ABSTRACT
Neurensin-2 (NRSN2) performs a pro-carcinogenic function in multiple cancers. However, the function of NRSN2 in HPV-infected laryngeal carcinoma (LC) remains unclear. HPV transfection was performed in LC cells. The mRNA and protein levels were monitored using RT-qPCR, immunoblotting, and IF. Cell viability and proliferation were found using the CCK-8 assay and Edu staining. Cell invasion, migration, and apoptosis were probed using the Transwell, wound healing, and flow cytometry, respectively. The autophagosome was observed using TEM. NRSN2 was overexpressed in HPV-transfected LC cells. Inhibition of NRSN2 restrained the autophagy and malignant behavior of HPV-transfected LC cells. Meanwhile, the inhibition of AMPK/ULK1 pathway limited the increased autophagy of HPV-transfected LC cells caused by NRSN2 overexpression. Furthermore, NRSN2 knockdown inhibits autophagy by suppressing AMPK/ULK1 pathway, thereby restraining the malignant behavior of HPV-transfected LC cells. Our research confirmed that HPV transfection increased the autophagy and malignant behavior of LC cells by regulating the NRSN2-mediated activation of the AMPK/ULK1 pathway, offering a new target for cure of LC.
Introduction
Head and neck cancer (HNC), with heterogeneity and high recurrence rate, is a malignant tumor occurring in the mucous epithelium of the upper respiratory tract.Citation1 Laryngeal carcinoma (LC) is one of the most common cancers of HNC, accounting for approximately one-third of total HNC.Citation2,Citation3 In recent years, the incidence of LC has continued to rise, with a 5-year overall survival rate of only 50%, and many patients are already in the advanced stage when discovered.Citation4,Citation5 The treatment methods for LC include total laryngectomy, laser transoral throat microsurgery, radiotherapy, and chemotherapy, among which surgical resection is the most effective method.Citation6,Citation7 However, due to the crucial role of the larynx in phonation, surgical resection may have a significant impact on the patient’s life.Citation8 Therefore, exploring potential diagnostic and therapeutic targets for LC is urgent. In recent years, more and more studies have found that among the many pathogenic factors of LC (smoking, drinking, air pollution, human papillomavirus (HPV) infection, etc.), HPV (especially HPV16) infection may be one of the most important factors.Citation9,Citation10 It is reported that the HPV infection rate of LC is high, and HPV infection has a positive effect on the development of LC.Citation9,Citation11 However, how HPV participates in the development of LC is still unknown.
Autophagy, a cellular degradation pathway, performs a vital function in sustaining cell homeostasis.Citation12 There is evidence that in many cases, the autophagy of cancer cell supports tumor growth.Citation13,Citation14 Lin C et al.Citation15 covered that inhibition of autophagy via blocking the AMPK/ULK1 pathway can restrain the development of prostate cancer. Autophagy inhibition has become a promising therapeutic target for LC.Citation16 The study of Guo Y et al.Citation17 covered that the inhibition of autophagy enhances the chemosensitivity of LC cells to cisplatin. Chen XH et al.Citation18 found that autophagy inhibition reduces the propagation and migration of LC stem cells. The research of Li G et al.Citation19 proved that miR-339-5p reduces the resistance of LC to cisplatin by inhibiting autophagy. In addition, studies have found that compared with clinically normal laryngeal tissues, the level of autophagy marker LC3B and the number of autophagosomes in HPV-transfected respiratory papilloma tissues are significantly higher.Citation20 This suggests that HPV may regulate laryngeal cancer progression by influencing autophagy.
Neurensin-2 (NRSN2), a protein localized on the cell membranes, has been reported to perform a cancer-promoting role in a variety of tumors in recent years.Citation21 For example, studies have found that high NRSN2 expression is associated with the malignant phenotype of ovarian cancer.Citation22 The report of Keremu A et al.Citation23 revealed that NRSN2 promotes the growth of osteosarcoma cells. Additionally, a research has displayed that NRSN2 accelerates the development of esophageal squamous cell carcinoma.Citation24 Nevertheless, the action of NRSN2 in LC is dim.
Hence, we hypothesized that NRSN2 expression is up-regulated and autophagy is enhanced in HPV-transfected LC cells, thus promoting the growth of HPV-transfected LC cells and inhibiting the apoptosis of HPV- transfected LC cells. We will establish a stable HPV-transfected LC cells and perform knockdown or overexpression of NRSN2 on these cells to explore the role of NRSN2 in HPV-mediated malignant behavior in LC, giving the diagnosis and treatment of LC a novel underlying target.
Methods
Cell culture and treatment
The LC cells TU212 and TU138 were cultivated in RPMI 1640 (Gibco) appended with 10% fetal bovine serum (FBS, Gibco), penicillin/streptomycin (Gibco), and humidity of 95% air and 5% CO2 at 37°C. For the inhibition of ULK1 and AMPK, the SBI-0206965 (SBI, 10 μM, MCE, China) and Compound C (Com, 10 μM, MCE) were applied to the cells for 24 h, respectively. Rapamycin (RAP, 20 μM, MCE) was added to activate the autophagy.
Generation of cells stably expressing HPV16E7
TU212 and TU138 cells were planted in the petri dish of 6 cm at a denseness of 1.5 × 10Citation6 cells and cultivated to about 90% denseness. Then, the recombinant vector pLXSN16E7 (Addgene, USA) containing the HPV16E7 genome was stably transfected into TU212 and TU138 cells via the Lipofectamine 3000 (Invitrogen, USA). 24 h later, the medium was appended with G418 (500 μg/mL, Invitrogen) to screen the cells stably expressing HPV16E7 (TU212/HPV and TU138/HPV) for 8 consecutive weeks. RT-qPCR and immunoblotting were applied to assay if the cells stably expressing HPV16E7 were successful or not.
Cell transfection
For the overexpression of NRSN2, NRSN2 overexpression vectors (ov-NRSN2) and the matching negative vectors (ov-NC) were constructed via Sangon Biotech Co., Ltd (China). For the NRSN2 knockdown, NRSN2 (sh-NRSN2, target sequence: 5’-AGGGTGTACAGCCACTATTTA-3’) knockdown vectors and the matching negative vector (sh-NC) were constructed via Sangon Biotech Co., Ltd. These vectors were transfected into TU212/HPV and TU138/HPV cells via the Lipofectamine 3000 (Invitrogen).
Transmission electron microscopy (TEM) to detect the autophagosomes
The cells of each group were prefixed in 2.5% glutaraldehyde for 2 h and then cleaned 3 times in 0.1 M phosphate buffer (PB) for 60 s each time. Fixed at 4°C for another 1 h in a 1% osmic acid. Then, it was washed with 0.1 M PB for 3 times, stained in 1% uranyl acetate under 25°C for 1 h, and cleaned in ddH2O for 3 times. The cells were progressively dehydrated in varying concentrations of ethanol solutions for 8 min, followed by cleaning in propylene oxide for 10 min. Later, the cells were coated with a compound of EPON812 resin and propylene oxide (2:1) under 4°C for 4 h, and then in pure EPON812 resin overnight. After roasting at 60°C for 24 h, the samples were cut into ultra-thin slices (70-nm thick) using the Ultracut UCT Microtome (Leica Biosystems, USA) and dyed in 1% uranium acetate for 20 min and lead citrate for 5 min. A Tecnai Spirit 120 kV TEM (FEI, USA) was applied to obtain the images.
Immunofluorescence (IF) staining
Cells of each group were inoculated in petri dishes with coverslip to prepare cell slides, which were fixed in 4% paraformaldehyde for 30 min, cleaned with PB saline (PBS) for 3 times, and penetrated in 0.2% Triton X-100 for 3 min. Then, the cell slide was cleaned with PBS for 3 times, and blocked with goat serum at 25°C for 30 min. The anti-LC3B (ab192890, 1:200, Abcam, UK) was appended and placed at 4°C for 12 h and then the cell slide was cleaned with PBS for 3 times. The cell slide was further mixed with the secondary antibody (ab150083, 1:1000, Abcam) in the dark for 2 h. After cleaned in PBS for 3 times, the cell slide was dyed with DAPI, and then photographed under an AX-70 fluorescent microscopy (Leica Microsystems Inc.).
Reverse transcription‑quantitative polymerase chain reaction (RT‑qPCR)
Using the Trizol (Invitrogen, USA), the overall RNA from the above treated cells was isolated. The SuperScript™ VILO™ cDNA Synthesis Kit (Thermo Fisher Scientific, USA) was applied to reverse the overall RNA into cDNA according to the instructions. Subsequently, the mRNA level of HPV16E7 and NRSN2 were probed via qPCR with SYBR Green PCR Master Mix (Takara, China) on the 7900HT Fast Real-Time PCR System (Applied Biosystems, USA). The reference gene for normalization was GADPH. The shift of the mRNA level of HPV16E7 and NRSN2 was quantified via 2−∆∆Ct method. The Forward (F) and Reverse (R) primer sequences of HPV16E7, NRSN2, and GAPDH were offered: NRSN2-F: 5’-GATGGCAAGTGGTATGGGGTC-3’, NRSN2-R: 5’-CGAGGACAGGCTGATCTTCC-3’; GAPDH-F: 5’-GGAGCGAGATCCCTCCAAAAT3’, GAPDH-R: 5’-GGCTGTTGTCATACTTCTCATGG-3’.
Immunoblotting
The RIPA lysate (Beyotime, China) was used to isolate the overall protein from the above treated cells and the concentration of overall protein was probed via BCA kit (Beyotime, China). 24 μg of overall protein was isolated via SDS-PAGE and electrotransferred onto PVDF membrane (Millipore, USA) at 4°C. Afterward, 5% skim milk was applied to incubate the PVDF membrane placed in at 25°C for 1 h. The primary antibodies were then cultivated with the PVDF membrane all-night at 4°C. The membrane was placed in specific secondary antibody for a further 2 h at 25°C. Finally, the protein bands were tested via the enhanced chemiluminescence (ECL) system (Thermo Fisher Scientific) and analyzed using Image J. The above antibodies including anti-HPV16E7 (ab308180, 1:1000), anti-NRSN2 (ab237739, 1:5000), anti-LC3B (ab192890, 1:2000), anti-p-ULK1(S555) (#5869, 1:1000), anti-p62 (ab109012, 1:4000), anti-p-AMPK(T172) (#2535, 1:1000), anti-β-actin (ab8227, 1:5000), and secondary antibody (ab6721, 1:10000) were acquired from Abcam and CST (USA).
5-ethynyl-2-deoxyuridine (EdU) staining
Using the BeyoClick™ EdU-555 Cell proliferation test kit (Beyotime, China) to confirm the cell proliferation. The cells of the different treatment groups were placed in 20 mmol/L EdU for 2 h. Then, the cells were immobilized at room temperature with 4% paraformaldehyde for 15 min. After scoured with PBS, the cells were permeated with 0.3% Triton X-100, then washed with PBS and placed in Click reaction solution at 25°C in the dark for 30 min. The cells were dyed with Hoechst 33,342 for 10 min and observed with an AX-70 fluorescent microscopy (Leica Microsystems Inc.).
Cell counting kit-8 (CCK-8) assay
A 96-well plate was applied to seed cells from each treatment group (at a denseness of 7.5 × 10Citation3 cells) and cultivated for 24 h, 48 h, and 72 h. Following this, the supernatant was dislodged, and 10 μl of CCK-8 solution was appended before being incubated for 2.5 h. Subsequently, using the SpectraMax 190 microplate reader (Molecular Devices, LLC., USA) to probe the absorbance at 450 nm.
Wound healing assay
Cells from each treatment group (at a denseness of 2.5 × 10Citation5 cells) were planted in 12-well plates and cultivated until confluent. Monolayers were scratched using a gun tip of 200 μl, washed by PBS, and then cultivated in serum-free RPMI 1640 for 24 h. The width of scratches was observed and probed by an AX-70 fluorescent microscopy (Leica Microsystems Inc.) at 0 h and 24 h, respectively.
Transwell assay
First, add the Matrigel (BD Biosciences) to the Transwell upper chamber. 4 h later, cells of each group were planted into the upper chamber and cultured in serum-free RPMI 1640 (2.5 × 10Citation4 cells). Then, the lower chamber was appended RPMI 1640 possessing 10% FBS. After 24 h cultivation, the cells in the lower side of the upper chamber were fixed with methanol for 0.5 h, and then dyed by 0.1% crystal violet (Thermo Fisher Scientific) for 1 h. Finally, the invasive cells were monitored under an AX-70 fluorescent microscopy (Leica Microsystems Inc.).
Flow cytometry
The cells of each treatment group were gathered and scoured with icy PBS, and then re-suspended with the binding solution (Beyotime). Annexin V-FITC and propyl iodide staining solution (Beyotime) were appended and incubated at 25°C for 20 min in the dark. Then, the cells were analyzed using BD LSRFortessa X20 (USA).
Statistical analysis
The SPSS 26.0 software (SPSS, Inc.) was applied to analyze the data, which was expressed as mean ± standard deviation (SD). The groups’ comparison and multiple groups’ comparison were analyzed by t-test and one-way ANOVA following Tukey’s test, respectively. All experiments were performed in triplicates at least. When the value of p < .05, the difference was identified as significance.
Results
The expression of NRSN2 was highly in HPV-transfected LC cells
The cells stably expressing HPV16E7 (TU212/HPV and TU138/HPV) were constructed. As shown in , the protein levels of HPV16E7 were highly in TU212/HPV and TU138/HPV, which indicated that the cells stably expressing HPV16E7 were successfully constructed. Then, the mRNA and protein levels of NRSN2 were assessed by RT-qPCR and immunoblotting. The consequences revealed that the HPV transfection observably increased the expression of NRSN2 (). Thus, it is indicated that HPV transfection can increase the expression of NRSN2 in LC cells.
Figure 1. The expression of NRSN2, cell proliferation and autophagy of HPV-transfected LC cells were enhanced.
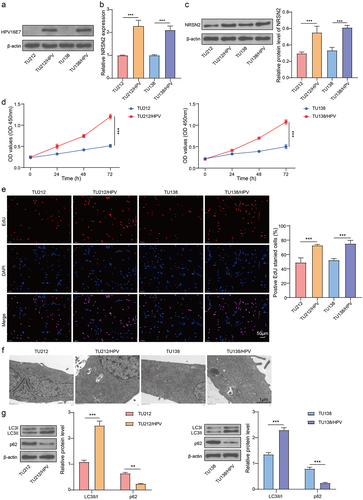
The proliferation and autophagy of HPV-transfected LC cells were enhanced
We further examined the proliferation and autophagy of parental cells (TU212 and TU138) and HPV-transfected cells (TU212/HPV and TU138/HPV). The result indicated that the cell vitality and EdU positive ratio of TU212/HPV and TU138/HPV were observably enhanced than those of TU212 and TU138 (). Furthermore, compared with TU212 and TU138, the TU212/HPV and TU138/HPV cells have more autophagosomes, as observed by TEM (). Moreover, the level of LC3B in TU212/HPV and TU138/HPV cells was markedly boosted than TU212 and TU138 cells, while the level of p62 in TU212/HPV and TU138/HPV cells was observably inhibited than TU212 and TU138 cells (). Hence, the consequences indicated that HPV transfection increased the proliferation and autophagy of LC cells.
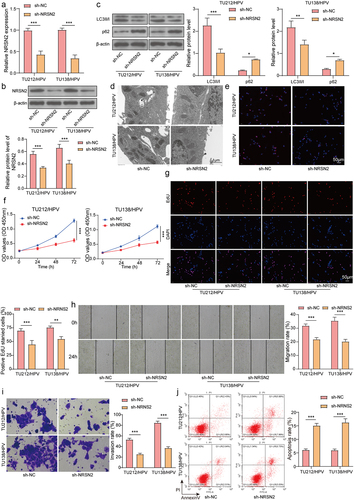
Inhibition of NRSN2 expression restrained the autophagy and malignant behavior of HPV-transfected LC cells
To investigate the function of NRSN2 in HPV-mediated malignant behavior in LC, we knocked down NRSN2 in TU212/HPV and TU138/HPV cells. As shown in , sh-NRSN2 transfection dramatically reduced the expression of NRSN2 compared with sh-NC transfection. Moreover, NRSN2 knockdown markedly decreased LC3B expression and increased p62 expression (). Similarly, the fluorescence intensity of LC3B and the number of autophagosome in TU212/HPV and TU138/HPV cells was visibly reduced by NRSN2 inhibition (). Furthermore, NRSN2 knockdown weakened the cell vitality and EdU positive ratio (). Meanwhile, the capacities of migration and invasion of TU212/HPV and TU138/HPV cells were observably inhibited via silencing NRSN2 (). Otherwise, NRSN2 knockdown aggrandized the apoptosis ratio of cells (). Altogether, the autophagy and malignant behavior of HPV-transfected LC cells were refrained by NRSN2 knockdown.
Figure 2. Inhibition of NRSN2 expression restrained the autophagy and malignant behavior of HPV-transfected LC cells.
Overexpression of NRSN2 upregulates autophagy via activating AMPK/ULK1 pathway
The AMPK/ULK1 pathway performs a vital impact in the activation of autophagy 15, 25. To test the function of AMPK/ULK1 pathway on NRSN2-mediated autophagy activation in TU212/HPV and TU138/HPV cells, we performed overexpression of NRSN2 and inhibition of AMPK/ULK1 in TU212/HPV and TU138/HPV cells. The result of Figures 3A and 3B showed that the NRSN2 overexpression was successful. In addition, we found that NRSN2 overexpression increased p-AMPK(T172) and p-ULK1(S555) levels, while Com (an inhibitor of AMPK) treatment decreased p-AMPK(T172) and p-ULK1(S555) levels (). Otherwise, the increased p-ULK1(S555) level caused by NRSN2 overexpression was decreased by SBI (an inhibitor of ULK1) (). Furthermore, the enhanced LC3B level contributed by NRSN2 overexpression was lessened by treatment of Com and SBI (). Broadly speaking, NRSN2 overexpression activates the autophagy by regulating AMPK/ULK1 pathway.
Figure 3. Overexpression of NRSN2 upregulates autophagy by activating AMPK/ULK1 pathway.
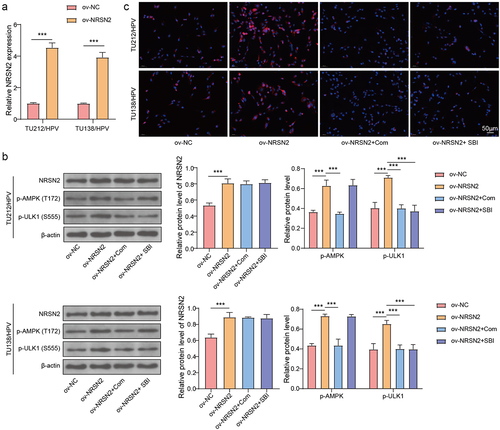
Figure 4. Knockdown of NRSN2 inhibits autophagy by suppressing AMPK/ULK1 pathway, thereby restraining the malignant behavior of HPV-transfected LC cells.
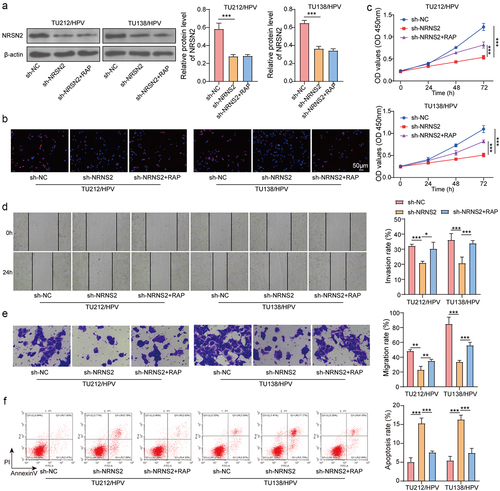
Knockdown of NRSN2 inhibits autophagy by suppressing AMPK/ULK1 pathway, thereby restraining the malignant behavior of HPV-transfected LC cells
To confirm the effect of autophagy on NRSN2-mediated malignant behavior of TU212/HPV and TU138/HPV cells, we performed inhibition of NRSN2 and autophagy in TU212/HPV and TU138/HPV cells. The outcome displayed that inhibition of NRSN2 obviously lessened the levels of NRSN2, while the autophagy activator RAP treatment had no impact on NRSN2 expression (). Moreover, the decreased LC3B level caused by NRSN2 inhibition was increased by treatment of RAP (). Otherwise, NRSN2 knockdown weakened the cell vitality, while RAP treatment restored the cell vitality (). The reduced migration and invasion of TU212/HPV and TU138/HPV cells caused by NRSN2 inhibition was increased by RAP treatment (). Meanwhile, NRSN2 inhibition increased the apoptosis ratio of cells, while treatment of RAP reversed these effects (). In summary, Knockdown NRSN2 restrains the malignant behavior of HPV-transfected LC cells by suppressing autophagy.
Discussion
In recent years, the incidence of LC has continued to raise, with a 5-year overall survival rate of only 50%, and many patients are already in the advanced stage when discovered.Citation4,Citation5 HPV infection performs a vital function in the progression of LC ,Citation9–11 Our research displayed that HPV transfection augmented the autophagy and growth of LC cells by regulating the NRSN2-mediated activation of the AMPK/ULK1 pathway.
NRSN2 has been reported to perform a cancer-promoting role in a variety of tumors in recent years.Citation21 Ren F et al. reported that NRSN2 enhanced the cell proliferation and tissue growth of breast cancer.Citation26 The research of Wang G et al. indicated that NRSN2 enhances the proliferation, invasion, and migration of colorectal cancer cells.Citation27 Besides, a research has revealed that inhibition of NRSN2 restrains the proliferation, migration, and invasion of esophageal squamous cell carcinoma.Citation24 Congruously, our research displayed that NRSN2 knockdown restrained the proliferation, migration, and invasion of HPV-transfected LC cells. Meanwhile, the apoptosis in HPV-transfected LC cells was increased via inhibition of NRSN2.
Autophagy, a cellular degradation pathway, has been reported supports tumor growth.Citation14 Huang HY et al. found that autophagy inhibitors significantly increased LC cell apoptosis and death both in vitro and in vivo.Citation28 The report of Chen L et al. showed that miR-101 lowered the autophagy, proliferation and enhanced the apoptosis in LC cells.Citation29 In addition, studies have indicated that compared with normal laryngeal tissues, HPV-infected respiratory papilloma tissues have more LC3B expression and autophagosomes.Citation20 In this study, we found that HPV transfection enhanced the autophagy of LC cells. Meanwhile, inhibition of NRSN2 lessened the proliferation, migration, and invasion and enhanced the apoptosis in HPV-transfected LC cells by reducing autophagy. AMPK/ULK1 pathway performs vital role in autophagy. The AMPK/ULK1 pathway performs a vital function in regulation of autophagy.Citation15,Citation25,Citation30 Conformably, our investigation demonstrated that inhibition of the AMPK/ULK1 pathway can reverse the increased autophagy and cell growth and decreased apoptosis caused by overexpression of NRSN2. However, we did not probe the molecular mechanics through which NRSN2 acts on AMPK/ULK1, that is a limitation in our study. We will continue to research how NRSN2 acts on AMPK/ULK1 further.
Conclusions
In summary, our study demonstrated that HPV transfection enhanced the autophagy and growth of LC cells by regulating the NRSN2-mediated activation of the AMPK/ULK1 pathway. Our current study provides a new potential target for the diagnosis and treatment of LC.
Author contributions
Fan Guo and Ruixia Ma are the guarantor of integrity of the entire study; Wulin Wen contributed to the study concepts, study design and definition of intellectual content; Zhipeng Mi contributed to the literature research, clinical studies and experimental studies; Chao Long contributed to the data acquisition and data analysis; Qiangyou Shi contributed to the statistical analysis; Meihua Yang contributed to the manuscript preparation, manuscript editing; Ruixia Ma contributed to the manuscript review.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Antra PP, Hungyo H, Jain A, Ahmad S, Tandon V. Unraveling molecular mechanisms of head and neck cancer. Crit Rev Oncol Hematol. 2022. 178:103778. doi:10.1016/j.critrevonc.2022.103778.
- Han X, Cheng X, Dai K, Bao W, Ding R, Wan Y. Identification of immunocell infiltrates and effective diagnostic biomarkers in laryngeal carcinoma. Medicine (Baltimore). 2023;102(3):e32548. doi:10.1097/MD.0000000000032548.
- Ke J, Jia L, Hu Y, Jiang X, Mo H, An X, Yuan W. Clinical and experimental study of a terahertz time-domain system for the determination of the pathological margins of laryngeal carcinoma. World J Surg Oncol. 2022;20(1):339. doi:10.1186/s12957-022-02788-8.
- Shen N, Duan XH, Wang XL, Yang QY, Feng Y, Zhang JX. Effect of NLK on the proliferation and invasion of laryngeal carcinoma cells by regulating CDCP1. Eur Rev Med Pharmacol Sci. 2019;23(14):6226–9. doi:10.26355/eurrev_201907_18441.
- Cavaliere M, Bisogno A, Scarpa A, D’Urso A, Marra P, Colacurcio V, De Luca P, Ralli M, Cassandro E, Cassandro C, et al. Biomarkers of laryngeal squamous cell carcinoma: a review. Ann Diagn Pathol. 2021; 54:151787. doi:10.1016/j.anndiagpath.2021.151787.
- Mannelli G, Lazio MS, Luparello P, Gallo O. Conservative treatment for advanced T3–T4 laryngeal cancer: meta-analysis of key oncological outcomes. Eur Arch Otorhinolaryngol. 2018;275(1):27–38. doi:10.1007/s00405-017-4799-x.
- Djukic V, Milovanovic J, Jotic AD, Vukasinovic M, Folic MM, Ivanov SY, Satueva DB. Laser transoral microsurgery in treatment of early laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2019;276(6):1747–1755. doi:10.1007/s00405-019-05453-1.
- Braz DS, Ribas MM, Dedivitis RA, Nishimoto IN, Barros AP. Quality of life and depression in patients undergoing total and partial laryngectomy. Clinics (Sao Paulo). 2005;60(2):135–142. doi:10.1590/S1807-59322005000200010.
- Li X, Gao L, Li H, Gao J, Yang Y, Zhou F, Gao C, Li M, Jin Q. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. J Infect Dis. 2013;207(3):479–488. doi:10.1093/infdis/jis698.
- Tsimplaki E, Argyri E, Sakellaridis A, Kyrodimos E, Xesfyngi D, Panotopoulou E. Oropharyngeal and laryngeal but not oral cancers are strongly associated with high-risk human papillomavirus in 172 Greek patients. J Med Virol. 2017;89(1):170–176. doi:10.1002/jmv.24614.
- Halec G, Holzinger D, Schmitt M, Flechtenmacher C, Dyckhoff G, Lloveras B, Höfler D, Bosch FX, Pawlita M. Biological evidence for a causal role of HPV16 in a small fraction of laryngeal squamous cell carcinoma. Br J Cancer. 2013;109(1):172–183. doi:10.1038/bjc.2013.296.
- Russell RC, Guan KL. The multifaceted role of autophagy in cancer. EMBO J. 2022;41(13):e110031. doi:10.15252/embj.2021110031.
- Cassidy LD, Narita M. Autophagy at the intersection of aging, senescence, and cancer. Mol Oncol. 2022;16(18):3259–3275. doi:10.1002/1878-0261.13269.
- Jain V, Singh MP, Amaravadi RK. Recent advances in targeting autophagy in cancer. Trends Pharmacol Sci. 2023;44(5):290–302. doi:10.1016/j.tips.2023.02.003.
- Lin C, Blessing AM, Pulliam TL, Shi Y, Wilkenfeld SR, Han JJ, Murray MM, Pham AH, Duong K, Brun SN, et al. Inhibition of CAMKK2 impairs autophagy and castration-resistant prostate cancer via suppression of AMPK-ULK1 signaling. Oncogene. 2021; 40(9):1690–1705. doi:10.1038/s41388-021-01658-z.
- Garcia-Mayea Y, Mir C, Munoz L, Benavente S, Castellvi J, Temprana J, Maggio V, Lorente J, Paciucci R, LLeonart ME, et al. Autophagy inhibition as a promising therapeutic target for laryngeal cancer. Carcinogenesis. 2019; 40:1525–1534. doi:10.1093/carcin/bgz080.
- Guo Y, Feng Y, Cui X, Wang Q, Pan X. Autophagy inhibition induces the repolarisation of tumour-associated macrophages and enhances chemosensitivity of laryngeal cancer cells to cisplatin in mice. Cancer Immunol Immunother. 2019;68(12):1909–1920. doi:10.1007/s00262-019-02415-8.
- Chen XH, Liu J, Zhong JT, Zhou SH, Fan J. Effect of GLUT1 inhibition and autophagy modulation on the growth and migration of laryngeal carcinoma stem cells under hypoxic and low-glucose conditions. Onco Targets Ther. 2021. 14:3069–3081. doi:10.2147/OTT.S300423.
- Li G, Cheng Z. miR-339-5p inhibits autophagy to reduce the resistance of laryngeal carcinoma on cisplatin via targeting TAK1. Biomed Res Int. 2021. 2021:9938515. doi:10.1155/2021/9938515.
- Papayannakos CJ, Zhu D, Jung B, Rana AA, DeVoti JA, Abramson AL, et al. Toll-like receptor agonists, poly(I: C) and flagellin, lead to IL-36gamma induction with divergent release kinetics and differentially alter autophagy in primary human keratinocytes. Eur Cytokine Netw. 2022;33:43–53.
- Tse SW, Tan CF, Park JE, Gnanasekaran J, Gupta N, Low JK, et al. Microenvironmental hypoxia induces dynamic changes in lung cancer synthesis and secretion of extracellular vesicles. Cancers Basel. 2020;12.
- Tang W, Ren A, Xiao H, Sun H, Li B. Highly expressed NRSN2 is related to malignant phenotype in ovarian cancer. Biomed Pharmacother. 2017. 85:248–255. doi:10.1016/j.biopha.2016.11.012.
- Keremu A, Maimaiti X, Aimaiti A, Yushan M, Alike Y, Yilihamu Y, Yusufu A. NRSN2 promotes osteosarcoma cell proliferation and growth through PI3K/Akt/MTOR and Wnt/β-catenin signaling. Am J Cancer Res. 2017;7(3):565–573.
- Yan Z, Xu T, Lu J, Wu Z, Li X, Xu J, Guo W, Dong Z. E2F1-activated NRSN2 promotes esophageal squamous cell carcinoma progression through AKT/mTOR pathway. Pathol Res Pract. 2022. 236:153963. doi:10.1016/j.prp.2022.153963.
- Jang JE, Eom JI, Jeung HK, Cheong JW, Lee JY, Kim JS, Min YH. Targeting AMPK-ULK1-mediated autophagy for combating BET inhibitor resistance in acute myeloid leukemia stem cells. Autophagy. 2017;13(4):761–762. doi:10.1080/15548627.2016.1278328.
- Ren F, Zhang W, Lu S, Ren H, Guo Y. NRSN2 promotes breast cancer metastasis by activating PI3K/AKT/mTOR and NF-kappaB signaling pathways. Oncol Lett. 2020. 19:813–823. doi:10.3892/ol.2019.11152.
- Wang G, Yang K. Neurensin‑2 promotes proliferation, invasion and migration of colorectal cancer cells via interaction with SOX12. Oncol Lett. 2020;20(6):1–1. doi:10.3892/ol.2020.12252.
- Huang HY, Li KN, Lau HC, Hsueh CY, Cong N, Zhang M. Dual inhibition of autophagy and PI3K/mTOR pathway as a potential therapeutic strategy against laryngeal squamous cell carcinoma. Transl Cancer Res TCR. 2022;11(5):1076–1088. doi:10.21037/tcr-21-2325.
- Chen L, Jia J, Zang Y, Li J, Wan B. MicroRNA-101 regulates autophagy, proliferation and apoptosis via targeting EZH2 in laryngeal squamous cell carcinoma. Neoplasma. 2019;66(4):507–515. doi:10.4149/neo_2018_180811N611.
- Zhang S, Xie Y, Yan F, Zhang Y, Yang Z, Chen Z, Zhao Y, Huang Z, Cai L, Deng Z, et al. Negative pressure wound therapy improves bone regeneration by promoting osteogenic differentiation via the AMPK-ULK1-autophagy axis. Autophagy. 2022; 18(9):2229–2245. doi:10.1080/15548627.2021.2016231.
