ABSRTRACT
Both Epidermal Growth Factor Receptor (EGFR) and the Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) play critical roles in tumorigenesis. We hypothesized co-targeting EGFR and VEGFR2 using a bispecific antibody might have significant therapeutic potential. Here,we designed and produced a human IgG-like bispecific antibody (Bi-Ab) based on the variable regions of cetuximab (an anti-EGFR antibody) and mAb-04 (an anti-VEGFR2 antibody developed in our lab) . The Bi-Ab was found to inhibit the proliferation, survival and invasion of cancer cells via ablating phosphorylation of receptor and downstream signaling. In vivo efficacy was demonstrated against established HT-29 and SKOV-3 xenografts grown in nude mice. Studies revealed our Bi-Ab was able to restrain xenografted tumor growth and prolong survival of mice through inhibiting cell proliferation,promoting apoptosis and anti-angiogenesis. In contrast to cetuximab or mAb-04 alone, our Bi-Ab exhibits enhanced antitumor activity and has equal or slightly superior activity to their combination (Combi). It shows promise as a therapeutic agent, especially for use against tumors EGFR and/or VEGFR2 over-expressing malignancies.
Abbreviations
| EGFR | = | epidermal growth factor receptor |
| VEGFR2 | = | vascular endothelial growth factor receptor 2 |
| NSCLC | = | non-small cell lung cancers |
| VEGF | = | vascular endothelial growth factor |
| MAPK | = | mitogen-activated protein kinase |
| AKT | = | protein kinase B |
| JNK | = | c-Jun N-terminal kinase; |
| CHO | = | chinese Hamster Ovary |
| NO | = | nitric oxide |
| PCR | = | Polymerase Chain Reaction |
| SPR | = | surface plasmon resonance |
Introduction
Epidermal Growth Factor Receptor (EGFR) is the cell-surface receptor for some extracellular protein ligands, such as epidermal growth factor family.Citation1 It has been involved in various malignancies such as non-small cell lung cancers (NSCLC) and colon cancers.Citation2-5 EGFR is activated through interaction with its specific ligands, then undergoes a transition from an inactive monomeric form to an active homodimer.Citation6 Moreover, EGFR may pair with another member of the ErbB receptor family to create an activated heterodimer. The dimerization irritates its intrinsic intracellular protein-tyrosine kinase activity, which promotes autophosphorylation of several tyrosine (Y) residues in the C-terminal domain of EGFR, including Y992, Y1045, Y1068, Y1148 and Y1173.Citation7 The downstream signaling proteins trigger several signal transduction cascades, principally the mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK) and protein kinase B (AKT) pathways.Citation8 Additionally, activation of EGFR improves angiogenesis by enhancing vascular endothelial growth factor (VEGF) levels and increasing cellular levels of nitric oxide (NO).Citation9
Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) is a member of VEGF receptor family, which regulate the formation of blood and lymphatic vessel.Citation10 VEGFR2 is expressed by endothelial cells and various cancers including breast cancer and NSCLC.Citation11-12 As the principal receptor leading angiogenesis in tumoral tissue, VEGFR2 has potent tyrosine kinase activity and is primarily associated with the induction of angiogenesis mediated by VEGF.Citation13 VEGF-induced homodimerization of VEGFR2 leads to a strong autophosphorylation of VEGFR2 on tyrosine residues.Citation14 Once autophosphorylated, VEGFR2 activates MAP-kinase and DNA synthesis, leading to pathological angiogenesis.Citation15 Other VEGFR2 dependent pathways reported include PI3K-PKB-AKT focal adhesion kinase, Src kinase, Rho family of GTPases, and other multifunctional docking proteins and adaptors.Citation16 Therefore, VEGFR2 could be used as a target for tumor therapy.Citation17-20
Cetuximab, a monoclonal antibody targeting EGFR, is used to treat a lot of cancers. Unfortunately, majority of patients acquire resistance in the end.Citation21 The EGFR-independent activation of the AKT and MAPK pathway (such as VEGFR2-inducing) may be one of the resistance mechanisms.Citation22 Although, blocking VEGF/VEGFR2 with antibody such as the ramucirumab (a VEGFR2 antibody) is a strategy for tumor therapy, EGF/EGFR-induced VEGF production may limit the effect of this strategy.Citation23
Based on the literature we hypothesized co-targeting EGFR and VEGFR2 may be beneficial in minimizing the activation of MAPK and AKT signaling and inhibition of angiogenesis for the treatment of EGFR related cancers. Previously, we reported a human anti-VEGFR2 antibody (mAb-04), which inhibits the activation of VEGFR2.Citation24 In this report, we designed and produced a bispecific antibody (Bi-Ab) with the variable regions from 2 different antibodies. The Bi-Ab is expected to demonstrate superior antitumor activity to the parental antibody and could therefore be applied to EGFR- and VEGFR2-overexpressing malignancies.
Results
Bi-Ab targets EGFR and VEGFR2
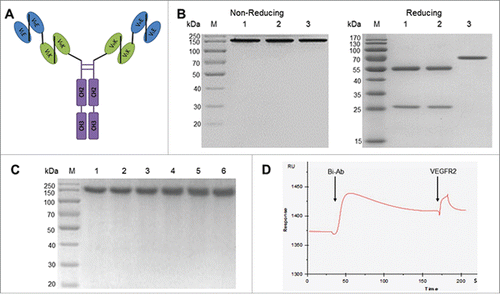
We generated Bi-Ab from the DNA sequences of anti-EGFR/anti-VEGFR2 taFv and the human IgG1 Fc fragment. The presence of hinge region in the Fc resulted in the production of stable dimmers () that showed stability at 37℃ for 15 d (). SDS-PAGE analysis of Bi-Ab under non-reducing conditions yielded a protein band of ~150kDa (similar to cetuximab and mAb-04). Under reducing conditions, Bi-Ab yielded a single protein band of ~78kDa with expected mobility. As controls, cetuximab and mAb-04 gave 2 major bands: the IgG light chain (~25kDa) and the IgG heavy chain (~50kDa). Results of SPR analysis showed that the Bi-Ab can bind both EGFR and VEGFR2 simultaneously. ()
Figure 1. The structure, stability and bispecificity of Bi-Ab. Structure of the anti-EGFR/VEGFR2 bispecific antibody (A) VLE, variable light region of cetuximab; VHE, variable heavy region of cetuximab; VLK, variable light region of mAb-04; VHK, variable heavy region of mAb-04. SDS-PAGE analysis of the purified Bi-Ab (B) Lane M, marker; lane 1, cetuximab; lane 2, mAb-04; lane 3, Bi-Ab. SDS-PAGE was used to analyze the thermostability of Bi-Ab (C) Lane M, marker; lane 1–6, sample incubated for 0, 3, 6, 9, 12 and 15 d at 37℃. The surface plasmon resonance spectroscopy analysis (D) VEGFR2 was injected after Bi-Ab were flowed over the EGFR-immobilized sensor chip. RU, resonance units.
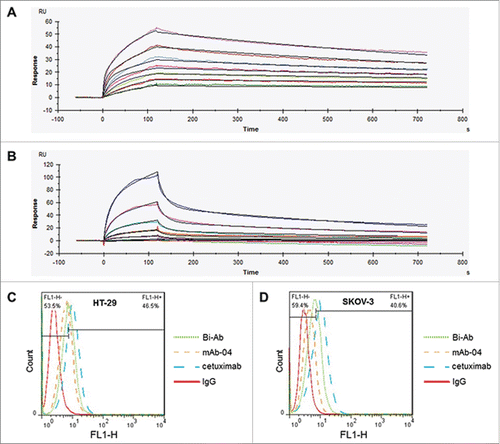
SPR analysis was employed to measure the binding affinities of Bi-Ab to EGFR and VEGFR2 (). The data indicated that the affinity of Bi-Ab to EGFR/VEGFR2 is similar to mAb-04 and cetuximab (). The binding affinities of cetuximab, mAb-04 and Bi-Ab to HT-29 or SKOV-3 cells were investigated with flow cytometer. All the 3 antibodies demonstrated relatively high binding affinity to HT-29 and SKOV-3 cells (). The binding levels of 10 μg/ml Bi-Ab with HT-29 / SKOV-3 cells were 46.5 / 40.6 %, that of mAb-04 and cetuximab were 33.5 / 27.1% and 61.4 / 64.0%, respectively.
Table 1 SPR analysis of the binding affinities of antibodies to EGFR or VEGFR2.
Figure 2. The binding of Bi-Ab to recombinant EGFR/VEGFR2 ectodomains and membrane-associated EGFR/VEGFR2. Surface plasmon resonance spectroscopy was used to analysis the binding kinetics of Bi-Ab to recombinant EGFR and VEGFR2 ectodomains ((A) and B). The equilibrium dissociation rate constant (KD) of Bi-Ab to EGFR or VEGFR2 were 1.45×10 −9M and 2.37×10 −9M respectively. Flow cytometry was used to investigate the binding of Bi-Ab to membrane-associated EGFR and VEGFR2 ((C)- F). The histogram overlay showed that Bi-Ab binds to HT-29 and SKOV-3 cells line with a relatively high binding levels.
Bi-Ab inhibits phosphorylation of EGFR and VEGFR2 and down-regulates PI3K/AKT and MAPK signaling
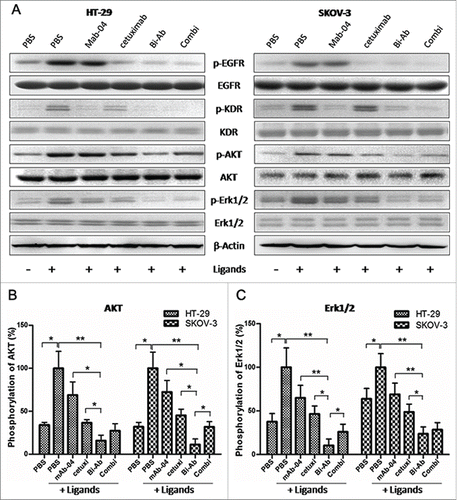
To analyze the synergistic blocking of both EGFR and VEGFR2 through the antibodies (Combi or Bi-Ab) in PI3K/AKT and MAPK signaling pathway, we examined the phosphorylation of EGFR, VEGFR2, AKT and Erk1/2 () in HT-29 and SKOV-3 cells treated with the antibodies. We first examined the effect of the antibodies on tyrosine phosphorylation of EGFR and VEGFR2 activated by EGF and VEGF. The phosphorylation of EGFR and VEGFR2 were blocked by cetuximab and mAb-04 respectively. Further, both EGFR and VEGFR2 were blocked by Bi-Ab or Combi. Either cetuximab or mAb-04 inhibited the phosphorylation of AKT and Erk1/2 incompletely in the presence of ligands (EGF and VEGF). However, the Bi-Ab and the Combi significantly inhibited the phosphorylation of AKT and Erk1/2 (). These results suggested that co-targeting EGFR and VEGFR2 with Bi-Ab has superior antitumor activity on co-expression high levels of EGFR and VEGFR2 in cancer cells.
Figure 3. Bi-Ab inhibits phosphorylation of EGFR and VEGFR2 and down-regulates AKT and MAPK signaling. (A) Western blot to show the inhibition on phosphorylation of EGFR, VEGFR2, AKT and Erk1/2 in HT-29 and SKOV-3 cells after antibodies treatment. ((B) and C) Phosphorylation level of AKT and Erk1/2 in HT-29 and SKOV-3 cells. The data presented as the mean ± SD, are from a representative experiment, n = 3. *P < 0.05; **P < 0.01.
Bi-Ab effectively inhibits proliferation and invasion of cancer cell, and preserves antibody-dependent cell-mediated cytotoxicity (ADCC) activity
MTT assay was used to analyze the effect of Bi-Ab on HT-29 and SKOV-3 cells proliferation. The results showed that, Bi-Ab treatment effectively inhibited the proliferation of HT-29 and SKOV-3 cells with EGF and VEGF stimulated in dose-dependent manner in vitro (). Notably, although Combi treatment showed enhanced inhibition of HT-29 and SKOV-3 proliferation compared with cetuximab or mAb-04 treatment alone, all the other treatments showed less potent than Bi-Ab, especially at high antibody concentrations (over 6nM for HT-29, over 125nM for SKOV-3). When stimulated with EGFR/VEGFR2, inhibition levels (%) of Bi-Ab on HT-29 / SKOV-3 was about 70 / 53 at most, that of mAb-04, cetuximab and Combi were 16 / 18, 37 / 27 and 44 / 39, respectively.
Figure 4. Bi-Ab showed the most effective inhibition of proliferation on HT-29 and SKOV-3 cells compared to mAb-04, cetuximab or Combi with EGF and VEGF stimulated ((A)and B). Three independent experiments were performed in triplicate, the means ± SD of triplicate experiment are shown, *P < 0.05; **P < 0.01 versus treatment with Bi-Ab treatment. Photomicrographs of transwell invasion assay indicated that Bi-Ab could effectively inhibit the invasion of HT-29 and SKOV-3 cells induced by EGF and VEGF ((C)and D). Quantitative analysis of the transwell invasion assay showing that Bi-Ab treatment significantly increased the inhibition of HT-29 and SKOV-3 cells invasion when compared to mAb-04 and cetuximab. The data presented as the mean ± SD, are from a representative experiment, 5 independent experiments were performed in triplicate, *P < 0.05; **P < 0.01. Percent ADCC of the antibodies on HT-29 and SKOV-3 (E). The data presented as the mean ± SD, each antibody was tested in triplicate, the assays were repeated once, n = 3, *P < 0.05.
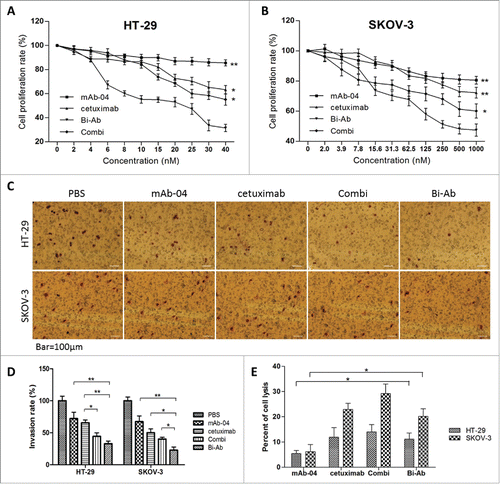
The effect of Bi-Ab on HT-29 and SKOV-3 cells invasion was analyzed by Transwell assay. The invasion was significantly reduced with the different antibodies, and the Bi-Ab demonstrated high inhibitory potential on HT-29 and SKOV-3 invasion than cetuximab and mAb-04 alone or Combi.().
Additionally, Bi-Ab showed comparable or slightly lower ADCC activity than cetuximab, however it was significantly higher than that of mAb-04, all the treatment conditions were less potent than that of Combi (). These data suggest that Bi-Ab remains effective in killing EGFR- and/or VEGFR2-overexpressing tumor cells through ADCC in vivo.
Bi-Ab induces cancer cell apoptosis, inhibits endothelial tube formation
Apoptosis assay was carried out to assess the ability of Bi-Ab to stimulate apoptosis in HT-29 and SKOV-3 cells. Annexin V-FITC/PI Vybrant apoptosis assay kit was used. The Bi-Ab demonstrated a more potent apoptotic stimulating potential than cetuximab, mAb-04 and Combi in SKOV-3 cells in vitro. However, HT-29 cells were insensitive to the treatment of all antibodies ().
Figure 5. The apoptosis was analyzed by flow cytometry, endothelial tube formation was performed using HUVECs tube formation assay. ((A)and C) Bi-Ab treatment increased apoptosis in SKOV-3 cells than cetuximab and mAb-04 alone or Combi, but not HT-29. (B) HUVEC tube-like photomicrographs showing the significant effects of Bi-Ab on HUVECs tube formation. (D) Similar to the Combi, Bi-Ab demonstrated relatively more potent restraining effect on tube formation by HUVEC cells compared to mAb-04 or cetuximab. Three independent experiments were performed in triplicate, the means ± SD of triplicate experiment are shown (*P <0 .05; **P <0 .01 vs. Bi-Ab treatment).
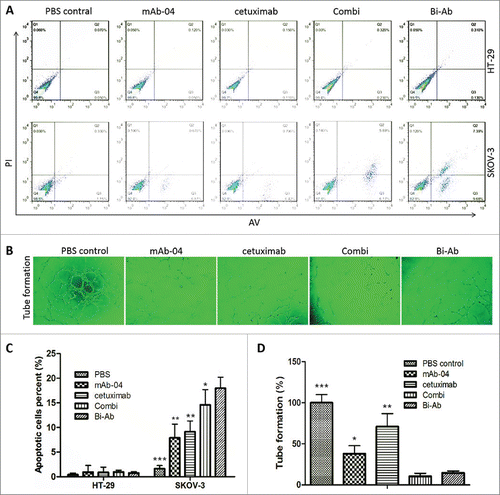
Since EGFR signaling and VEGFR2 signaling have been demonstrated to enhance angiogenesis,Citation10,25 the tube formation assay was carried out to investigate the anti-angiogenic potential of Bi-Ab, as against cetuximab or mAb-04 on tube formation by HUVEC cells. Similar to the Combi, Bi-Ab demonstrated relatively more potent restraining effect on tube formation by HUVEC cells compared to mAb-04 or cetuximab ().
Bi-Ab shows potent antitumor effect in HT-29 and SKOV-3 xenograft models
Balb/C nude mice xenografted with HT-29 and SKOV-3 tumors were treated with antibodies. PBS-treated tumors grew rapidly, whereas tumors were inhibited in different extent with the different antibodies (). Compared with PBS, mAb-04 or cetuximab treatment, Bi-Ab treatment significantly inhibited the growth of HT-29 or SKOV-3 tumors xenografts.
The survival rates of HT-29 and SKOV-3 tumor-bearing mice were compared following the 5 different treatment regimens (). Median survival times and terminal survival rate of HT-29/SKOV-3 tumor-bearing mice for the 5 different groups are shown in . These studies showed that the Bi-Ab treatment did not only demonstrate better inhibition of tumor growth but also prolonged median survival of xenograft-bearing animals.
Table 2 Median survival and 100-day survival rate (%).
Effect of Bi-Ab on proliferation, apoptosis and angiogenesis in vivo
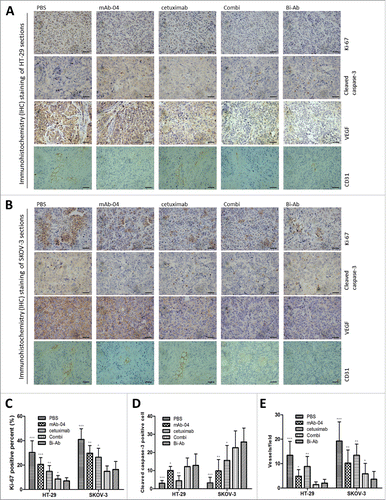
To further investigate the anti-tumor mechanisms of Bi-Ab in vivo, the proliferation, apoptosis and angiogenesis in the tissue sections of the tumors were immunohistochemically analyzed. Cell proliferation and apoptosis were evaluated with Ki-67 and cleaved caspase-3 staining methods respectively. In addition, anti-VEGF and CD31 antibodies were used to evaluate tumor angiogenesis (). These results are consistent with the in vitro studies. Bi-Ab and the Combi, significantly reduced the percentage of Ki-67-positive cells compared to cetuximab or mAb-04 alone (). Contrary to the cell line study, the results of cleaved caspase-3 staining demonstrated that Bi-Ab treatment induced significant apoptosis in HT-29 and SKOV-3 tumors (). The VEGF and CD31 staining showed that, treatment with Bi-Ab significantly reduce blood vessel density compared with mAb-04 or cetuximab treatment ().
Figure 6. The Bi-Ab shows potent antitumor effect on HT-29 and SKOV-3 tumor xenografts in nude mice. ((A) and B) Bi-Ab suppressed tumor growth, tumor diameter was measured with a vernier caliper (*P < 0.05; **P < 0.01; ***P <0 .005 versus treatment with Bi-Ab). The survival rates of HT-29 and SKOV-3 tumor-bearing mice ((C)and D). The median survival and terminal survival rate were shown in .
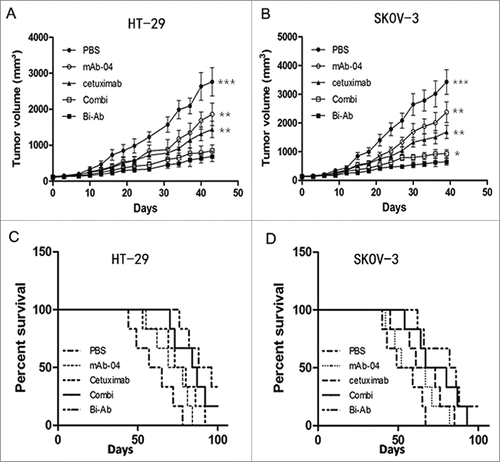
Figure 7. Immunohistochemical analysis was used to measure the effect of Bi-Ab treatment on proliferation, apoptosis and angiogenesis in vivo. In HT-29 (A) and SKOV-3 (B) tumors, proliferation and apoptosis were evaluated with Ki-67 and cleaved caspase-3 staining methods respectively, VEGF and CD31 were used to evaluate tumor angiogenesis. (C) Proliferative cells (Ki-67 positive cells) decreased in Bi-Ab-treated HT-29 and SKOV-3 tumors, and it was more effective compared to mAb-04- and cetuximab-treated tumors. (D) Apoptotic cells (cleaved caspase-3 positive cells) increased in Bi-Ab-treated HT-29 and SKOV-3 tumors. (E) The density of CD31-positive blood vessels decreased in Bi-Ab-treated HT-29 and SKOV-3 tumors. The data presented as the mean ± SD, are from a representative experiment, n = 5, *P < 0.05; **P < 0.005; ***P < 0.0005 vs. Bi-Ab treatment.
Discussion
EGFR has been involved in various malignancies such as NSCLC, colon, breast, head and neck, and pancreatic cancers. VEGFR2 is over-expressed in various cancers such as breast cancer and non-small cell lung cancer. It has been reported that inhibition of EGFR or VEGFR2 signaling restrains the proliferation of ovarian tumors.Citation23,26 In addition, the combination of EGFR and VEGFR2 antagonists enhanced anti-tumor effect, which suggests EGFR and VEGFR2 co-targeted therapy has therapeutic potential in colon tumors.Citation27-28 In this article, we designed a bispecific antibody based on a known EGFR antibody and our VEGFR2 antibody. Its efficacy was evaluated both in vitro assays and nude mice tumor xenograft models, indicating co-targeting EGFR and VEGFR2 may be of clinical relevance in treating EGFR and/or VEGFR2 overexpressing malignant.
The functional affinity of Bi-Ab to EGFR or VEGFR2 were measured by SPR. The result showed that there were disparity between the level of signal in the SPR experiments and the predicted Rmax based on the molecular weights of the ligand and analyte. This may be explained by the inactivation of ligands in immobilization and the steric inhibition of binding for all the ligands and analytes are biomacromolecules. Further investigation will be needed in future.
It has been reported that, both EGFR and VEGFR2 contribute to the activation of MAPK and AKT signaling in cancer cells and angiogenesis in tumors.Citation8,16 Activation of MAPK and AKT pathways mediated by mutations or amplification of cell surface receptors is a frequent event in human cancers, which leads to molecular alterations in genes encoding key components of the pathways.Citation29–31 These intracellular signaling pathways regulate proliferation, motility, differentiation and survival of cancer cells. The PI3K and MAPK pathways interact in multiple ways that limit the activity of targeting the MAPK or the PI3K pathways.Citation32–33 In cancer cells which express both EGFR and VEGFR2, MAPK and AKT signals are regulated by both EGFR and VEGFR2. Hence, simultaneous targeting to both receptors would yield an enhanced inhibition in the signal transduction. We found that both Bi-Ab and Combi treatment inhibited phosphorylation of AKT and Erk1/2 more thoroughly compared with cetuximab or mAb-04 treatment alone (). Interestingly, the effect of co-targeting EGFR- and VEGFR2-overexpressing cancer cells with Bi-Ab has slightly superior activity to the Combi, which is presumably due to the enhanced inhibition of AKT and MAPK signal pathway and ligand-induced EGFR phosphorylation in HT-29 cells and SKOV-3 cells (). Nonetheless, intensive understanding will be further needed through investigating whether heterodimer exists between EGFR and VEGFR2 and whether it contributed to this result via steric to antibodies (cetuximab and mAb-04).
ADCC, an important mechanism of action for antitumor antibodies, is mediated by Fc fragment. ADCC assay demonstrates that the Fc mediated cytotoxicity activity of Bi-Ab was significantly higher than mAb-04 and slightly lower than cetuximab. This phenomenon may be explained by the same Fc fragment in antibodies and the different binding affinity of antibodies to cancer cells ().
Angiogenesis is a critical step in tumor progression, providing nutrients and oxygen for the proliferation and metastasis of the tumor cells. VEGF and its receptors (VEGFR1, VEGFR2 and Neuropilin1) are key regulators of angiogenesis and have been the key targets for anti-angiogenesis therapy in recent times.Citation34 In this study, targeting VEGFR2 was used to suppress angiogenesis. Surprisingly, co-targeting EGFR and VEGFR2 was more effective to suppress angiogenesis than VEGFR2-targeting alone, which may be due to the decrease in the production of VEGF by EGFR-inhibition (). Although, VEGFR2 is the main receptor of VEGF in angiogenesis, VEGF also induces angiogenesis through VEGFR1 and Neuropilin1.Citation35–36 As such, targeting EGFR decreases VEGF production, resulting in angiogenesis suppression from synergistic VEGFR2-targeting.Citation9
Our current data showed that none of the treatments induced apoptosis in HT-29 cells (). The failure of Bi-Ab on stimulating apoptosis of HT-29 is partly due to coexpression of multiple EGFR family members such as EGFR, HER2 and HER4, especially HER2.Citation37 HER2 stimulate S-phase proliferation and inhibit apoptosis by increasing the expression of Wilms' Tumor 1 protein in cancer cells.Citation38 Degradation of HER2 induces apoptosis in HER2-overexpressing cancer cells.Citation39-40
Our data suggest that Bi-Ab has anti-angiogenic effect in vivo. Antiangiogenic therapy lessened leakage of plasma proteins from microvessels in the tumor tissue, and reduced vascular density and blood flow in total tumor. This may contribute to the initiation of tumor cell apoptosis.Citation41 It has also been reported that, anti-VEGFR2 antibody was able to promote apoptosis in vivo.Citation42 These could account for the reason why the Bi-Ab faliled in promoting apoptosis in HT-29 cells in vitro, but stimulated apoptosis in HT-29 tumor xenograft ().
In conclusion, co-targeting EGFR and VEGFR2 with Bi-Ab or Combi has great anti-cancer effect both in vitro and in vivo. We found that Bi-Ab has equal or slightly enhanced efficacy compared with the Combi. These results indicate that antitumor synergy of combined EGFR and VEGFR2 targeted therapy is more effective than single targeting in inhibiting EGFR- and/or VEGFR2 over-expressing tumors. With lower cost in manufacture and improved efficacy, the Bi-Ab has more potential in targeted therapy for EGFR and/or VEGFR2 over-expressing tumors.
Materials and methods
Construction of Bi-Ab
Employing the reported method, we effectively generated an anti-EGFR/anti-VEGFR2 tandem single-chain variable fragment (taFv) with variable regions of cetuximab targeting EGFR and mAb-04 targeting VEGFR2. The DNA of the taFv was then fused to the Fc fragment of human IgG1 with linker (GGGGS) through overlap PCR. The fusion DNA and pMH3 vector were digested with NotI and XbaI and the resulting fragments joined using T4 DNA ligase. Nucleotide sequence of the recombinant vector (pMH3-taFv-Fc) was confirmed by Sangon Biotech. (Shanghai, China).
Expression and purification of Bi-Ab
After confirmed, the recombinant expression vector (pMH3-taFv-Fc) was transfected into CHO-s cell line by electroporation. After screening stable transfectants with 1mg/ml G418, Bi-Ab was expressed by CHO-s cells, and purified from the culture supernatants using Protein A affinity purification. Subsequent to purification, samples of Bi-Ab (200mM in PBS) were incubated for 0, 3, 6, 9, 12 and 15days at 37 °C respectively and then analyzed by SDS-PAGE.
Surface plasmon resonance spectroscopy
The functional affinity between Bi-Ab and EGFR or VEGFR2 were analyzed with surface plasmon resonance (SPR) spectroscopy using Biacore system (Biacore X100, GE Healthcare). EGFR/VEGFR2 (ligand) was immobilized on Sensor Chip CM5 (GE Healthcare, BR-1000–12) up to 1000 resonance units. Different concentrations of Bi-Ab or cetuximab/mAb-04 (analyte, 40–0.625nM, fold2- serially diluted) in running buffer (HBS-EP, pH7.4) flowed over the ligand on Sensor Chip CM5. Because the Bi-Ab being bivalent to both EGFR and VEGFR2 (), association rate constant ka and dissociation rate constant kd were determined by Bivalent analyte with the assumption of a 2:1 binding model.
Flow cytometry
After washing twice with phosphate buffered saline (PBS), 5×105 HT-29 or SKOV-3 cells were incubated at 4℃ for 1 h with 10μg/ml antibodies (mAb-04, cetuximab, Bi-Ab or isotypical IgG) in PBS containing 2% fetal bovine serum (FBS). Cell surface bound antibodies were detected with FITC-conjugated goat anti-human IgG antibody (SANGON, Shanghai, China) by incubation at 4℃ for 1 h followed by washing twice with PBS. The cells binding assay was performed with a BD FACS flow cytometer and FlowJo software.
Proliferation assays
HT-29 or SKOV-3 cells were plated on 96-well plates with a final concentration of 2,000 cells per well. After overnight incubation, cells were treated with different concentrations of the antibodies (in media supplemented with 2% FBS containing or not containing 10ng/ml EGF and 10ng/ml VEGF). The plates were then incubated at 37℃ with 5% CO2 for 48 h. The cell proliferation level was detected with MTT assay and the inhibitory rates expressed as percentages of the vehicle control (100%).
Transwell invasion assays
Five×104 HT-29 or SKOV-3 cells with or without the antibodies (100nM) were suspended in serum-free medium and added to the upper chamber of 24-well transwell chambers (Millipore, PIEP12R48) coated with 20μl Matrigel (BD Biosciences, 356234) on the bottom. The lower chambers were filled with 600 μl McCoy's 5A (for HT-29 cells) / RPMI-1640 (for SKOV-3 cells) containing 10% FBS, 10ng/ml EGF and VEGF, incubated for 24h (HT2–9) or 12h (SKOV-3). Invaded cells were stained with 1% (w/v) crystal violet and washed thrice with water. Images were then captured with an OLYMPUS inverted microscope at ×100 magnification and cells counted with Image-pro-plus program.
Apoptosis assays
At 70% confluence, HT29 cells and SKOV3 cells were treated with the antibodies (100nM) for 48 hours. They were harvested with trypsin, washed with PBS and centrifuged at 500g for 5 min at 4℃. The apoptotic or necrotic death induced by the antibodies in cells was measured with Annexin V-FITC / PtdIns Vybrant apoptosis assay kit (KeyGEN BioTECH, KGA108). The data of apoptosis was determined with a BD FACS flow cytometer, and FlowJo software was used for the data analysis.
Tube formation assay
Matrigel (BD Biosciences) was coated on a 96-well tissue culture plate and incubated at 37℃ for 1 h to solidify. HUVECs (2×104 ) in 100μl ECM supplemented with 2% (v/v) FBS and 1% (v/v) ECGS containing 1ng EGF and VEGF were added in each well. It was then incubated with antibodies (50nM) for 8h. After incubation, endothelial tube formation was photographed and quantified.Citation24
ADCC assay
Target cells (HT-29 or SKOV-3, 2 × 104 per well) were incubated with 5μg/ml antibodies at 37°C for 30 minutes in McCoy's 5A (for HT-29 cells) / RPMI-1640 (for SKOV-3 cells) containing 10% FBS and 2mM GlutaMax™. Subsequently, effector cells (PBMCs, isolated from healthy human donor blood) were added at an effector/target ratio of 50:1. After overnight incubation, lactate dehydrogenase release was measured and then cell-mediated cytotoxicity of the target cells measured as follows: Cytotoxicity (expressed as a percentage) = (experimental lysis - spontaneous lysis) / (maximum lysis - spontaneous lysis) × 100.
Western blotting
At 80% confluence, cells were incubated in serum-free medium overnight, then incubated with the antibodies for 30 min, followed by induction with ligands (10ng/ml EGF and VEGF) for 30 min. Cells were then lysed and harvested with RIPA buffer (Beyotime, P0013C). Proteins were resolved by electrophoresis then transferred onto PVDF membranes.Citation24 The membranes were blocked and incubated with the following primary mAbs purchased from Cell Signal Technology: rabbit anti-P44/42 MAPK (4695), rabbit anti-Phospho-p44/42 MAPK (4376), rabbit anti-AKT (4691), rabbit anti-Phospho-AKT (4060), rabbit anti-Phospho- VEGFR2 (2478), rabbit anti- VEGFR2 (9698), rabbit anti-EGFR (4267), rabbit anti-Phospho-EGFR (3777), and rabbit anti-â-Actin (4970). Anti-rabbit antibody conjugated to horseradish peroxidase (HRP) and enhanced ECL chemiluminescence reagent (Millipore, WBKLS0500) were used to visualize protein bands. The membranes were then exposed with Bio-Rad detection system.
In vivo tumor xenograft studies
Six-weeks-old nude BALB/c female mice were purchased from the Animal Center of the Yangzhou University. The animal study was done under the protocol approved by the Animal Center of the Yangzhou University. SKOV-3 (5×106 ) or HT-29 (3×106 ) cells were resuspended in 100μl PBS and injected subcutaneously into each nude mouse. Mice were randomly allocated into 6 groups (n = 6 for each treatment group): PBS control, 5mg/kg mAb-04, 5mg/kg cetuximab, Combi (5mg/kg MAb-04 and cetuximab), 5mg/kg Bi-Ab. After the xenograft tumors had reached 50 mm3, drugs were injected intraperitoneally (I.P.) into each mouse 3 times a week for the duration of the treatment regimen.Citation43 Tumor diameter was measured with caliper, and the volume calculated with the formula: ð/6 × larger diameter × (smaller diameter).Citation2 Mice were killed when the tumor load reached 4,000 mm3, and tumor tissues were harvested.
Immunohistochemistry (IHC) staining and analysis
Xenograft tissues samples (n = 6 for each treatment group) were harvested (from 2.10), fixed in 4% formaldehyde/PBS and embedded in paraffin. Sections were incubated with rabbit anti-cleaved caspase-3 (Cell Signaling Technology, 9661), rabbit anti-Ki-67 (Cell Signaling Technology, 9027), rabbit anti-VEGF (Santa Cruz Biotechnology, sc-152), or rabbit anti-CD31 (Cell Signaling Technology, 3528). The sections were then stained with Peroxidase/DAB and examined in 5 randomly selected areas from each slide at ×100 magnification. Necrotic areas were excluded from analysis.
Statistical analysis
Excel and SPSS 17.0 software were used to analysis the data we got. Results were presented as mean ± SD of the mean (s.e.m.). Student's t-test was used to determine the statistical significance, GraphPad Prism 5 software program were used for figures production and log rank test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC81102364, NSFC81273425 and NSFC81473125) and Specialized Research Fund for the Doctoral Program of Higher Education (20130096110007). China Scholarship Council and Jiangsu Province Qinglan Project (2014). A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004; 59(2 Suppl):21-6; PMID:15142631; http://dx.doi.org/10.1016/j.ijrobp.2003.11.041
- Lee CC, Shiao HY, Wang WC, Hsieh HP. Small-molecule EGFR tyrosine kinase inhibitors for the treatment of cancer. Expert Opin Investig Drugs 2014; 23(10):1333-48; PMID:24921970; http://dx.doi.org/10.1517/13543784
- Chen C, Zhang Y, Zhang Y, Li J, Tsao SW, Zhang MY. Superior antitumor activity of a novel antibody cotargeting human epidermal growth factor receptor 2 and type I insulin-like growth factor receptor, Mol Cancer Ther 2014 Jan; 13 (1):90-100; PMID:24227890; http://dx.doi.org/10.1158/1535-7163
- Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest 2007; 117(8):2051-8; PMID:17671639; http:dx.doi.org/10.1172/JCI32278
- Luca T, Barresi V, Privitera G, Musso N, Caruso M, Condorelli DF, Castorina S. In vitro combined treatment with cetuximab and trastuzumab inhibits growth of colon cancer cells. Cell Prolif 2014; 47(5):435-47; PMID:25131935; http://dx.doi.org/10.1111/cpr.12125
- Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor, Biochemistry 1987; 26 (5):1443-51; PMID:3494473
- Batzer AG, Rotin D, Ureña JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol 1994 Aug; 14(8):5192-201; PMID:7518560
- Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 2005; 1:2005.0010; PMID:16729045; http:dx.doi.org/10.1038/msb4100014
- Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther 2011 Jul; 131(1):80-90; PMID:21439312; http://dx.doi.org/10.1016/j.pharmthera.2011.03.012
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 2006 May; 7(5):359-71; PMID:16633338; http://dx.doi.org/10.1038/nrm1911
- Guo S, Colbert LS, Fuller M, Zhang Y, Gonzalez-Perez RR. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim Biophys Acta 2010 Aug; 1806(1):108-21; PMID:20462514; http://dx.doi.org/10.1016/j.bbcan.2010.04.004
- Tanno S, Ohsaki Y, Nakanishi K, Toyoshima E, Kikuchi K. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer 2004 Oct; 46(1):11-9; PMID:15364128; http://dx.doi.org/10.1016/j.lungcan.2004.03.006
- Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ 2010 Mar; 17(3):499-512; PMID:19834490; http://dx.doi.org/10.1038/cdd.2009.152
- Lamalice L, Houle F, Jourdan G, Huot J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 2004 Jan 15; 23(2):434-45; PMID:14724572; http;//dx.doi.org/10.1038/sj.onc.1207034
- Dougher M, Terman BI. Autophosphorylation of VEGFR2 in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene 1999 Feb 25; 18(8):1619-27; PMID:10102632
- Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med 2013 Feb; 273(2):114-27; PMID:23216836; http://dx.doi.org/10.1111/joim.12019
- Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 2006 Jun 19; 94(12):1823-32; PMID:16773076
- Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol 2005 May 10; 23(14):3243-56; PMID:15886312; http://dx.doi.org/10.1200/JCO.2005.18.853
- Markman M. Antiangiogenic drugs in ovarian cancer. Expert Opin Pharmacother 2009 Oct; 10(14):2269-77; PMID:19671017; http://dx.doi.org/10.1517/14656560903120907
- Kösem M, Tuncer I, Kotan C, Ibiloğlu I, Oztürk M, Türkdoğan MK. Significance of VEGF and microvascular density in gastric carcinoma. Hepatogastroenterology 2009 Jul-Aug; 56(93):1236-40; PMID:19760978
- Iida M, Brand TM, Starr MM, Huppert EJ, Luthar N, Bahrar H, Coan JP, Pearson HE, Salgia R, Wheeler DL. Overcoming acquired resistance to cetuximab by dual targeting HER family receptors with antibody-based therapy. Mol Cancer 2014 Oct 24; 13:242; PMID:25344208; http://dx.doi.org/10.1186/1476-4598-13-242
- Troiani T, Napolitano S, Vitagliano D, Morgillo F, Capasso A, Sforza V, Nappi A, Ciardiello D, Ciardiello F, Martinelli E. Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition. Clin Cancer Res 2014 Jul 15; 20(14):3775-86; PMID:24812410; http://dx.doi.org/10.1158/1078-0432.CCR-13-2181
- Becker MA, Farzan T, Harrington SC, Krempski JW, Weroha SJ, Hou X, Kalli KR, Wong TW, Haluska P. Dual HER/VEGF receptor targeting inhibits in vivo ovarian cancer tumor growth. Mol Cancer Ther 2013 Dec; 12(12):2909-16; PMID:24130056; http://dx.doi.org/10.1158/1535-7163.MCT-13-0547
- Xie W, Li D, Zhang J, Li Z, Acheampong DO, He Y, Wang Y, Chen Z, Wang M. Generation and characterization of a novel human IgG1 antibody against vascular endothelial growth factor receptor 2. Cancer Immunol Immunother 2014 Sep; 63(9):877-88; PMID:24893856; http://dx.doi.org/10.1007/s00262-014-1560-9
- McMellen ME, Wakeman D, Erwin CR, Guo J, Warner BW. Epidermal growth factor receptor signaling modulates chemokine (CXC) ligand 5 expression and is associated with villus angiogenesis after small bowel resection. Surgery 2010 Aug; 148(2):364-70; PMID:20471049; http://dx.doi.org/10.1016/j.surg.2010.03.020
- Cho YR1, Choi SW, Seo DW. The in vitro antitumor activity of Siegesbeckia glabrescens against ovarian cancer through suppression of receptor tyrosine kinase expression and the signaling pathways. Oncol Rep 2013 Jul; 30(1):221-6; PMID:23673404; http://dx.doi.org/10.3892/or.2013.2468
- Tonra JR, Deevi DS, Corcoran E, Li H, Wang S, Carrick FE, Hicklin DJ. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res 2006 Apr 1; 12(7 Pt 1):2197-207; PMID:16609035; http://dx.doi.org/10.1158/1078-0432.CCR-05-1682
- Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009 May 15; 15(10):3484-94; PMID:19447865; http://dx.doi.org/10.1158/1078-0432.CCR-08-2904
- Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010 Feb 20; 28(6):1075-83; PMID:20085938; http://dx.doi.org/10.1200/JCO.2009.25.3641
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 2007 Apr; 7(4):295-308; PMID:17384584; http://dx.doi.org/10.1038/nrc2109
- De Luca A, Maiello MR, D'Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 2012 Apr; 16 Suppl 2:S17-27; PMID:22443084; http://dx.doi.org/10.1517/14728222.2011.639361
- Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res 2010 Jul 1; 16(13):3329-34; PMID:20472680; http://dx.doi.org/10.1158/1078-0432.CCR-09-3064
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 2011 Jan 18; 19(1):58-71; PMID:21215704; http://dx.doi.org/10.1016/j.ccr.2010.10.031
- Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action. Angiogenesis 2010 Mar; 13(1):1-14; PMID:20012482; http://dx.doi.org/10.1007/s10456-009-9160-6
- Gelfand MV, Hagan N, Tata A, Oh WJ, Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH, Gu C. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. Elife 2014 Sep 22; 3:e03720; PMID:25244320; http://dx.doi.org/10.7554/eLife.03720
- Ohba T, Cates JM, Cole HA, Slosky DA, Haro H, Ando T, Schwartz HS, Schoenecker JG. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol Cancer Res 2014 Aug; 12(8):1100-11; PMID:24759089, http://dx.doi.org/10.1158/1541-7786.MCR-14-0037
- Xu H, Yu Y, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar AP. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol Cancer Ther 2005 Mar; 4(3):435-42; PMID:15767552; http:dx.doi.org/10.1158/1535-7163.MCT-04-0280
- Tuna M, Chavez-Reyes A, Tari AM. HER2/neu increases the expression of Wilms' Tumor 1 (WT1) protein to stimulate S-phase proliferation and inhibit apoptosis in breast cancer cells. Oncogene 2005 Feb 24; 24(9):1648-52; PMID:15674342; http;dx.doi.org/10.1038/sj.onc.1208345
- Faltus T, Yuan J, Zimmer B, Krämer A, Loibl S, Kaufmann M, Strebhardt K. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia 2004 Nov-Dec; 6(6):786-95; PMID:15720805; http://dx.doi.org/10.1593/neo.04313
- Liu K, Chen H, You Q, Shi H, Wang Z. The siRNA cocktail targeting VEGF and HER2 inhibition on the proliferation and induced apoptosis of gastric cancer cell. Mol Cell Biochem 2014 Jan; 386(1-2):117-24; PMID:24158524; http://dx.doi.org/10.1007/s11010-013-1850-0
- Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol 2003 Apr; 13(2):159-67; PMID:12654259; http://dx.doi.org/10.1016/S1044-579X(02)00133-5
- Xuan ZX, Li LN, Zhang Q, Xu CW, Yang DX, Yuan Y, An YH, Wang SS, Li XW, Yuan SJ. Fully human VEGFR2 monoclonal antibody BC001 attenuates tumor angiogenesis and inhibits tumor growth. Int J Oncol 2014 Dec; 45(6):2411-20; PMID:25269419; http://dx.doi.org/10.3892/ijo.2014.2690
- Hsu YH, Wei CC, Shieh DB, Chan CH, Chang MS.Anti-IL-20 monoclonal antibody alleviates inflammation in oral cancer and suppresses tumor growth. Mol Cancer Res 2012 Nov; 10(11):1430-9; PMID:23002091; http://dx.doi.org/10.1158/1541-7786.MCR-12-0276
