand break repair proteins (specifically base excision repair proteins) to the site of DNA damage, signals the extent of DNA damage, and relaxes the chromatin for increased access to DNA breaks. Therefore, PARP has a major role in single strand DNA damage repair through the base excision repair pathway.Citation12 Studies evaluating PARP inhibition were first published nearly a decade ago.Citation13,14 PARP inhibition, combined with a double-strand DNA repair deficiency such as a BRCA mutation or ‘BRCAness’, was demonstrated to cause synthetic lethality.Citation15 Most preclinical and subsequent clinical studies with PARP inhibitors, specifically AZD2281 (Olaparib), have been performed in BRCA-mutated breast and ovarian carcinomas with significant anti-tumor effects reported.Citation16 Use of this inhibitor has since expanded into other malignancies including brain, lung, and esophageal cancers, and leukemia.Citation17-20 Currently, AZD2281 is in a phase 3 clinical trial (SOLO1 and SOLO2) for BRCA-mutated ovarian cancer.
Although MPNSTs do not have a characterized defect in BRCA1/2, they have a genetically complex karyotype that would suggest deficient DNA damage repair and a high level of inherent DNA damage and genomic instability.Citation21,22 Furthermore, MPNSTs have been shown to have a higher expression of DNA repair genes compared to plexiform neurofibromas, the precursor lesion of NF1-associated MPNST.Citation23 In this study, we treated sporadic and NF1-associated MPNST cell lines with the PARP inhibitor AZD2281 and observed a substantial anti-MPNST effect, including decreased cell proliferation and the induction of apoptosis. Subsequent in vivo experiments using AZD2281 in multiple xenograft mouse models of MPNST resulted in substantially decreased local tumor growth and slowed metastatic progression. In addition, AZD2281 treatment of mice with experimental lung metastases significantly prolonged survival. These data indicate that PARP inhibition is unfavorable to MPNST tumorigenicity and support further investigation into PARP inhibition as a possible therapeutic option for MPNST patients.
Materials and methods
Clinically annotated tissue microarray
This study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. A clinically annotated tissue microarray (TMA) containing 115 human atypical and plexiform neurofibromas (n = 24) and MPNSTs (primary, n = 38; recurrent, n = 32; MPNST, n = 21) samples was used for immunohistochemical staining. The TMAs were constructed as previously described.Citation24
Cell lines
MPNST cell lines used in this study included 2 NF1-associated S462 (provided by Dr. Brian Rubin, The Cleveland Clinic and The Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH) and ST88 (provided by Dr. Jonathan Fletcher, Brigham and Women's Hospital, Boston, MA) and 2 sporadic MPNST cell lines STS26T (provided by Dr. Steven Porcelli, Albert Einstein College of Medicine, Bronx, NY) and MPNST724 (provided by Dr. Jonathan Fletcher). Primary human adult Schwann cell cultures were purchased from ScienCell Research Laboratories. MPNST cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Schwann cells were grown in Schwann cell media (#1701, ScienCell Research Laboratories) supplemented with 5% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% Schwann cell growth supplement (#1752, ScienCell Research Laboratories). DNA fingerprinting (short tandem repeat) was conducted as previously described to verify the identity of all MPNST cell lines.Citation25
Antibodies and reagents
The PARP inhibitors AZD2281 and ABT888 were purchased from ChemieTek (CT-A2281) and (CT-A888). For in vitro studies, the drugs were dissolved in dimethyl sulfoxide (DMSO) and stored at −80°C. For in vivo experiments, AZD2281 (50 mg/kg/d) was dissolved in PBS, 10% DMSO, and 10% 2-hydroxypropyl-β-cyclodextrin. Commercially available antibodies were used for Western blot or immunohistochemical (IHC) detection of PARP1 (9542, Cell Signaling Technology), PARP2 (PA1-4280, Thermo Fisher Scientific Pierce), PAR (4335-AMC-050, Trevigen Inc.) and (ALX-084-220-R100, Enzo Life Sciences, Inc.), Ki67 (CRM325, Biocare Medical), cleaved caspase 3-CC3 (CP229, Biocare Medical), cyclin B1 (752, Santa Cruz Biotechnology), and β-actin-HRP (47778-HRP, Santa Cruz Biotechnology). TUNEL was detected using the TdT-FragEL™ DNA Fragmentation Detection Kit according to the manufacturer's instructions (QIA33, EMD Millipore).
MTS assays
CellTiter96 AQueous One Solution Cell Proliferation Assays [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] (G3580, Promega) were used to evaluate cell proliferation as per the manufacturer's instructions. Briefly, cells were seeded in 96-well plates in 100 µL of media. Attached cells were subsequently treated with AZD2281 or ABT888 and incubated with drug for 96 hours. MTS reagent was added to each well and incubated for 1–2 hours at 37°C. Absorbance was measured at a wavelength of 490 nm. The absorbance values of treated cells were represented as a percentage of the absorbance of untreated cells.
Clonogenic assay
Clonogenic assays were conducted by plating 100–500 MPNST cells in 6-well plates. Cells were then treated with DMSO, ABT888, or AZD2281 in DMEM for approximately 2 weeks. The media was removed, and the cells were washed twice with phosphate-buffered saline (PBS). The cells were fixed with 5% gluteraldehyde for 20 minutes and then washed and stained with 0.1% crystal violet in 20% methanol for at least 1 hour. The wells were washed with water and allowed to dry. Pictures were captured digitally and individual clones were counted. Clones of treated cells were represented relative to the untreated control.
Western blot analysis
Western blot analyses were conducted according to previously published standard methods.Citation26
Cell cycle/Annexin V-FITC FACS
Cell cycle progression was measured by propidium iodide (PtdIns) staining/fluorescence activated cell sorting (FACS) analysis. Cells were treated with AZD2281 at the indicated dilutions for 24 hours and subsequently fixed in 70% ethanol. Fixed cells were resuspended in a PI staining solution (75 ug/mL PtdIns and 10 ug/mL RNAse A). For evaluation of apoptosis, cells were treated with AZD2281 for 96 hours. Apoptosis was measured via Annexin V-FITC/PI staining and FACS analysis with the FITC Annexin V Apoptosis Detection Kit I (556547, BD Biosciences) per the manufacturer's recommendations. Cells reported as apoptotic include those in early and late phase apoptosis. Cell cycle and apoptosis samples were analyzed with the use of a Gallios flow cytometer (Beckman Coulter Inc.) located in the Flow Cytometry and Cellular Imaging Core at MD Anderson. Data from these experiments were analyzed with the use of the Multicycle program in FCS Express (De Novo Software).
PARP activity assay
PARP activity assays were performed by using the Trevigen Universal PARP Colormetric Assay Kit (4677-096-K, Trevigen Inc.) according to the manufacturer's instructions with the following modifications. Cells were seeded in 6-well plates at approximately 50% confluency. The cells were then treated with AZD2281 and incubated for 24 hours at 37°C. Endogenous PARP activity was established without AZD2281 pretreatment. Cellular protein was extracted by scraping using the Cell Extraction Buffer (1X PARP Buffer [20X PARP buffer provided in kit diluted in water], 0.4M NaCl, 0.9% Triton X-100, 0.4 nM PMSF, cOmplete Protease Inhibitor Cocktail Tablets [11697498001, Roche]). The suspension was centrifuged at 4°C for 10 minutes at >10,000g; the supernatant was extracted and the protein concentration was measured.
Histone-coated strip wells were hydrated by using 1X PARP buffer for 30 minutes at room temperature; 3 wells per condition were used. Conditions included positive and negative control wells, and experimental wells. The positive control wells contained diluted PARP-HSA enzyme (provided in kit), 1X PARP buffer, and 1X PARP cocktail (10X PARP cocktail provided in kit, 10X Activated DNA provided in kit, and 1X PARP buffer) at a final volume of 50µl. The negative control wells contained DMSO, 1X PARP buffer, and 1X PARP cocktail at a final volume of 50 µl. The experimental wells included 10µg pretreated cell lysate in a total volume of 25 μl, and 1X PARP cocktail at a final volume of 50 µl. The wells were incubated for 1 hour at room temperature. The remainder of the experiment was performed according to the manufacturer's instructions. The absorbance values of 3 wells from each experiment were averaged and the absorbance values of treated cells were represented as a percentage of the absorbance of untreated cells. The data were represented as the average of 2 experiments with the standard error of the mean (SEM).
In vivo animal models
All animal procedures and care were approved by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Usage Committee (IACUC). Animals received humane care as per the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals.” Animal models were utilized as previously described.Citation27,28 Trypan blue confirmed viable MPNST724 cells (2 × 106 in 0.1 mL of PBS/mouse) or STS26T cells (2 × 106 in 0.1 mL of PBS/mouse) were injected into the flank of 6–week-old female hairless severe combined immunodeficient (SCID) outbred mice (Crl:SHO-PrkdcscidHrhr, Strain Code: 474) (474-SCID Hairless, Charles River Laboratories) (MPNST724- vehicle n = 9 and AZD2281 n = 8; STS26T- n = 8/treatment group), and growth was measured twice weekly. When average tumor volumes reached 50 mm3, the mice were assigned to 2 treatment groups: (a) control (vehicle only) and (b) AZD2281. AZD2281 (50 mg/kg/d, intraperitoneal injection [IP]) resuspended in PBS, 10% DMSO, and 10% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO) was used. Mice were monitored for tumor size, body-weight, and well-being, and were euthanized when control group tumors reached an approximate volume of 1500 mm3. Tumors were resected, weighed, and fixed in formalin and paraffin-embedded for IHC studies.
An experimental lung metastasis MPNST model was used to evaluate metastatic growth. This model has been described previously.Citation28 Viable STS26T cells were injected as cell suspensions (1 × 106 in 0.1 mL of PBS/mouse) into the tail vein of 6–week-old female hairless severe combined immunodeficient (SCID) outbred mice (Crl:SHO-PrkdcscidHrhr, Strain Code: 474). Mice were monitored for 3 weeks (a time point by which 95%–100% of mice develop established lung metastases).Citation29 Mice were then assigned to the following treatment groups: (a) control (vehicle only) and (b) AZD2281 (50 mg/kg/d, IP). AZD2281 resuspended in PBS, 10% DMSO, and 10% 2-hydroxypropyl-β-cyclodextrin was used. Mice were euthanized when physical signs of disease were present (weight loss >20% of baseline, difficulty of breathing, hunched posture, pallor, impaired ambulation and lethargy). Lungs were resected, weighed, and examined for macroscopic lung metastases; these tissues were then fixed in formalin and paraffin-embedded for IHC studies. Survival studies proceeded as above; however, mice were sacrificed individually when euthanasia was required.
IHC analysis
PARP1 and PARP2 immunostaining was scored by 2 independent observers (A.J.L and G.A.A) for TMA analyses. Intensity scores were designated as negative (0), weak (1 and 2), or strong (3). The percentage of cells staining positive was evaluated on a scale of 0–100%. A combined score ranging from 0–300 was obtained from the product of the intensity and cells-positive scores. Xenograft-derived specimens were analyzed for markers of cell proliferation (Ki67), cell cycle arrest (cyclin B1), inhibitor specificity (PAR), and apoptosis (CC3 and TUNEL). Representative images of the TMA and xenograft- derived specimens were captured at 200× and 400× total magnification, respectively, using an Olympus microscope (BX41) and camera (DP72) with Olympus cellSens Standard v1.5 software.
Statistical analyses
Differences among expression of PARP1 and PARP2 between tumor types were evaluated using Mann-Whitney U tests. Univariate Cox proportional hazards models were used to assess the potential prognostic significance of PARP1 expression in primary and recurrent MPNST samples and further visualized utilizing univariate Kaplan-Meier curves. Two-sided p-values <0.05 were considered statistically significant. All analyses were performed using SPSS version 21.
Cellular assays were repeated at least 2 times; the mean and SEM were calculated for each assay. The mean and standard deviation were calculated for the xenograft experiment. Significance of findings was assessed using a 2-tailed Student's t-test (p < 0.05 = *; p < 0.001 = ***).
Results
PARP1 and PARP2 are highly expressed in MPNST tissue samples
To determine the expression of PARP1 and PARP2 in human tumor samples, we performed IHC on a clinically annotated TMA containing neurofibroma, primary MPNST, recurrent MPNST, and metastatic MPNST patient samples (). The majority of MPNST samples were positive for PARP staining. Overall, moderate to high expression of PARP1 and PARP2 was observed (). While there was differential expression of PARP1 and PARP2 between primary, recurrent, and metastatic MPNST samples in terms of percent positive cells, nuclear intensity, and combined score, the clinical relevance of these minor variations is negligible as the overall expression levels remain high (Table S1 and S2).
Figure 1. PARP expression and activity is enhanced in MPNST cell lines compared to normal Schwann cells. (A) MPNST TMA stained for PARP1 and PARP2. Images were captured at 200× magnification. (B) PARP1 staining correlated to patient outcomes. (C) PAR immunoblot of MPNST cell panel and normal Schwann cells. (D) Endogenous PARP activity in untreated MPNST cells and normal Schwann cells (n = 4). Error bars represent standard error of the mean; (*= p < 0.05).
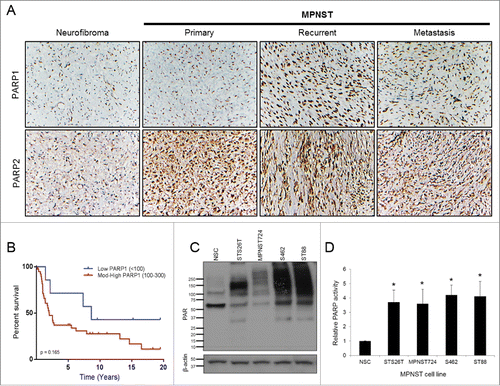
Table 1. Mean expression of PARP1 and PARP2 combined score (0–300) and percentage of tumors with positive staining in neurofibroma and MPNST.
In addition, we evaluated whether PARP1 expression correlated with the survival outcomes of MPNST patients. Although not statistically significant, patients with tumors expressing low levels of PARP1 had a better survival when compared to patients that had tumors with moderate to high expression (p = 0.165; HR 0.51, 95% CI 0.19–1.32) (, Supplemental Table S3). PARP2 was not evaluated by log-rank comparison due to its homogenously uniform expression.
PARP activity is enhanced in MPNST cell lines
PARP1 and PARP2 modify acceptor proteins through PARylation and thus influence their cellular function. Therefore, PAR expression can be used as a marker of PARP catalytic activity. We performed western blot analysis to interrogate a panel of MPNST cell lines (sporadic: MPNST724 and STS26T; NF1-associated: S462 and ST88) for endogenous PAR expression. MPNST cell lines have enhanced PAR expression, suggestive of increased PARP activity compared with normal Schwann cells (NSCs) (). Because PARP activity is stimulated by DNA damage, PARP activity can indicate the relative levels of endogenous DNA damage in MPNST cell lines. To validate the level of endogenous PARP activity in MPNST cell lines, untreated MPNST cell lines and NSCs were used for the modified Universal PARP Colormetric Assay. PARP activity was found to be more than 3-fold higher in MPNST cell lines compared to NSCs ().
AZD2281 is a highly effective single-agent inhibitor of MPNST in vitro
Before assessing the effect of AZD2281 on MPNST tumorigenicity, we wanted to establish the efficacy of this inhibitor to block PARP activity. A panel of MPNST cell lines was treated with increasing doses of AZD2281 for 24 hours and evaluated by the modified Universal PARP Colormetric Assay. AZD2281 treatment resulted in a dose-dependent decrease in PARP activity in all MPNST cell lines (). Treatment of MPNST cell lines with AZD2281 for 96 hours resulted in a dose-dependent decrease in cell proliferation as assessed by an MTS assay (). No significant effect on NSC proliferation was seen with the use of this treatment schema (Supplemental Fig. S1). No significant variation in sensitivity between NF1-associated and sporadic MPNST was found. Additionally, AZD2281 decreased the clonogenic capacity of MPNST cell lines in a dose-dependent manner (Supplemental Fig. S2). To confirm the specificity of PARP inhibitors in MPNST, we also treated 2 cell lines (MPNST724 and S462) with the PARP inhibitor ABT888. While the sensitivity to this inhibitor was less than AZD2281, ABT888 attenuated MPNST cell proliferation and clone forming ability (Fig. S3).
Figure 2. AZD2281 significantly inhibits MPNST cell growth in vitro. (A) A panel of MPNST cell lines were pretreated for 24 hours with AZD2281 and subjected to a modified PARP activity assay (26T, ST88, and 462 n = 3; 724 n = 2). (B) MPNST cell lines were treated for 96 hours with AZD2281 and cell proliferation was assessed by MTS assay (26T, 724, and 462 n = 3; ST88 n = 4). (C) PI FACS analysis after 24 hour treatment with AZD2281 in MPNST cell lines (26T and ST88 n = 3; 724 and 462 n = 4). (D) Annexin V-FITC/PI FACS analysis after 96 hour AZD2281 treatment in MPNST cell lines (n = 3). Error bars represent standard error of the mean; (*=p < 0.05; ***=p < 0.001).
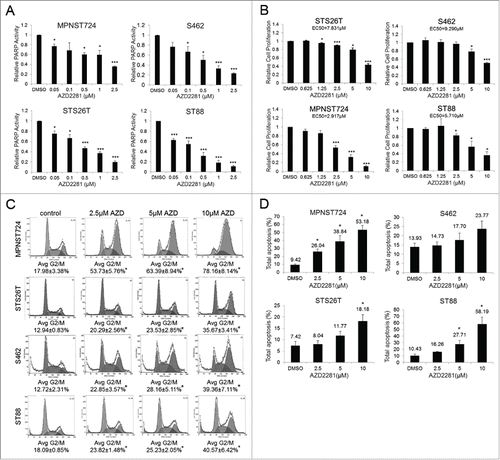
Based on the reduced cell proliferation and clone-forming ability observed, we wanted to evaluate cell cycle distribution and apoptosis after AZD2281 treatment. FACS analysis of MPNST cells after a 24 hour treatment with AZD2281 revealed a significant increase in the G2/M phase of the cell cycle (). This analysis also revealed that AZD2281 induced apoptosis in MPNST cells after 96 hours of treatment (). These in vitro responses prompted us to evaluate the functional effects of PARP inhibition on MPNST tumor growth.
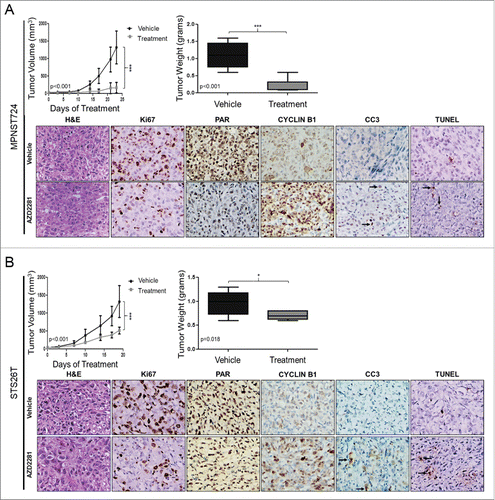
AZD2281 decreases MPNST local tumor growth
To evaluate the effect of AZD2281 on MPNST local tumor growth, we utilized 2 subcutaneous xenograft mouse models using the MPNST724 and STS26T cell lines. Treatment began when average tumor volumes reached 50 mm3. We then treated mice with daily 50 mg/kg AZD2281 (n = 8) or vehicle (n = 9) (IP). AZD2281 treatment significantly reduced tumor volume and weight compared to the vehicle group (). The effects of AZD2281 treatment on xenograft proliferation and cell death were validated by IHC analyses. AZD2281 resulted in a decrease in cell proliferation as measured by Ki67 staining. Furthermore, a decrease in the intensity of PAR staining was observed after AZD2281 treatment, supporting the specificity of the inhibitor for PARP enzymatic activity. Increased cyclin B1 staining was observed, suggesting an accumulation of cells in the G2/M phase. Furthermore, an increase in tumor cell apoptosis (TUNEL and CC3 positivity) was found after PARP inhibition. These results support our observations in vitro: MPNST724 cells were found to be slightly more sensitive to PARP inhibition than STS26T cells.
Figure 3. AZD2281 significantly inhibits MPNST local tumor growth in vivo. (A) Seventeen female hairless SCID mice were injected with 2 × 106 MPNST724 cells and treated with AZD2281 for 23 d. Treatment groups included vehicle (10% HPCB, 10% DMSO, and PBS) (n = 9) and 50 mg/kg/day AZD2281 (n = 8). Tumor volume and weight were assessed. Tumor samples stained for Ki67, PAR, cyclin B1, CC3, and TUNEL. (B) Mouse experiment proceeded as above excepting 16 mice (n = 8 per treatment group) were injected with STS26T cells and treated with AZD2281 for 19 d. Original photos were captured at 400× magnification. Error bars represent standard deviation; (*=p < 0.05; ***=p < 0.001).
PARP inhibition reduces experimental MPNST lung metastases
MPNSTs have a strong propensity to metastasize, primarily to the lung, within 2 y of initial disease presentation; therefore, we evaluated whether PARP inhibition would affect metastatic growth.Citation9,30 Because our MPNST xenograft mouse models do not spontaneously metastasize, we utilized an experimental lung metastasis model as previously described.Citation28 All mice in the vehicle group had macroscopic MPNST lung metastases, whereas 3 out of 7 (43%) had metastases in the treatment group. However, microscopic lung metastases were detected in all samples, regardless of treatment. No significant difference in lung weight between treatment groups was observed (Supplemental Fig. S4).
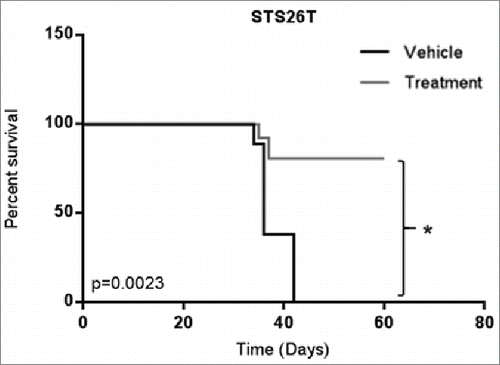
AZD2281 long-term treatment increases survival
We then evaluated whether AZD2281 treatment affected survival in a metastatic setting. To answer this question, we utilized the experimental lung metastasis model used in the prior section, but sacrificed the animals individually when euthanasia was mandated due to weight loss >20% of baseline, difficulty of breathing, hunched posture, pallor, impaired ambulation, and lethargy. Overall, treatment continued for 60 d at which point 5 out of 7 mice in the AZD2281 treatment group were alive versus 0 out of 7 mice in the control group (). These results indicated that treatment with AZD2281 significantly increased the survival of mice with metastatic disease.
Figure 4. AZD2281 significantly improves survival in an in vivo model of metastatic MPNST. Female hairless SCID mice were injected with STS26T cells in the tail vein and treated with AZD2281 for 60 d. Treatment groups included vehicle (10% HPCB, 10% DMSO, and PBS) and 50 mg/kg/day AZD2281. (* = p<0.05).
Discussion
MPNST is a devastating malignancy for which there are currently limited therapeutic options. Surgical resection has been the standard of care for this subtype of soft tissue sarcoma for decades and continues to be the most effective curative treatment. MPNSTs have a complex karyotype indicating a high baseline level of genomic instability. In addition, chromosomal aberrations and specific recurring genetic alterations have been established (such as those noted in NF1, TP53, CDKN1B, PDGFRA, and HGF), as well as deregulated receptor tyrosine kinase activity, which could be targeted for therapeutic gain in MPNST.Citation31-33 Several clinical trials have been conducted in sarcoma patients, including those with MPNST. However, the observed responses seen with targeted kinase inhibitors, such as sorafenib and imatinib, have been minimal.Citation1,34-37
PARP inhibitors have been shown to be highly effective in malignancies with defects in homologous recombination, especially those with BRCA1 mutations, or in those that have ‘BRCAness’.Citation11 AZD2281 is a well-established PARP inhibitor that has been used alone and in combination with conventional chemotherapies and radiotherapy in preclinical studies in breast and lung cancer and glioma models among others.Citation20,38,Citation39 Based on these substantial anti-tumorigenic effects, this inhibitor is now in Phase III clinical trials.
In this study, we determined the effect of the PARP inhibitor, AZD2281, in MPNST. We found that AZD2281 is highly effective as a single agent in MPNST in vitro. Specifically, we observed that AZD2281 treatment decreased cell proliferation at doses comparable to, or even less than, those used in models with established sensitizing genetic aberrations over similar, or shortened, time points.Citation40-42 Furthermore, we observed that AZD2281 significantly enhanced apoptosis. All of the MPNST cell lines that we tested have similar sensitivity profiles; however, the mechanism of sensitivity of MPNST cell lines to PARP inhibition is out of the scope of this article. Further research in MPNST is warranted to identify which DNA repair nodes contribute to MPNST sensitivity to PARP inhibition. Identification of these nodes can potentially provide insights on how to properly select MPNST patients that could benefit from this therapeutic strategy.
In this study we treated MPNST724 and STS26T xenograft mouse models starting at an average volume of 50 mm3 to determine the effect of PARP inhibition on local tumor growth. While other studies with xenograft models treat tumors when they reach approximately 200 mm3, due to the rapid growth rate of untreated MPNST xenografts, we began treatment at a smaller volume in order to treat the mice for a meaningful amount of time before euthanasia was required.Citation28,38 AZD2281 treatment of MPNST724 and STS26T xenograft mouse models resulted in a significant decrease in tumor growth when compared to the untreated group. Although no regression of disease was seen, PARP inhibition seemed to slow the progression of tumor growth. Additionally, the IHC analysis revealed a decrease in cell proliferation as determined by lower Ki67 expression; specific targeting of PARP enzymatic activity as evidenced by decreased PAR staining; a possible G2/M phase cell cycle arrest indicated by increased cyclin B1 staining; and increased CC3 and TUNEL indicating induced apoptosis. These results indicate the potential efficacy of PARP inhibition for the treatment of local MPNST disease.
We observed a decrease in the development of macroscopic lung metastases after PARP inhibition. Furthermore, survival was approximately 70% (5/7) for animals that received AZD2281 treatment compared to 0% (0/7) in the control group. These results highlight the utility of PARP inhibition as a potential MPNST therapeutic strategy to prevent, or at least decrease, tumor growth and/or metastatic spread. Additional studies of PARP inhibition in MPNST should be conducted to further validate the efficacy of AZD2281 with clinical endpoints in mind.
In this study, we evaluated the expression of PARP1 and PARP2 in neurofibroma and MPNST human tumor samples using a clinically annotated MPNST TMA. Overall, we found that all MPNST samples had high levels of PARP1 and PARP2 expression. The enhanced level of PARP expression as well as its ubiquitous expression pattern, supports the use of PARP inhibitors in MPNST. While PARP2 expression was too homogenous for further evaluation, moderate to high PARP1 expression correlated with a worse patient prognosis. Similar findings have been demonstrated in patients with breast cancer in which PARP1 overexpression correlated with reduced disease-specific survival.Citation43,44 This suggests that MPNST patients with a high level of PARP1 expression would warrant a more aggressive treatment plan or closer monitoring. While these findings are intriguing, further evaluation is necessary to determine the clinical implications of PARP staining in MPNST. Future studies should consider larger MPNST populations and include clinicopathologic factors to correlate to expression to find more associations for PARP expression.
Our study provides evidence that PARP inhibition, specifically with AZD2281, is an effective single agent therapy for MPNST. Current treatment methods are often ineffective and are typically limited to surgical excision for this orphan disease; therefore, targeted therapies are even more crucial. Future research endeavors should further investigate the clinical applicability of AZD2281 in MPNST, and determine the mechanisms underlying MPNST sensitivity to PARP inhibition.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2015CBT8474R1-f05-z-4c.pptx
Download MS Power Point (1.2 MB)2015CBT8474R1-file002.docx
Download MS Word (17.2 KB)Funding
Funding for this research was provided in part by NIH/NCI K08CA160443 (to K.T.), the Sally M. Kingsbury Sarcoma Research Foundation (to K.T.), the Amschwand Foundation (supporting C.M.), Children's Tumor Foundation (supporting C.K.), and the Texas Neurofibromatosis Foundation (to K.T.).
References
- Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, Maki RG. Malignant peripheral nerve sheath tumors. Oncologist 2014; 19:193-201; PMID:24470531; http://dx.doi.org/10.1634/theoncologist.2013-0328
- Gupta G, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Focus 2007; 22:E12; PMID:17613203; http://dx.doi.org/10.3171/foc.2007.22.6.13
- Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet 1999; 89:1-6; PMID:10469430; http://dx.doi.org/10.1002/(SICI)1096-8628(19990326)89:1%3c1::AID-AJMG3%3e3.0.CO;2-8
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986; 57:2006-21; PMID:3082508; http://dx.doi.org/10.1002/1097-0142(19860515)57:10%3c2006::AID-CNCR2820571022%3e3.0.CO;2-6
- Blakely ML, Spurbeck WW, Pappo AS, Pratt CB, Rodriguez-Galindo C, Santana VM, Merchant TE, Prichard M, Rao BN. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672-5; PMID:10359161; http://dx.doi.org/10.1016/S0022-3468(99)90353-6
- Carli M, Ferrari A, Mattke A, Zanetti I, Casanova M, Bisogno G, Cecchetto G, Alaggio R, De Sio L, Koscielniak E, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 2005; 23:8422-30; PMID:16293873; http://dx.doi.org/10.1200/JCO.2005.01.4886
- Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, Zhang W, McCutcheon IE, Slopis JM, Lazar AJ, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg 2009; 249:1014-22; PMID:19474676; http://dx.doi.org/10.1097/SLA.0b013e3181a77e9a
- Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys 1998; 42:351-60; PMID:9788415; http://dx.doi.org/10.1016/S0360-3016(98)00223-5
- Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A, Mussi C, Lozza L, Collini P, Olmi P, Casali PG, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006; 107:1065-74; PMID:16881077; http://dx.doi.org/10.1002/cncr.22098
- McCabe N, Lord CJ, Tutt AN, Martin NM, Smith GC, Ashworth A. BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of Poly (ADP-Ribose) polymerase: an issue of potency. Cancer Biol Ther 2005; 4:934-6; PMID:16251802; http://dx.doi.org/10.4161/cbt.4.9.2141
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka MZ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006; 66:8109-15; PMID:16912188; http://dx.doi.org/10.1158/0008-5472.CAN-06-0140
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays 2004; 26:882-93; PMID:15273990; http://dx.doi.org/10.1002/bies.20085
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434:917-21; PMID:15829967; http://dx.doi.org/10.1038/nature03445
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005; 434:913-7; PMID:15829966; http://dx.doi.org/10.1038/nature03443
- Plummer ER. Inhibition of poly(ADP-ribose) polymerase in cancer. Curr Opin Pharmacol 2006; 6:364-8; PMID:16753340; http://dx.doi.org/10.1016/j.coph.2006.02.004
- Meindl A, Ditsch N, Kast K, Rhiem K, Schmutzler RK. Hereditary breast and ovarian cancer: new genes, new treatments, new concepts. Dtsch Arztebl Int 2011; 108:323-30; PMID:21637635; http://dx.doi.org/10.3238/arztebl.2011.0323
- Nasuno T, Mimaki S, Okamoto M, Esumi H, Tsuchihara K. Effect of a poly(ADP-ribose) polymerase-1 inhibitor against esophageal squamous cell carcinoma cell lines. Cancer Sci 2014; 105:202-10; PMID:24219164; http://dx.doi.org/10.1111/cas.12322
- Faraonia I, Compagnone M, Lavorgna S, Angelini DF, Cencioni MT, Piras E, Panetta P, Ottone T, Dolci S, Venditti A, et al. BRCA1, PARP1 and γH2AX in acute myeloid leukemia: role as biomarkers of response to the PARP inhibitor olaparib. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis 2015; 1852:462-72; PMID:25483710; http://dx.doi.org/10.1016/j.bbadis.2014.12.001
- van Vuurden DG, Hulleman E, Meijer OL, Wedekind LE, Kool M, Witt H, Vandertop PW, Wurdinger T, Noske DP, Kaspers GJ, et al. PARP inhibition sensitizes childhood high grade glioma, medulloblastoma and ependymoma to radiation. Oncotarget 2011; 2:984-96; PMID:22184287; http://dx.doi.org/10.18632/oncotarget.362
- Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, Giri U, Peyton M, Fan YH, Diao L, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012; 2:798-811; PMID:22961666; http://dx.doi.org/10.1158/2159-8290.CD-12-0112
- Fang Y, Elahi A, Denley RC, Rao PH, Brennan MF, Jhanwar SC. Molecular characterization of permanent cell lines from primary, metastatic and recurrent malignant peripheral nerve sheath tumors (MPNST) with underlying neurofibromatosis-1. Anticancer Res 2009; 29:1255-62; PMID:19414372
- Upadhyaya M. Genetic basis of tumorigenesis in NF1 malignant peripheral nerve sheath tumors. Front Biosci (Landmark Ed) 2011; 16:937-51; PMID:21196210; http://dx.doi.org/10.2741/3727
- Peacock JD, Cherba D, Kampfschulte K, Smith MK, Monks NR, Webb CP, Steensma M. Molecular-guided therapy predictions reveal drug resistance phenotypes and treatment alternatives in malignant peripheral nerve sheath tumors. J Transl Med 2013; 11:213; PMID:24040940; http://dx.doi.org/10.1186/1479-5876-11-213
- Lazar AJ, Das P, Tuvin D, Korchin B, Zhu Q, Jin Z, Warneke CL, Zhang PS, Hernandez V, Lopez-Terrada D, et al. Angiogenesis-promoting gene patterns in alveolar soft part sarcoma. Clin Cancer Res 2007; 13:7314-21; PMID:18094412; http://dx.doi.org/10.1158/1078-0432.CCR-07-0174
- Torres KE, Zhu QS, Bill K, Lopez G, Ghadimi MP, Xie X, Young ED, Liu J, Nguyen T, Bolshakov S, et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res 2011; 17:3943-55; PMID:21540237; http://dx.doi.org/10.1158/1078-0432.CCR-11-0193
- Jin Z, Lahat G, Korchin B, Nguyen T, Zhu QS, Wang X, Lazar AJ, Trent J, Pollock RE, Lev D. Midkine enhances soft-tissue sarcoma growth: a possible novel therapeutic target. Clin Cancer Res 2008; 14:5033-42; PMID:18698021; http://dx.doi.org/10.1158/1078-0432.CCR-08-0092
- Lopez G, Liu J, Ren W, Wei W, Wang S, Lahat G, Zhu QS, Bornmann WG, McConkey DJ, Pollock RE, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res 2009; 15:3472-83; PMID:19417021; http://dx.doi.org/10.1158/1078-0432.CCR-08-2714
- Lopez G, Torres K, Liu J, Hernandez B, Young E, Belousov R, Bolshakov S, Lazar AJ, Slopis JM, McCutcheon IE, et al. Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res 2011; 71:185-96; PMID:21084276; http://dx.doi.org/10.1158/0008-5472.CAN-10-2799
- Ren W, Korchin B, Lahat G, Wei C, Bolshakov S, Nguyen T, Merritt W, Dicker A, Lazar A, Sood A, et al. Combined vascular endothelial growth factor receptor/epidermal growth factor receptor blockade with chemotherapy for treatment of local, uterine, and metastatic soft tissue sarcoma. Clin Cancer Res 2008; 14:5466-75; PMID:18765538; http://dx.doi.org/10.1158/1078-0432.CCR-08-0562
- Goertz O, Langer S, Uthoff D, Ring A, Stricker I, Tannapfel A, Steinau HU. Diagnosis, treatment and survival of 65 patients with malignant peripheral nerve sheath tumors. Anticancer Res 2014; 34:777-83; PMID:24511012
- Jhanwar SC, Chen Q, Li FP, Brennan MF, Woodruff JM. Cytogenetic analysis of soft tissue sarcomas. Recurrent chromosome abnormalities in malignant peripheral nerve sheath tumors (MPNST). Cancer Genet Cytogenet 1994; 78:138-44; PMID:7828144; http://dx.doi.org/10.1016/0165-4608(94)90081-7
- Mertens F, Dal Cin P, De Wever I, Fletcher CD, Mandahl N, Mitelman F, Rosai J, Rydholm A, Sciot R, Tallini G, et al. Cytogenetic characterization of peripheral nerve sheath tumours: a report of the CHAMP study group. J Pathol 2000; 190:31-8; PMID:10640989; http://dx.doi.org/10.1002/(SICI)1096-9896(200001)190:1%3c31::AID-PATH505%3e3.0.CO;2-
- Rahrmann EP, Watson AL, Keng VW, Choi K, Moriarity BS, Beckmann DA, Wolf NK, Sarver A, Collins MH, Moertel CL, et al. Forward genetic screen for malignant peripheral nerve sheath tumor formation identifies new genes and pathways driving tumorigenesis. Nat Genet 2013; 45:756-66; PMID:23685747; http://dx.doi.org/10.1038/ng.2641
- Bradford D, Kim A. Current treatment options for malignant peripheral nerve sheath tumors. Curr Treat Options Oncol 2015; 16:328; PMID:25777573; http://dx.doi.org/10.1007/s11864-015-0328-6
- Maki RG, D'Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009; 27:3133-40; PMID:19451436; http://dx.doi.org/10.1200/JCO.2008.20.4495
- Kim A, Dombi E, Tepas K, Fox E, Martin S, Wolters P, Balis FM, Jayaprakash N, Turkbey B, Muradyan N, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer 2013; 60:396-401; PMID:22961690; http://dx.doi.org/10.1002/pbc.24281
- Robertson KA, Nalepa G, Yang FC, Bowers DC, Ho CY, Hutchins GD, Croop JM, Vik TA, Denne SC, Parada LF, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol 2012; 13:1218-24; PMID:23099009; http://dx.doi.org/10.1016/S1470-2045(12)70414-X
- Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 2008; 105:17079-84; PMID:18971340; http://dx.doi.org/10.1073/pnas.0806092105
- Dungey FA, Loser DA, Chalmers AJ. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-Ribose) polymerase: mechanisms and therapeutic potential. Int J Radiat Oncol Biol Phys 2008; 72:1188-97; PMID:18954712; http://dx.doi.org/10.1016/j.ijrobp.2008.07.031
- Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer MJ, Smith G, Powell JE, Rudzki Z, Kearns P, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood 2010; 116:4578-87; PMID:20739657; http://dx.doi.org/10.1182/blood-2010-01-265769
- Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A, Dillon KJ, Drzewiecki J, Garman S, Gomez S, et al. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin- 1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem 2008; 51:6581-91; PMID:18800822; http://dx.doi.org/10.1021/jm8001263
- Williamson CT, Muzik H, Turhan AG, Zamo A, O'Connor MJ, Bebb DG, Lees-Miller SP. ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol Cancer Ther 2010; 9:347-57; PMID:20124459; http://dx.doi.org/10.1158/1535-7163.MCT-09-0872
- Goswami J, Goyal S, Wu H, Moran MS, Haffty BG. Poly(ADP-ribose) polymerase-1 (PARP-1) expression in patients treated with breast-conserving surgery and radiation therapy (BCS plus RT). J Clin Oncol 2010; 28: 15s: 582
- Gennari ASM, Varesco L, et al. Prognostic significance of BRCA1, PARP1 and PARP2 in sporadic breast cancer. J Clin Oncol 2009; 27s:e22114
