ABSTRACT
Tumor-derived exosomes are highly correlated with tumor progression and angiogenesis. This study was designed to probe the role of tumor-derived exosomal miR-1247-3p in mediating the angiogenesis in bladder cancer. Exosomes isolation from the culture medium of normal or bladder cancer cell lines was performed using a differential centrifugation method. miR-1247-3p expression in exosomes and cells was detected by quantitative real-time PCR (qRT-PCR). The effect of exosomes on the angiogenesis of human umbilical vein endothelial cells (HUVECs) was assessed using cell counting kit-8 (CCK-8), transwell and tube formation assays. The interaction between miR-1247-3p and forkhead box protein O1 (FOXO1) was studied using luciferase reporter and RNA pull down assays. Exosomes were successfully isolated from T24, UM-UC-3, and SV-HUC-1 cells, as confirmed by corresponding identifications. Functional experiments revealed that exosomes derived from T24 and UM-UC-3 cells significantly enhanced the abilities of proliferation, migration, angiogenesis, and vascular endothelial-derived growth factor (VEGF) secretion in HUVECs. miR-1247-3p was highly expressed in exosomes derived from T24 and UM-UC-3 cells, and exosomes derived from miR-1247-3p inhibitor-transfected cells reduced HUVEC viability, migration, tube formation, and VEGF level. FOXO1 was confirmed as a direct target of miR-1247-3p. Rescue assays suggested that the effect of miR-1247-3p inhibition on the viability, migration, and angiogenesis of HUVECs was partly abrogated by the knockdown of FOXO1. Our data suggest that miR-1247-3p is up-regulated in tumor-derived exosomes, thereby inhibiting FOXO1 expression and facilitating angiogenesis in bladder cancer.
Introduction
As a common malignant tumor in the urogenital tract, bladder cancer seriously affects men’s health in China.Citation1 Due to the absence of specific diagnosis and therapy, a majority of patients are diagnosed with advanced stage upon discovery. The advanced patients with bladder cancer are prone to develop tumor metastasis and the prognosis remains poor.Citation2 Angiogenesis is the main cause of advanced bladder cancer, leading to tumor neovascularization and distant metastasis.Citation3 With efforts made on angiogenic inhibitors in clinical, the result has not been satisfactory. Thus, it is of necessity to delineate the molecular mechanisms concerning the process of bladder cancer to find potentially novel therapeutic targets.
Exosomes are 30–100 nm extracellular vesicles with various biological functions.Citation4 Studies revealed that exosomes are secreted by tumor cells and play a crucial role in intercellular communication, which contributes to the tumor angiogenesis and progression.Citation5,Citation6 The tumor-derived exosomes are the important regulatory factors on angiogenesis and malignant phenotypes in many cancers, but the effect of bladder cancer cells-derived exosomes on angiogenesis remains unclear. Researches showed that the exosomes affect the communication and angiogenesis depend on the proteins or nucleic acids they carry, such as microRNA (miRNA), which were reported to regulate the angiogenesis and tumor progression.Citation7,Citation8
miRNAs are endogenous noncoding RNAs with 18–22 nt length that regulate target genes to influence the development of various diseases. Numerous studies have demonstrated that exosomes carry miRNA regulate tumor angiogenesis by interfering target genes.Citation9,Citation10 The significant role of exosomal miRNAs in cancer has been widely studied. Zeng et al. demonstrated that cancer-derived exosomal miR-25-3p could promote the angiogenesis in colorectal cancer.Citation11 He et al. confirmed that exosomal miR-205 facilitated metastasis by inducing angiogenesis in ovarian cancer.Citation12 Liu et al. clarified that miR-1247-3p expression was up-regulated in bladder cancer, and this elevation enhanced cell proliferation and invasion.Citation13 Whereas, the effect and underlying mechanism of miR-1247-3p on bladder cancer-related angiogenesis is unclear.
Using the Targetscan website (http://www.targetscan.org/), forkhead box protein O1 (FOXO1) was predicted as a target of miR-1247-3p. A previous study has shown that FOXO1 inhibits tumor growth and angiogenesis in gastric cancer.Citation14 Another recent study has confirmed that downregulation of FOXO1 facilitates tumor proliferation and angiogenesis in colorectal adenocarcinoma.Citation15 Clinical evidence has demonstrated that FOXO1 expression is decreased and hippocampal angiogenesis was increased in major depressive disorder mouse model.Citation16 These above studies indicated the negative regulatory effect of FOXO1 on angiogenesis. In addition, researches have proven that silencing FOXO1 promotes the proliferation and migration of bladder cancer cells.Citation17,Citation18 Based on the above evidence, we hypothesized that bladder cancer-derived exosomes expedited angiogenesis through miR-1247-3p/FOXO1 signaling axis, which may be used as a candidate target for anticancer therapy.
Results
Isolation and identification of exosomes from bladder cancer cells
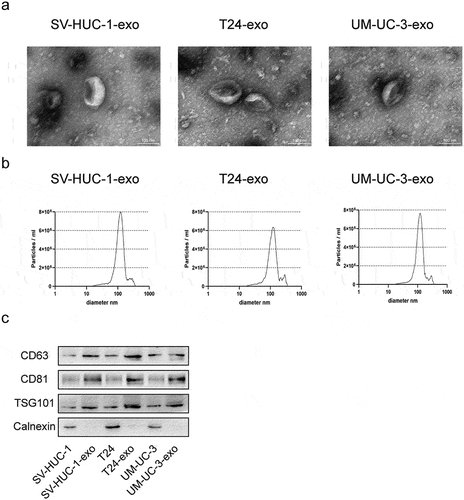
Exosomes derived from tumor cells play a major role in the progression of cancer.Citation19 To investigate the regulatory mechanism of exosomes in bladder cancer, we isolated the exosomes from the culture medium of normal urinary transitional epithelial cell (SV-HUC-1) and human bladder cancer cells (UM-UC-3 and T24). Exosomes were visualized and identified using transmission electron microscopy (TEM) and Flow nano analysis. Round or oval membranous vesicles were observed, with a disk-shaped structure and intact vesicles, approximately 100 nm in size (). The exosomes were also verified by western blot analysis via detection of the specific markers CD63, CD81, tumor susceptibility gene 101 (TSG101) and negative marker Calnexin. As indicated in , the vesicles were evidently expressed with CD63, CD81, and TSG101, while Calnexin was almost not expressed. These data suggested the successful isolation of the exosomes derived from bladder cancer cells.
Figure 1. Isolation and identification of exosomes from bladder cancer cells.
Bladder cancer cells-derived exosomes facilitate angiogenesis
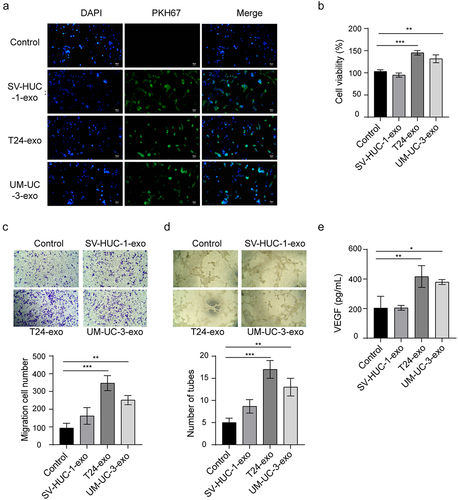
To understand the impact of tumor-derived exosomes on angiogenesis in bladder cancer, exosomes derived from SV-HUC-1 (SV-HUC-1-exo), T24 (T24-exo), and UM-UC-3 (UM-UC-3-exo) cells were incubated with human umbilical vein endothelial cells (HUVECs), respectively. The uptake of exosomes by HUVECs was evaluated by fluorescent labeling of the exosomes with PKH67. As indicated in , no fluorescence signals were observed in phosphate-buffered saline (PBS) group, while prominent green fluorescence signals were appeared after incubation with exosomes. There was no significant difference in fluorescence signals among exosomes treatment groups. The effect of bladder cancer cells-derived exosomes on the proliferation and migration of HUVECs were assessed by cell counting kit-8 (CCK-8) and transwell assays. It was noted that the exosomes derived from T24 and UM-UC-3 cells significantly enhanced the ability of HUVECs to proliferate and migrate when compared to SV-HUC-1-exo or PBS group (). Furthermore, the T24-exo and UM-UC-3-exo significantly facilitated angiogenesis, as evidenced by an increased total tube number and elevated level of angiogenic modifier vascular endothelial-derived growth factor (VEGF) in the supernatants (). Collectively, these findings indicated that the bladder cancer cells-derived exosomes promoted angiogenesis of endothelial cells.
Figure 2. Effect of bladder cancer cells-derived exosomes on angiogenesis.
Bladder cancer cells-derived exosomal miR-1247-3p facilitates angiogenesis
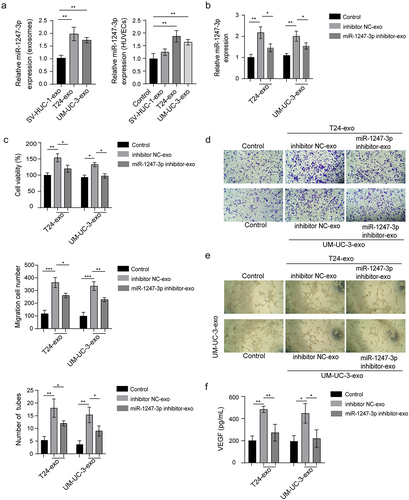
Previous evidence has demonstrated that the exosomal miR-1247-3p promotes lung metastasis of hepatoma carcinoma.Citation20 We next sought to probe the mechanism by which exosomes regulate bladder cancer. To address this, we first examined exosomal miR-1247-3p expression in bladder cancer cell lines. The result demonstrated that miR-1247-3p was highly expressed in T24-exo and UM-UC-3-exo, as compared to SV-HUC-1-exo. After the co-culture, in comparison with PBS or SV-HUC-1 group, the T24-exo and UM-UC-3-exo groups showed a significant increase of miR-1247-3p expression in HUVECs (). To validate the effect of exosomal miR-1247-3p on angiogenesis, T24, and UM-UC-3 cells were transfected with miR-1247-3p inhibitor following by the isolation of exosomes from supernatants and incubation with HUVECs. miR-1247-3p expression was markedly reduced by exosomes from miR-1247-3p inhibitor transfected bladder cancer cells compared to the inhibitor negative control (NC) group (). The CCK-8 and transwell assays showed a distinct decrease of HUVEC viability and migration after co-culture with exosomes derived from miR-1247-3p inhibitor – transfected cells (). Likewise, the exosomal miR-1247-3p inhibition restrained the angiogenesis of endothelial cells, showing the reduction of HUVEC tube formation and VEGF level in the supernatants after incubation with the T24-exo and UM-UC-3-exo in prior transfection of miR-1247-3p inhibitor (). These data revealed that the bladder cancer cells-derived exosomal miR-1247-3p could facilitate the angiogenesis.
Figure 3. The role of bladder cancer cells-derived exosomal miR-1247-3p in angiogenesis.
miR-1247-3p directly targets FOXO1 3ʹ-UTR
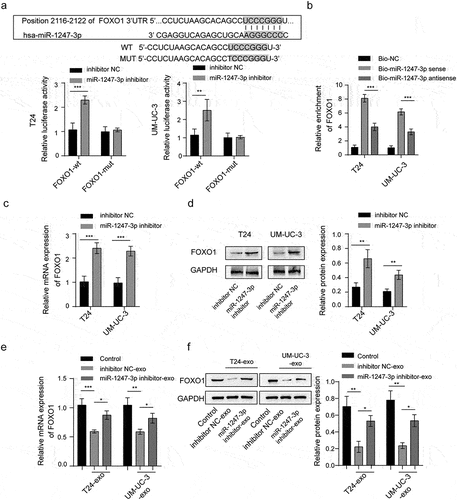
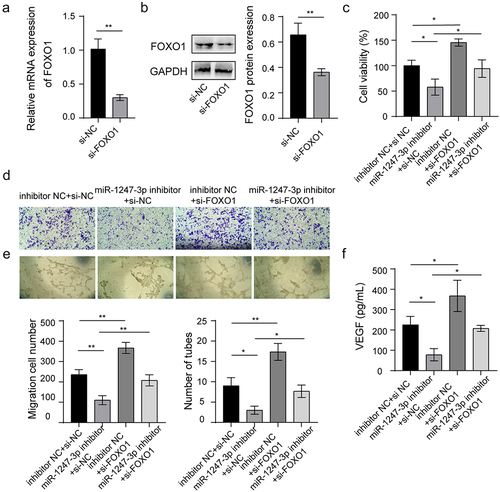
Given the importance of miR-1247-3p on angiogenesis in bladder cancer, we continued to explore the predicted binding target of miR-1247-3p. The bioinformatics analysis exhibited that miR-1247-3p had direct-binding site to 3’-untranslated region (3’−UTR) of FOXO1. To further identify this binding, the wild-type, and mutation of FOXO1 3ʹ-UTR (FOXO1-wt and FOXO1-mut) luciferase reporter plasmids were constructed, and these plasmids were co-transfected into T24 or UM-UC-3 cells with miR-1247-3p inhibitor or inhibitor NC. miR-1247-3p inhibition dramatically enhanced the luciferase activity of FOXO1-wt 3ʹ-UTR, while failed to alter the luciferase activity of FOXO1-mut 3ʹ-UTR, indicating that miR-1247-3p directly targets the FOXO1 3ʹ-UTR (). RNA pull-down was also carried out to confirm the interaction between miR-1247-3p and FOXO1. The data indicated that FOXO1 was preferentially enriched by biotinylated miR-1247-3p (Bio-miR-1247-3p) sense compared with Bio-miR-1247-3p antisense group (). Next, we found that the inhibition of miR-1247-3p up-regulated FOXO1 mRNA and protein levels in T24 and UM-UC-3 cells (). Subsequently, the exosomes derived from supernatants of miR-1247-3p inhibitor-transfected cells were co-cultured with HUVECs. As revealed in , the exosomes treatment led to a dramatic decrease of FOXO1 mRNA and protein levels, while this inhibitory effect was partly reversed by exosomes preliminarily processed with miR-1247-3p silencing, further suggesting that the exosomal miR-1247-3p targets FOXO1 3ʹ-UTR to repress its expression.
Figure 4. miR-1247-3p targets FOXO1 directly in bladder cancer cells.
miR-1247-3p regulates angiogenesis through targeting FOXO1
To validate whether miR-1247-3p affects angiogenesis of endothelial cells via targeting FOXO1, the impact of FOXO1 silencing on angiogenesis was determined. The knockdown of FOXO1 in HUVECs was induced by transfection of small interfering RNA (siRNA)-mediated knockdown of FOXO1 (si-FOXO1), and the result showed a marked decrease of FOXO1 mRNA and protein levels compared to the siRNA negative control (si-NC) group (). Next, miR-1247-3p inhibitor and si-FOXO1 were transfected or co-transfected into HUVECs. It was observed that miR-1247-3p inhibition dramatically decreased the viability and migration of HUVECs, while the simultaneous knockdown of miR-1247-3p and FOXO1 partially abrogated the impact of miR-1247-3p inhibition alone (). Ultimately, we found that miR-1247-3p silencing inhibited tube formation of HUVECs and reduced VEGF level in the supernatants, while these changes were neutralized in part after simultaneous knockdown of miR-1247-3p and FOXO1 (). Above all, these findings revealed that miR-1247-3p-induced angiogenesis of endothelial cells via regulating FOXO1.
Figure 5. miR-1247-3p regulates angiogenesis through targeting FOXO1.
Discussion
Tumor-derived exosomes participate in the progression of angiogenesis in bladder cancer.Citation21 miRNA is the most extensively studied factor in exosomes. Hence, it is necessary to explore a novel therapeutic target in bladder cancer, which is a disease with high mortality rate and serious impact on people’s lives. In this study, we confirmed a novel role that bladder cancer-derived exosomes facilitated the abilities of proliferation, migration, and angiogenesis in HUVECs through delivery of miR-1247-3p. miR-1247-3p directly targets FOXO1 and negatively regulates its expression. To our knowledge, this is the first research to provide the evidence of miR-1247-3p/FOXO1 signaling axis in exosomes-treated bladder cancer.
The process of angiogenesis involves the creation of new blood vessels from existing vessels.Citation22 Emerging evidence demonstrate that exosomes play a significant role in facilitating tumorigenesis by affecting angiogenesis and immunity. Li et al. discovered that hepatocellular carcinoma-derived exosomes foster angiogenesis of HUVECs via a paracrine mechanism.Citation23 Mo et al. demonstrated that exosomes derived from lung cancer markedly facilitated the proliferation and angiogenesis of HUVECs.Citation24 This study firstly isolated and identified the exosomes from culture medium of bladder cancer cell lines. Through TEM, Flow nano analysis, and exosomes-specific markers detection, the successful isolation of exosomes was confirmed. Then, the exosomes were incubated with HUVECs, and the uptake of exosomes was identified by fluorescent labeling of the exosomes with PKH67, showing prominent green fluorescence signals after incubation. T24-exo and UM-UC-3-exo obviously increased the ability of the proliferation, migration, and angiogenesis of HUVECs when compared to SV-HUC-1-exo or PBS group. These findings suggested that the bladder cancer cells-derived exosomes accelerated the angiogenesis. Nevertheless, the mechanism by which exosomes affect angiogenesis in bladder cancer remains to be further explored.
Exosomes carry bioactive factors to exert various functional regulations, among which miRNA is the most investigated molecule. Qiu et al. have shown that the exosomal miR-519a-3p aggravates liver metastasis by inducing angiogenesis in gastric cancer.Citation25 Ma et al. revealed that exosomal miR-3157-3p accelerated angiogenesis and metastasis in lung cancer.Citation26 miR-1247-3p serves as an oncogenic factor in many cancers, such as lung adenocarcinoma,Citation27 oral squamous cell carcinoma,Citation28 and bladder cancer.Citation13 Here, we discovered that miR-1247-3p was highly expressed in T24-exo and UM-UC-3-exo, and also highly detected in HUVECs after incubation with T24-exo and UM-UC-3-exo, suggesting the delivery of miR-1247-3p from exosomes to HUVECs. It was next noted that the viability, migration, total tube number, and VEGF level in HUVECs were reduced after incubation with exosomes from miR-1247-3p inhibitor-transfected cells. These data suggested that bladder cancer-derived exosomes promoted angiogenesis via delivery of miR-1247-3p.
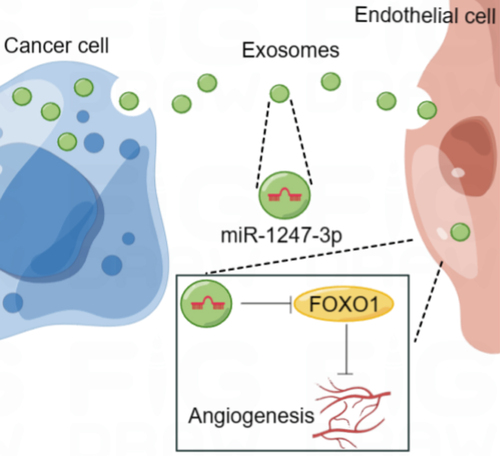
FOXO1 is an angiogenesis-related molecule that has been shown to regulate various cancers, including colorectal adenocarcinoma, gastric cancer, and bladder cancer.Citation15,Citation18 Here, FOXO1 was confirmed as a target gene of miR-1247-3p, and the rescue assays indicated that the effect of miR-1247-3p inhibition on cell viability, migration, and angiogenesis was partially abrogated by the knockdown of FOXO1. Similar to our work, Dai et al. demonstrated that cancer-associated fibroblasts derived extracellular vesicles (EVs) delivered miR-135b-5p to down-regulate FOXO1 and promoted proliferation and angiogenesis of HUVECs.Citation15 Shang et al. emphasized that exosomal miR-183-5p promoted angiogenesis and aggravated colorectal cancer by affecting FOXO1.Citation29 These studies may support our work to some certain extent. Collectively, we conclude that tumor-derived exosomal miR-1247-3p facilitates angiogenesis in bladder cancer through targeting FOXO1 (). Furthermore, by using the Targetscan website, FOXO1 was predicted as a target of miR-1247-3p in rats, mice, and human. The interaction between miR-1247-3p and FOXO1 may also exist in other cancers or diseases, but further research is needed to confirm it.
Figure 6. Schematic illustration of the functional role and potential mechanism of exosomal miR-1247-3p in angiogenesis of bladder cancer (by figdraw).
In summary, we reported that exosomes derived from bladder cancer cells and exosomal miR-1247-3p facilitated the proliferation, migration, and angiogenesis of HUVECs, and these effects could be partly reversed by FOXO1 overexpression in HUVECs. Our data may offer a theoretical basis for applications of exosomes in bladder cancer intervention. This study also has some limitations. We have not verified the role of exosomal miR-1247-3p in animal models, which requires further investigation. In addition, the other targets of miR-1247-3p and the role of other exosomal miRNAs in bladder cancer still needs to be further elucidated.
Materials and methods
Cell culture
The SV-HUC-1, UM-UC-3, and T24 cells were obtained from Shanghai Institutes of Biological Sciences (Shanghai, China). HUVECs were gained from ScienCell Research Laboratories (Carlsbad, CA, USA). The UM-UC-3, T24, and HUVECs were grown in RPMI 1640 medium (Thermo Fisher, Scientific, Waltham, MA, USA) consisting of 10% fetal bovine serum (FBS, Thermo Fisher), 100 U/mL penicillin, and 100 µg/mL streptomycin (Invitrogen, Carlsbad, CA, USA). SV-HUC-1 cells were grown in F-12K medium (Gibco, Grand Island, NY, USA) containing equal concentration of penicillin, streptomycin, and FBS to the mentioned cells. All the cells were incubated in 37°C environment containing 5% CO2.
Cell transfection
The miR-1247-3p inhibitor and si-FOXO1, and the inhibitor NC and si-NC were synthesized from RiboBio Co., Ltd (Guangzhou, China). Following the standard instructions, UM-UC-3 and T24 or HUVECs were transfected with miR-1247-3p inhibitor or inhibitor NC, and HUVECs were transfected with si-FOXO1 or si-NC with the Lipofectamine 3000 reagent (Invitrogen). 48 h later, cells were harvested for further experiments.
Isolation and identification of exosomes
Exosome isolation from culture medium was conducted using a differential ultracentrifugation method. In short, the conditioned medium of SV-HUC-1, UM-UC-3 and T24 cells was collected and centrifuged at 10,000×g at 4°C for 30 min. After discarding the pellet, the obtained supernatants were moved into a centrifuge tube and continued centrifugation at 100,000×g twice at 4°C, each for 70 min. The harvested supernatants were removed, and the pellet was resuspended in PBS. Then, the resuspended sediments were filtered with a 0.22 μm strainer and carried out centrifugation at 100,000×g for 1 h to obtain exosomes. The exosome pellets were diluted in PBS for following experiments.
To confirm the successful isolation of the exosomes, TEM (Tecnai, Philips, Netherlands) was conducted to identify the morphology of exosomes, containing the quantity, shape, and size. The size distribution of exosomes was detected by Flow nano analysis method on a Flow Nano Analyzer. The characteristic exosome markers (CD63, CD81, and TSG101) and negative marker Calnexin were determined by western blot analysis. The concentration of exosomes was evaluated using a bicinchoninic acid (BCA) protein kit (Beyotime, Shanghai, China).
For cell treatment, HUVECs were seeded with 3 × 105 cells/well, and then cultured for 48 h with 100 μg/mL derived exosomes from transfected cell supernatants.
Exosomes uptake by HUVECs
HUVECs were seeded in chamber slides and cultured overnight. The medium containing 10 µg fluorescent PKH67-labeled exosomes was incubated with HUVECs for 24 h. Cells incubated with PBS were served as the control group. After the incubation was finished, the cells were rinsed and fixed. DAPI (4’,6-diamidino-2-phenylindole) solution (10 µM) diluted in HBSS was then incubated with cells for 30 min. The uptake of PKH67-labeled exosomes in HUVECs was observed by laser confocal microscopy.
CCK-8 assay
The viability potential of HUVECs was evaluated using CCK-8 (Solarbio, Beijing, China) method. After finishing the transfection and treatment with exosomes, HUVECs (5 × 103/well) were seeded in a 96-well plate (Corning, NY, USA) with 10 μL CCK-8 solution stimulation in the following. Then, the cells were continued culture for 2 h. Under a microplate reader (BioTek, Biotek Winooski, Vermont, USA), the absorbance at 450 nm was measured.
Transwell assay
To assess the cell migration ability, 200 μL of culture medium containing HUVECs was put into the upper chamber with 1 × 105 cells and treated with exosomes for 48 h. The lower chamber was added with 600 μL of culture medium containing 10% FBS. Cells were then incubated at 37°C for 24 h, and the cells on the upper membrane surface were removed and the migrated cells were fixed and stained with 1% crystal violet. Finally, the images were captured, and cell number was counted using a microscope (Nikon, Tokyo, Japan).
Tube formation assay
To validate the impact of exosomes on angiogenic ability of HUVECs, the exosomes were directly co-cultured with HUVECs. Briefly, the 24-well plates were coated with 200 μL Matrigel (BD Bioscience, San Jose, CA, USA) and placed in a 37°C incubator for 30 min to solidfy. Then, HUVECs were seeded into the 24-well plates with 5 × 106 cells/well. Following 48 h of incubation, the total tube number was recorded and calculated in six random fields under a computer-assisted microscope.
Enzyme-linked immunosorbent assay (ELISA)
The VEGF contents in the supernatants of HUVECs were examined by an ELISA assay. In brief, the harvested culture supernatants were centrifuged at 1000 rpm for 5 min to remove the cell debris. Then, the concentration of VEGF level was evaluated using human ELISA kits (Solarbio) followed the standard guidelines.
Quantitative real-time PCR (qRT-PCR)
Trizol reagent (Invitrogen) was used for the extraction total RNA from cells, and an exoRNeasy Midi Kit (Qiagen, Duesseldorf, Germany) was used to separate exosomal RNA from culture medium. A total of 1 μg RNA were reverse transcribed with a reverse transcription kit (Promega, Madison, WI, USA). The qRT-PCR detection was conducted on the ABI 7500 system (Applied Biosystems, Waltham, MA, USA) using the SYBR Green Mix (Bio-Rad, Hercules, CA, USA). Relative expressions of miR-1247-3p and FOXO1 were calculated using the 2–∆∆Ct method. The endogenous controls were the U6 and GAPDH. The primer sequences were as follows: miR-1247-3p: 5ʹ-GGA ACG UCG AGA CUG GAG C-3ʹ (forward), 5ʹ-CGG CCC AGT GTT CAG ACT AC-3ʹ (reverse); U6: 5ʹ-CTC GCT TCG GCA GCA CAT-3ʹ (forward), 5ʹ-TTT GCG TGT CAT CCT TGC G-3ʹ (reverse); FOXO1: 5ʹ-CTA CGA GTG GAT GGT CAA GAG C-3ʹ (forward), 5ʹ-CCA GTT CCT TCA TTC TGC ACA CG-3ʹ (reverse); GAPDH: 5ʹ-GTC TCC TCT GAC TTC AAC AGC G-3ʹ (forward), 5ʹ-ACC ACC CTG TTG CTG TAG CCA A-3ʹ (reverse).
Western blotting
The cells or exosomes were lysed, and the isolated proteins were denatured in loading buffer after concentration detection. The separation of proteins was carried out by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis and then transferred to the polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked and incubated with antibodies containing anti-CD63 (ab134045, 1:2000, Abcam, Cambridge, MA, USA), anti-CD81 (ab109201, 1:2000, Abcam), anti-TSG101 (ab125011, 1:3000, Abcam), anti-Calnexin (ab92573, 1:20000, Abcam), anti-FOXO1 (ab179450, 1:2000, Abcam) and anti-GAPDH (ab9485, 1:2500, Abcam) antibodies at 4°C overnight. After washing with TBST, the membranes were incubated with secondary antibody goat anti-rabbit IgG (ab6721, 1:5000, Abcam) for 2 h. Subsequently, the bands were visualized by the enhanced chemiluminescence (ECL) reagent (Millipore).
Luciferase reporter assay
The binding of FOXO1 3ʹ-UTR and miR-1247-3p was predicted by Targetscan website. A density of 5 × 104/mL UM-UC-3 and T24 cells were seeded into a 24-well plate. The next day, the wild-type and mutation of FOXO1 3ʹ-UTR (FOXO1-wt and FOXO1-mut) luciferase reporter plasmids were constructed, and these newly established vectors were co-transfected into cells with miR-1247-3p inhibitor or inhibitor NC by using Lipofectamine 3000 reagent. Following transfection for 48 h, the luciferase activity was examined referring to the standard protocols.
RNA pull‐down assay
Transfection of the biotinylated-miR-1247-3p sense, biotinylated-miR-1247-3p antisense or biotinylated-NC (GenePharma, Suzhou, China) was performed in UM-UC-3 and T24 cells. After 48 h, the transfected cells were collected and lysed, followed incubation with streptavidin agarose beads (Thermo Fisher Scientific). The RNA complex bound to the beads was eluted by wash buffer, and the abundance of FOXO1 was then measured by qRT-PCR.
Statistical analysis
Data analysis was conducted with the SPSS 24.0 software (IBM, Armonk, NY, USA) and expressed as mean ± standard deviation (SD). Graphs were produced by GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis was conducted using student’s t-test or one-way analysis of variance (ANOVA) following the Tukey’s test. Experiments were independently repeated three times. P value less than 0.05 was considered statistically significant.
Highlights
Bladder cancer cells-derived exosomes facilitate angiogenesis.
Bladder cancer cells-derived exosomes facilitate angiogenesis through delivery of miR-1247-3p.
Exosomal miR-1247-3p regulates angiogenesis of HUVECs by targeting FOXO1.
List of Abbreviation
| miRNA | = | microRNA |
| FOXO1 | = | forkhead box protein O1 |
| VEGF | = | vascular endothelial-derived growth factor |
| HUVECs | = | human umbilical vein endothelial cells |
| FBS | = | fetal bovine serum |
| PBS | = | phosphate-buffered saline |
| DAPI | = | 4’,6-diamidino-2-phenylindole |
| SDS-PAGE | = | sodium dodecyl-sulfate polyacrylamide gel electrophoresis |
| PVDF | = | polyvinylidene fluoride |
| ELISA | = | enzyme-linked immunosorbent assay |
| qRT-PCR | = | quantitative real-time PCR |
| CCK-8 | = | cell counting kit-8 |
| TEM | = | transmission electron microscopy |
| TSG101 | = | tumor susceptibility gene 101 |
| NC | = | negative control |
| 3’-UTR | = | 3’-untranslated region |
| si-NC | = | small interfering RNA (siRNA) negative control |
| si-FOXO1 | = | small interfering RNA (siRNA)-mediated knockdown of FOXO1 |
| EVs | = | extracellular vesicles |
| BCA | = | bicinchoninic acid |
| SD | = | standard deviation |
| ANOVA | = | analysis of variance |
Author contributions
Guarantor of integrity of the entire study: Zonglai Liu, Dan Du, Shizhong Zhang
Study concepts: Zonglai Liu, Dan Du, Shizhong Zhang
Study design: Zonglai Liu, Dan Du, Shizhong Zhang
Definition of intellectual content: Zonglai Liu
Literature research: Zonglai Liu
Clinical studies: Zonglai Liu, Dan Du
Experimental studies: Zonglai Liu
Data acquisition: Zonglai Liu
Data analysis: Zonglai Liu
Statistical analysis: Zonglai Liu
Manuscript preparation: Zonglai Liu
Manuscript editing: Zonglai Liu
Manuscript review: Zonglai Liu
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplemental Material
Download MS Word (39.7 KB)Acknowledgments
We would like to thank the anonymous reviewers who have helped to improve the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384047.2023.2290033
Additional information
Funding
Notes on contributors
Zonglai Liu
Zonglai Liu, the deputy chief physician of the Urology Department at the Second People’s Hospital of Three Gorges University, graduated from Wuhan University with a major in clinical medicine. He also holds a master’s degree in clinical medicine from Three Gorges University (academic track). Additionally, he serves as the deputy director of the Yichang Urology branch of the Doctors Association.
Dan Du
Dan Du is the chief physician of the Urology Department at the Second People’s Hospital of Three Gorges University. He also serves as the Chairman of the third and fourth committees of the Yichang Urological Surgery Society, and as the honorary chairman of the fifth committee.
Shizhong Zhang
Shizhong Zhang is a professor, master, and doctoral supervisor at China Three Gorges University. He is also the Executive Director of the Physiology Society of Hubei Province. Zhang received his master’s degree in physiology from Shanxi Medical University in 2000, his doctorate in physiology from Zhejiang University in 2006, and completed a post-doctoral program at the University of Michigan from 2011 to 2012. Currently, he serves as the Deputy Dean of the School of Basic Medicine at China Three Gorges University Medical School.
References
- Liu J, Xie J, Huang Y, Xie J, Yan X. TFPI-2 inhibits the invasion and metastasis of bladder cancer cells. Progres en urologie : j de l’Association francaise d’urologie et de la Societe francaise d’urologie. 2021;31(2):71–11. doi:10.1016/j.purol.2020.07.243.
- Ashrafizadeh M, Hushmandi K, Hashemi M, Akbari ME, Kubatka P, Raei M, Koklesova L, Shahinozzaman M, Mohammadinejad R, Najafi M, et al. Role of microRna/epithelial-to-mesenchymal transition axis in the metastasis of bladder cancer. Biomolecules. 2020;10(8):1159. doi:10.3390/biom10081159.
- Elayat G, Punev I, Selim A. An overview of angiogenesis in bladder cancer. Curr Oncol Rep. 2023;25(7):709–728. doi:10.1007/s11912-023-01421-5.
- Thakur A, Parra DC, Motallebnejad P, Brocchi M, HJ C. 2022. Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater. 10:281–294. doi:10.1016/j.bioactmat.2021.08.029.
- Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int J Mol Sci. 2020;21(16):5840. doi:10.3390/ijms21165840.
- Xu K, Zhang C, Du T, Gabriel ANA, Wang X, Li X, Sun L, Wang N, Jiang X, Zhang Y. 2021. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother Biomed Pharmacotherapie. 134:111111. doi:10.1016/j.biopha.2020.111111.
- Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–1762. doi:10.1016/j.cmet.2021.08.006.
- Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, Li HM, Zhang WS, Chen CY, Xie H. Xie H: exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8(1):169–184. doi:10.7150/thno.21234.
- Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, Zou Y, Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38(1):310. doi:10.1186/s13046-019-1313-x.
- Mahati S, Fu X, Ma X, Zhang H, Xiao L. 2021. Delivery of miR-26a using an exosomes-based nanosystem inhibited proliferation of hepatocellular carcinoma. Front Mol Biosci. 8:738219. doi:10.3389/fmolb.2021.738219.
- Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395. doi:10.1038/s41467-018-07810-w.
- He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, Wu X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–8220. doi:10.7150/thno.37455.
- Liu H, Chen D, Bi J, Han J, Yang M, Dong W, Lin T, Huang J. Circular RNA circUBXN7 represses cell growth and invasion by sponging miR-1247-3p to enhance B4GALT3 expression in bladder cancer. Aging. 2018;10(10):2606–2623. doi:10.18632/aging.101573.
- Kim SY, Ko YS, Park J, Choi Y, Park JW, Kim Y, Pyo JS, Yoo YB, Lee JS, Lee BL. Forkhead transcription factor FOXO1 inhibits angiogenesis in gastric cancer in relation to SIRT1. Cancer Res Treat. 2016;48(1):345–354. doi:10.4143/crt.2014.247.
- Dai X, Xie Y, Dong M. Cancer-associated fibroblasts derived extracellular vesicles promote angiogenesis of colorectal adenocarcinoma cells through miR-135b-5p/FOXO1 axis. Cancer Biol Ther. 2022;23(1):76–88. doi:10.1080/15384047.2021.2017222.
- Zhang S, Lu Y, Shi W, Ren Y, Xiao K, Chen W, Li L, Zhao J. 2022. SIRT1/FOXO1 axis-mediated hippocampal angiogenesis is involved in the antidepressant effect of Chaihu Shugan San. Drug Des Devel Ther. 16:2783–2801. doi:10.2147/DDDT.S370825.
- Wang W, Jiang X, Xia F, Chen X, Li G, Liu L, Xu Q, Zhu M, Chen C. HYOU1 promotes cell proliferation, migration, and invasion via the PI3K/AKT/FOXO1 feedback loop in bladder cancer. Mol Biol Rep. 2023;50(1):453–464. doi:10.1007/s11033-022-07978-x.
- Li F, Xie W, Fang Y, Xie K, Liu W, Hou L, Tan W. HnRNP-F promotes the proliferation of bladder cancer cells mediated by PI3K/AKT/FOXO1. J Cancer. 2021;12(1):281–291. doi:10.7150/jca.50490.
- Chen S, Chen X, Luo Q, Liu X, Wang X, Cui Z, He A, He S, Jiang Z, Wu N, et al. Retinoblastoma cell-derived exosomes promote angiogenesis of human vesicle endothelial cells through microRNA-92a-3p. Cell Death Disease. 2021;12(7):695. doi:10.1038/s41419-021-03986-0.
- Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9(1):191. doi:10.1038/s41467-017-02583-0.
- Yuan F, Yin XY, Huang Y, Cai XW, Jin L, Dai GC, Zang YC, Sun Y, Liu XL, Xue BX. Exosomal miR-93-5p as an important driver of bladder cancer progression. Transl Androl Urol. 2023;12(2):286–299. doi:10.21037/tau-22-872.
- Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao HL, Li H, Zhang SR, Xu JZ, Qi ZH, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22(1):15–36. doi:10.1007/s10456-018-9645-2.
- Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, Yang X, Fang F, Xia Q, Zhang Z, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 2019;18(1):18. doi:10.1186/s12943-019-0948-8.
- Mo F, Xu Y, Zhang J, Zhu L, Wang C, Chu X, Pan Y, Bai Y, Shao C, Zhang J. Effects of hypoxia and radiation-induced exosomes on migration of lung cancer cells and angiogenesis of umbilical vein endothelial cells. Radiat Res. 2020;194(1):71–80. doi:10.1667/RR15555.1.
- Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. 2022;41(1):296. doi:10.1186/s13046-022-02499-8.
- Ma Z, Wei K, Yang F, Guo Z, Pan C, He Y, Wang J, Li Z, Chen L, Chen Y, et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Disease. 2021;12(9):840. doi:10.1038/s41419-021-04037-4.
- Lin J, Zheng X, Tian X, Guan J, Shi H. Shi H: miR-1247-3p targets STAT5A to inhibit lung adenocarcinoma cell migration and chemotherapy resistance. J Cancer. 2022;13(7):2040–2049. doi:10.7150/jca.65167.
- Zheng S, Li L, Li N, Du Y, Zhang N. 2020. 1, 6-O, O-Diacetylbritannilactone from inula britannica induces anti-tumor effect on oral squamous cell carcinoma via miR-1247-3p/LXRalpha/ABCA1 signaling. OncoTargets Ther. 13:11097–11109. doi:10.2147/OTT.S263014.
- Shang A, Wang X, Gu C, Liu W, Sun J, Zeng B, Chen C, Ji P, Wu J, Quan W, et al. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging. 2020;12(9):8352–8371. doi:10.18632/aging.103145.
