ABSTRACT
The introduction of novel immunotherapies has significantly transformed the treatment landscape of genitourinary (GU) cancers, even becoming the standard of care in some settings. One such type of immunotherapy, immune checkpoint inhibitors (ICIs) like nivolumab, ipilimumab, pembrolizumab, and atezolizumab play a pivotal role by disturbing signaling pathways that limit the immune system’s ability to fight tumor cells. Despite the profound impact of these treatments, not all tumors are responsive. Recent research efforts have been focused on understanding how cancer cells manage to evade the immune response and identifying the possible mechanisms behind resistance to immunotherapy. In response, ICIs are being combined with other treatments to reduce resistance and attack cancer cells through multiple cellular pathways. Additionally, novel, targeted strategies are currently being investigated to develop innovative methods of overcoming resistance and treatment failure. This article presents a comprehensive overview of the mechanisms of immunotherapy resistance in GU cancers as currently described in the literature. It explores studies that have identified genetic markers, cytokines, and proteins that may predict resistance or response to immunotherapy. Additionally, we review current efforts to overcome this resistance, which include combination ICIs and sequential therapies, novel insights into the host immune profile, and new targeted therapies. Various approaches that combine immunotherapy with chemotherapy, targeted therapy, vaccines, and radiation have been studied in an effort to more effectively overcome resistance to immunotherapy. While each of these combination therapies has shown some efficacy in clinical trials, a deeper understanding of the immune system’s role underscores the potential of novel targeted therapies as a particularly promising area of current research. Currently, several targeted agents are in development, along with the identification of key immune mediators involved in immunotherapy resistance. Further research is necessary to identify predictors of response.
ARTICLE HIGHLIGHTS
Immunotherapy has transformed the treatment landscape for many cancer types, including genitourinary malignancies such as renal and bladder cancers.However, not all patients or tumor types, such as prostate cancer, respond to this type of treatment.
Understanding the mechanisms of immunotherapy resistance is critical for developing strategies to overcome these challenges.
Primary resistance, which is present at the onset of treatment, can bedue to genetic abnormalities or immune system dysregulation. These factors alter the interactions between host cells and cancer cells.
Adaptive resistance develops during therapy due to dynamic changes in the levels of growth factors, cytokines, and the tumor microenvironment (TME).
Acquired resistance mainly occurs at the genetic and translational levels, involving the downregulation of critical human leukocyte antigen (HLA) molecules and interference with mutational repair.
Future therapies may focus on detailed genetic profiling of patients to guide treatment selection and on the use of immune profile monitoring to assist in assessing responsiveness, alongside developing novel targeted therapies and ICIs.
Further research is needed to identify predictors of response to ICIs.
Introduction
Genitourinary (GU) malignancies, including prostate, testicular, penile, bladder, ureteral, and kidney cancers, represent 24% of new cancer cases, totaling 468,100 new diagnoses annually. Based on the Cancer Statistics Explorer Network SEER*Explorer tool, the 5-year survival rate for bladder cancer at any stage is 77.9%, but this rate drops dramatically to 8.3% for metastatic disease.Citation1 A similar trend is observed in prostate cancer, where the 5-year survival rate for any stage of the disease is 97.1%, while the 5-year survival rate for metastatic disease falls to 34.1%.Citation2 Despite the annual increase in survival rates of GU cancers thanks to the development of new treatment options, better screening methods, and rapid initiation of therapy, improved treatment strategies remain necessary for metastatic disease in GU cancers.
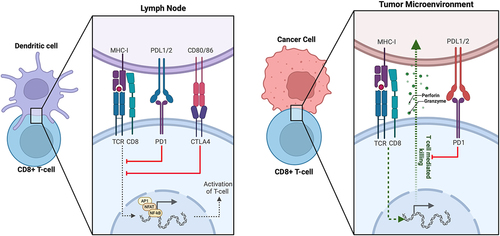
Immune checkpoint inhibitors (ICIs) were first approved by the FDA in 2011 for the treatment of late-stage melanoma. After being evaluated in numerous clinical trials for the treatment of various cancer types, including GU malignancies, the use of ICIs has shifted the landscape of cancer therapy.Citation3 ICIs are monoclonal antibodies that target specific regulatory immune receptors on the surface of cells. The mechanism of action of these agents is based on the inhibition of signals that attenuate the immune response, causing the immune system to exert a more robust immune response against tumor cells. The top three most widely used checkpoint inhibitor classes target immune inhibitory receptors: cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1), and programmed cell death receptor ligand-1 (PD-L1).Citation4 demonstrates the ligand-receptor interactions involved in immunological recognition of cancer cells on a cellular level. While ICIs have generally demonstrated good tolerability and effectiveness in GU malignancies, a significant number of patients do not respond to therapy, resulting in highly variable outcomes.Citation5 In this review, we examine the current mechanisms of resistance to immunotherapy in GU cancers and explore ongoing investigations into novel methods of overcoming this resistance.
Immunotherapy resistance
Despite the remarkable success of immunotherapy in treating bladder and renal malignancies, not all patients or tumor types respond favorably to these treatments, and many develop resistance. Clinically, this resistance may present as progressive tumor growth, worsening symptoms, or a decline in overall health indicators or functional status. In some cases, resistance can also be monitored through changes in tumor markers, inflammatory markers, immune cell activity, or genetic alternations that can be assessed through laboratory tests and tissue biopsies.Citation6 Imaging studies can offer additional visual evidence of resistance by monitoring disease progression or response to therapy. Identifying these indicators is crucial for adapting treatment plans to enhance patient outcomes.
Primary resistance
Primary resistance can be a formidable barrier to the initial success of immunotherapy treatments. Unlike adaptive or acquired resistance, which develops over time in response to the selective pressure of anti-tumor immunity, primary resistance occurs at the outset of therapy administration. It is rooted in the intrinsic properties of the cancer cells themselves, often manifested through genetic aberrations or dysregulation of factors that typically regulate the immune response. This complex interplay of genetic and micro-environmental factors enables the cancer to withstand immunological attacks.Citation7 Exploring the mechanisms of primary resistance uncovers a range of biological factors, from immune pathway disruptions to microbiome effects, that contribute to a cancer’s resistance to immunotherapy.
Tumor-intrinsic genetic and genomic factors
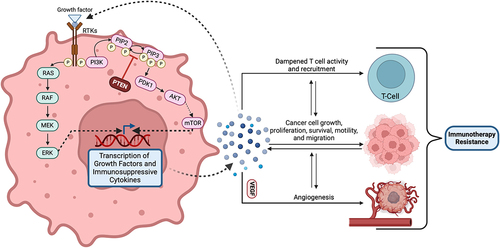
Intrinsic tumor factors encompass genetic and molecular alterations within the tumor cells that can inherently impede the efficacy of immunotherapies. Alterations in the mitogen-activated protein kinase (MAPK) pathway may represent a key intrinsic factor contributing to primary resistance in cancer cells. Oncogenic activation of the MAPK pathway is known to lead to the secretion of cytokines such as VEGF and IL-10, known to dampen T-cell activity and recruitment to tumor sites. This has been demonstrated to reduce the efficacy of T-cell-mediated immunotherapies.Citation8 Similarly, the loss of a tumor suppressor gene PTEN, which enhances PI3K signaling to promote malignant cell proliferation, is common in many cancers and effectively contributes to resistance against checkpoint inhibitors. The absence of PTEN is associated with reduced expression of immune response factors like IFN-γ (Interferon-gamma) and granzyme-B in immune cells, as well as reduced CD8+ T-cell infiltration. PTEN mutations have also been linked to a higher rate of ICI therapy resistance, suggesting an interplay between these mutations and an immunosuppressive tumor microenvironment (TME).Citation9 Alterations of these pathways have also been explored in genitourinary cancers. In renal cell carcinoma (RCC), PTEN mutations, though less common, have been linked to abnormal AKT activation, while MAPK pathway alterations are being explored for their prognostic potential.Citation10,Citation11 In bladder cancer, studies have focused on the prevalence and clinical significance of PTEN deletions, suggesting their association with disease progression, though the incidence of PTEN mutations appears low.Citation12 On the other hand, prostate cancer presents a higher frequency of PTEN genomic aberrations, seen in up to 20% of primary prostate tumors and 50% of castration-resistant tumors, highlighting their role in disease progression and resistance to therapies.Citation13
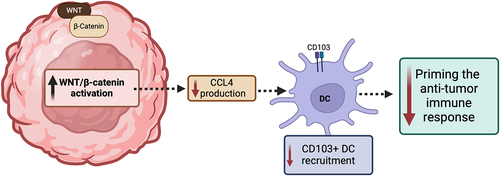
The ability of cancer-promoting signaling pathways to prevent T-cells from acting on tumor cells is also seen in the activation of WNT signaling, which is caused by the stabilization of β-catenin. In GU cancers, aberrant WNT signaling, notably in germ cell tumors, prostate, and bladder cancers, has been linked to tumor progression and therapeutic resistance. This emphasizes its potential as a target for novel treatments,Citation14–17 Tumors in murine models with elevated β-catenin lacked a particular type of dendritic cells (DCs) known as CD103+ DCs, due to diminished expression of CCL4, a cytokine.Citation18 These DCs are particularly important for engaging and activating CD8+ T-cells, given their enhanced ability to efficiently traffic to lymph nodes and cross-present tumor antigen to CD8+ T-cells, priming the anti-tumor immune response.Citation19,Citation20 Additionally, ICIs were found to be more effective in targeting tumors with loss of β-catenin compared to those expressing β-catenin.Citation18 Similarly, β-catenin signaling pathway-related genes were expressed to a greater degree in non-T-cell inflamed tumors. Studies in patients with melanoma found that individuals who initially responded to anti-PD-1 therapy but then developed metastases (i.e., developed resistance) exhibited new elevations of β-catenin expression and subsequent loss of T-cell infiltration. Mutations that enhance β-catenin signaling were notably prevalent in tumors lacking T-cell inflammation, with the Wnt/β-catenin pathway activated in the majority (90%) of these non-inflamed tumors.Citation21 demonstrates the effect of increased Wnt/β-catenin activation on the tumor response.
Alterations in antigen processing and presentation
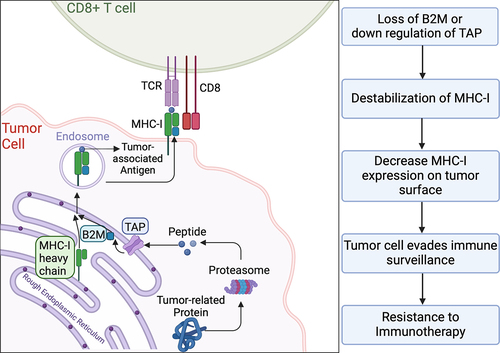
Studies have also identified the loss of β2-microglobulin (B2M) as a potential molecular pathway of tumor cells to evade immunity. Major histocompatibility complex class I (MHC-I) molecules play a pivotal role in presenting tumor neoantigens to immune cells, and B2M is essential for the proper assembly and presentation of MHC-I molecules on the cell surfaces.Citation22 Its absence hinders CD8+ T-cell recognition, impairing a key pathway of immune surveillance.Citation23 While B2M loss is somewhat less common in renal cancers compared to bladder and prostate cancers, renal cell carcinomas may exhibit other alterations that affect MHC- I presentation. This highlights the diverse mechanisms of immune evasion in genitourinary tumors. Furthermore, Gettinger et al. found that homozygous loss of B2M leads to the downregulation of MHC-I in cancer cells, further facilitating resistance to ICIs.Citation24 demonstrates how loss of B2M destabilizes the MHC-I.
An in vivo experiment demonstrated that immunocompetent mice when treated with anti-PD-1 therapy and injected with B2M-knockout or B2M-wild type cancer cells as a control group. These knockout cancer cells exhibited less sensitivity to PD-1 blockade compared to those in the control group. Further observations indicated a marked decrease in the destructive capability of CD8+ T-cells against these B2M-depleted cancer cells. This suggests that the absence of B2M facilitates evasion from ICIs by altering antigen presentation on the cell surface.Citation24 Additionally, comprehensive RNA sequencing, combined with flow cytometric evaluation in human patients with melanoma revealed that lower levels of MHC-I molecules are associated with resistance to PD-1 inhibitors. Interestingly, this resistance was also linked to increased levels of transforming growth factor-β (TGF- β) and cancer-associated fibroblast (CAF) signatures.Citation22
Microbiome-mediated effects
Recent findings have underscored the significant influence of the microbiome and intratumoral microbes on anti-tumor immune responses and the effectiveness of ICIs. Multiple retrospective studies have provided evidence linking the gut microbiota with prostate cancer, showing an increased incidence of prostate cancer in patients with inflammatory bowel disease (IBD) compared to matched controls,Citation25–27 In a study with 20 male participants, a higher presence of Bacteroides massiliensis was observed in the gastrointestinal microbiota of those diagnosed with prostate cancer while individuals without the disease exhibited a greater prevalence of Faecalibacterium prausnitzii and Eubacterium rectale. Notably, F. prausnitzii, a member of the Firmicutes phylum, is recognized for its production of butyrate, a short-chain fatty acid that exhibits anti-inflammatory effects, particularly in cases of colitis. In another study, researchers compared the gut microbiota of mice with prostate tumors, created by injecting a specific human metastatic cancer cell line (CRPC 22Rv1-M4), and healthy mice. The findings showed that mice with prostate tumors exhibited a higher concentration of certain bacterial families, specifically Akkermansiaceae, Bifidobacteriaceae, and Enterococcaceae. Furthermore, the study noted an increase in bacterial groups known for their steroid-producing ability in the mice injected with prostate cancer cells. This increase was associated with a more rapid progression of the disease.Citation28
Research has displayed the impact of prostate cancer-specific therapies, such as androgen receptor-targeted therapy (ATT), on gastrointestinal microbes. These microbes are not only affected by such treatments but also contribute to the development of resistance to these therapies. In patients with castration-resistant prostate cancer, gut microbes have the capability to convert pregnenolone into androgenic steroids. This has been demonstrated in mouse models, which have shown that androgen deprivation therapy leads to significant changes in the composition of the gut microbiome. This includes an increased presence of Ruminococcus and Bacteroides species, particularly observed in mice with castration-resistant disease.Citation29 This finding has been further corroborated through comprehensive metagenomic sequences in human cohorts of both hormone-sensitive and castration-resistant prostate cancer patients. A similar study examining the stool microbiota of 30 individuals across various stages of prostate cancer identified an elevated presence of bacteria involved in steroid biosynthesis within the gut microbiota of patients undergoing androgen-targeted treatments, compared to those not receiving any treatment. Species such as A. muciniphila, Ruminococcaceae, and Lachnospiraceae spp. were notably more prevalent in the stool samples of patients undergoing oral ATT.Citation30
Various studies have identified a diverse array of microbes within different types of cancers in addition to prostate cancers. These microbes are believed to play a role in cancer progression, from initiation to metastasis, and may even influence patient survival outcomes. For instance, in pancreatic cancer, certain microbial profiles have been correlated with longer survival.Citation31 In preclinical models, responses to therapies such as anti-CTLA-4 were found to be dependent on bacterial species, as mice that were germ-free or antibiotic-treated did not respond to the therapy.Citation32 These findings were further supported by clinical reports indicating that patients treated with ICIs who also received antibiotics experienced decreased overall survival (OS) and progression-free survival (PFS). Similar findings were observed for anti-PD-1, where other microbes were also implicated in influencing the therapy’s effectiveness.Citation33 It has been suggested that tumor cells may have the ability to present peptides derived from internal bacteria on their surface using human MHC molecules. This process potentially allows them to be recognized by T-cells that infiltrate the tumor. This mechanism might be a pathway through which bacteria present in tumors directly modulate the body’s immune defense against cancer.Citation34 In the context of RCC, studies highlighted the gut microbiota’s significant role in enhancing clinical responses to ICIs.Citation35,Citation36 For instance, De Rosa et al. observed in patients with advanced RCC that a diminished response to treatment was associated with the abundance of certain bacteria types such as Erysipelotrichaceae bacterium and Clostridium hathewayi.Citation37
These studies have expanded their scope to include bladder and prostate cancer, revealing that gut microbiome composition influences PD-1 blockade efficacy. In prostate cancer, recent literature highlights the microbiota’s role in the disease’s pathogenesis and treatment strategies. It has been indicated that specific species within the urinary and gut microbiome are associated with an increased risk of developing prostate cancer and its subsequent progression.Citation38 This evidence underscores the critical role of the microbiota in carcinogenesis, suggesting that modulating the host microbiota could offer new avenues for improving early detection rates and developing new treatment strategies for prostate cancer.Citation39
PD-L1 mediated resistance
In addition to tumor genetic factors, immunological factors further complicate the landscape of primary resistance. The expression of PD-L1, a protein commonly found on the surface of cancer cells, plays a crucial role in enabling tumors to evade the immune system. This mechanism is also positively associated with response to ICIs across various types of cancers.Citation40 Tumors that express PD-L1 can bind to PD-1 receptors on T-cells, thereby facilitating immune system evasion by leveraging this natural negative feedback mechanism design to dampen T-cell activation and activity. Given its canonical role in negatively regulating an activated immune system, the presence of PD-L1 may also indicate an attenuated, yet activated, immune microenvironment.Citation41 Consequently, tumors with low or no expression of PD-L1 may tend to be resistant to anti-PD-1 and anti-PD-L1 treatments, as seen in breast and lung cancers which have historically demonstrated low PD-L1 expression.Citation42,Citation43 Despite this trend, the absence of PD-L1 does not necessarily preclude a positive response to such therapies, nor does its presence guarantee the treatment success. This suggests that the efficacy of immunotherapy is determined by a broader spectrum of factors beyond just PD-L1 expression.Citation40
Influence of tumor mutational burden
Tumor mutational burden (TMB) is another well-studied potential biomarker, that predicts the efficacy of immunotherapies, such as PD-1 and CTLA-4 inhibitors, across a spectrum of cancers. This metric quantifies the number of mutations within a megabase of tumor DNA and has shown a significant correlation with treatment success in certain tumor types.Citation44 For example, lung cancer patients with higher TMB levels experienced better outcomes with nivolumab, as shown by exome analyzes.Citation45 Furthermore, the KEYNOTE-158 study demonstrated that a high TMB contributed to the effectiveness of pembrolizumab, leading to its FDA approval for TMB-high tumors. Despite the strong link between TMB and treatment response, tumors with lower mutational loads, such as those found in prostate and pancreatic cancers, typically exhibit less favorable responses to immunotherapies.Citation45 Nevertheless, certain studies point out exceptions, showing that low TMB does not universally predict poor outcomes.Citation46 In cases like metastatic renal cell carcinoma and polyomavirus-positive Merkel cell carcinoma, patients have still responded positively to ICIs despite their low TMB.Citation47 Thus, although a high TMB is generally associated with better response to ICIs, the interplay between mutational burden and immunotherapy efficacy is complex and influenced by factors beyond TMB alone.
Adaptive resistance
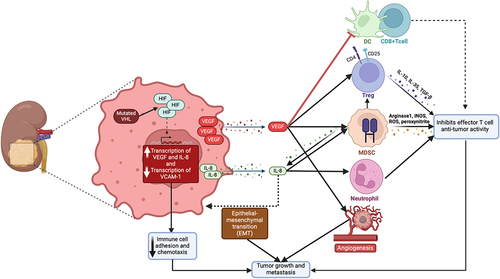
Adaptive resistance primarily involves dynamic tumor modifications, whereby tumors counteract the immune system’s attacks during therapy. This phenomenon not only complicates treatment strategies but also underscores the urgent need for innovative approaches to overcome or prevent tumor adaptations and developed resistance to current therapies. For example, dynamic expression of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) by tumor cells have been identified in several preclinical models and some human cancers as another strategy to modulate the TME in favor of tumor survival and cancer cell proliferation.Citation48 VEGF plays a particularly crucial role in renal cancers, making it a common target for therapeutic strategies. In RCC, its role in promoting angiogenesis is pivotal, as it not only sustains tumor growth but also contributes to creating an immunosuppressive microenvironment, a hallmark of this cancer type’s progression.Citation49 Angiogenesis, the formation of new blood vessels, is essential for supplying the tumor with necessary nutrients and oxygen. Moreover, it enables the tumor to exert its influence on the immune system, further emphasizing the multifaceted impact of VEGF in cancer dynamics.
VEGF is also known to contribute to the creation of an immunosuppressive environment.Citation50 It directly inhibits the maturation and function of dendritic cells, crucial orchestrators of the immune response, thereby impairing the T-cell activation.Citation51 Furthermore, VEGF facilitates the recruitment of regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs), both essential in maintaining the immune suppressive TME.Citation52 By promoting these suppressive cell populations, VEGF helps shield the tumor from immune surveillance. RCC, especially its most common form, clear cell renal cell carcinoma (ccRCC), the VEGF pathway plays a crucial role due to the inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene. This leads to the upregulation of hypoxia-inducible factors (HIF), which in turn drive VEGF overexpression, a key factor in the progression of RCC.Citation53
IL-8 works synergistically with VEGF to create an immunosuppressive TME. Acting as a chemokine, it attracts neutrophils and MDSCs to the tumor site. These cells then release proteases and reactive oxygen species, creating a hostile environment that inhibits T-cell function and thus further impairs the anti-tumor immune response.Citation54 Additionally, IL-8 is implicated in enhancing tumor invasiveness and metastasis by promoting the epithelial-mesenchymal transition (EMT) of tumor cells, which facilitates their spread throughout the body.Citation55 Together, VEGF and IL-8 orchestrate a microenvironment that not only favors tumor growth and spread but also renders strategically less accessible to immune effector cells. Specifically, in the context of GU tumors, the role of IL-8 is crucial as it contributes to a TME that facilitates tumor progression and metastasis. This is evidenced by elevated IL-8 expression observed not only in various tumor cell lines and tissues but also in the peripheral blood of cancer patients. Specifically, the interaction of IL-8 with components of the TME, such as fibroblasts, endothelial cells, and immune cells, plays a significant in molding the immune landscape within GU tumors. This interaction affects both tumor progression and therapeutic responses. Moreover, elevated IL-8 expression leads to the recruitment of myeloid cell lines including MDSCs, CD15+ monocytes, and neutrophils, which have been shown to suppress adaptive T-cell antitumor immunity,Citation56–58 Transcriptomic analysis of circulating and tumor-infiltrating IL-8-producing MDSCs has revealed an increased expression of pro-inflammatory myeloid genes coupled with downregulation of antigen-presentation and interferon-inducible genes, further underscoring the role of IL-8 in compromising adaptive immunity in the context of the GU tumors.Citation59
Interferon-gamma (IFN-γ) plays a dual-faceted role in tumor progression and immune surveillance. Produced by CD8+ T-cells, IFN-γ enhances antigen presentation, facilitates immune cell recruitment, and exerts anti-proliferative effects on tumors. However, continuous exposure to this factor can lead to genetic instability in tumors, followed by altered DNA repair and editing genes. This genetic reshuffling may result in tumor escape from immune detection and the development of resistance.Citation60 Central to this resistance mechanism is the mutation or downregulation of components in the IFN-γ signaling pathway, particularly the JAK-STAT signaling cascade. Specific mutations, such as those in the IFN-γ receptor kinases JAK1 and JAK2, are associated with resistance to immunotherapies, including anti-CTLA-4 and anti-PD-1 treatments, which may be mediated through decreased sensitivity to IFN-γ. For instance, tumors harboring these mutations often exhibit a lack of PD-L1 expression upon IFN-γ exposure, making them less susceptible to T-cell-mediated destruction.Citation61 IFN-γ plays a pivotal role in tumor immunity by enhancing antigen presentation, facilitating immune cell recruitment, and exerting anti-proliferative effects on tumors. It inhibits angiogenesis in tumor tissue and induces apoptosis in Tregs, as well as stimulates the activity of M1 proinflammatory macrophages, which are all crucial for combating tumor progression. Its pleiotropic nature, encompassing both antiviral, antitumor, and immunomodulatory functions, allows IFN-γ to coordinate both innate and adaptive immune responses. However, understanding this complex dual role in promoting pro-tumorigenic and antitumor immunity within the TME is critical, especially in the context of GU tumors.Citation62
The TME plays a major role in enabling tumor immune evasion and resistance to immunotherapy, primarily through the secretion of various immunomodulatory substances. In the context of immunotherapy, outcomes are significantly influenced by factors such as the decline in immune function associated with aging, overall tumor mutational burden, and mutations in specific tumor suppressor/DNA damage repair genes. Among these, midkine, a protein linked with negative outcomes and decreased responsiveness to therapies targeting the PD-1/PD-L1 axis in melanoma, has also been identified in the TME of GU tumors. High midkine expression correlates with a TME that supports the presence of Tregs and tumor-associated macrophages (TAMs), both of which contribute to tumor progression and are linked to a poorer prognosis.Citation63
In RCC, alterations in the VHL gene cause abnormal HIF accumulation and the activation of angiogenic pathways, affecting both disease progression and severity. The TME in RCC, characterized by inflammation and the presence of immune cells, necessitates a shift in treatment focus toward modifying the microenvironment rather than directly targeting the tumor cells. Prostate adenocarcinoma, the primary treatment approach involves hormone-based therapies such as chemical castration or androgen deprivation therapy (ADT). However, resistance to ADT often necessitates combined therapeutic approaches, influenced by the complex cellular interactions within the TME. In bladder cancer, the variability in the immune profile of its TME further complicates the prediction of immunotherapy efficacy.Citation64
In the context of cytokines within the TME, TGF-β emerges as a multifaceted regulator, affecting a wide range of cellular interactions. Elevated levels of TGF-β have been associated with reduced effectiveness of anti-PD-1 therapy and overall poorer patient outcomes.Citation65 Moreover, the influence of TGF-β’s on stromal components, such as cancer-associated fibroblasts, has been linked to decreased T-cell infiltration, which potentially leads to an environment that excludes these vital anti-tumor immune cells.Citation66 Adenosine, another immune-modulating agent found within the TME, has been observed to contribute to creating an immunosuppressive environment in GU cancers that adversely affecting patient prognosis.Citation67 Clinical trials are currently investigating new therapies that target adenosine pathways alongside anti-PD-1 treatment.Citation65 Despite progress in understanding these dynamic immune components within the TME of GU cancers, significant knowledge gaps persist. A deeper understanding of these mechanisms holds the potential for the development of prognostic and predictive biomarkers, thereby increasing the precision of immunotherapy and improving therapeutic outcomes in GU cancers.
In addition to the tumor-immune cell interactions within the TME, metabolic dynamics, such as hypoxia, play a crucial role in influencing tumor immunity. Hypoxia is one of the hallmarks of the TME, promoting unregulated cell proliferation in tumors and erratic vascular growth, thus limiting essential resources like oxygen. These hypoxic conditions within tumors lead to diminished MHC-I expression in tumor cells and dendritic cells.Citation68 Additionally, within the RCC TME, tumor-associated immune cells including T-cells and NK cells, often exhibit compromised mitochondrial function.Citation69 Notably, hypoxia promotes the secretion of T-cell suppressors like adenosine and galectin-1. While adenosine increases intracellular cAMP levels which leads to immune suppression, galectin-1 plays a crucial role in cell adherence, tissue invasion, and vascular formation.Citation70,Citation71 Furthermore, hypoxia within the TME attracts TAMs to these hypoxic zones. TAMs, in turn, are known to increase resistance to several cancer treatments and elevate the risk of cancer recurrence.Citation72
Mutations in the VHL gene, commonly seen in ccRCC, impair the function of the VHL protein. These mutations are crucial in the development of ccRCC by altering the cell’s response to hypoxia, which is critical for regulating angiogenesis, cell proliferation, and tumor progression.Citation73 The VHL gene is key in regulating HIFs, including HIF1α and HIF2α. depicts the down-stream effects of VHL dysfunction. Normally, VHL mediates the breakdown of HIFα subunits under oxygen-rich conditions. However, with dysfunctional VHL, as often occurs in ccRCC, there’s continuous activation of HIF-1, which promotes the angiogenic nature of these tumors.Citation74 Furthermore, a study investigating the role of VHL in ccRCC found that the loss of VHL, the presence of hypoxia, or the inactivation of prolyl hydroxylase domain-containing proteins reduces vascular cell adhesion molecule 1 (VCAM-1) levels through a transcriptional mechanism. VCAM-1 is essential for immune cell adhesion and chemotaxis, and its reduced expression is linked to tumor aggression. Interestingly, in ccRCC samples with nonmissense VHL mutations, there’s an inverse correlation between VCAM-1 level and indicators of tumor aggressiveness such as tumor stage, symptoms, and microvascular invasion, suggesting that higher VCAM-1 level, with functional VHL protein, might enhance antitumor immunity.Citation75 These insights underscore the significant role of VHL alterations in affecting immune cell function and the tumor microenvironment, leading to a TME that is suppressive to the immune system and presents obstacles to successful immunotherapy in RCC.
In a study conducted by Brauch et al. on 227 sporadic ccRCC tumors analyzed for VHL-altering events, it was found that the combined rate of VHL mutation and promoter hypermethylation was 45%.Citation75 The study further identified that the presence of VHL mutation or hypermutation events was significantly associated with poor prognostic markers, notably for the pT3 tumor stage. Another study examining 187 patients with ccRCC revealed that VHL mutations were associated with worse patient outcomes at Stage III. However, in Stage IV cases, this correlation was not significant. This suggests that the impact of VHL mutations is more pronounced in earlier stages when assessing the potential for metastatic disease.Citation76 This highlights the complexity and adaptability of RCC tumors in evading immune surveillance, especially considering the near-universal presence of alterations in hypoxia sensing mechanisms, such as VHL mutations, in these tumors.
CAFs play a pivotal role in shaping the TME of GU cancers, particularly in terms of the immunosuppressive features of that environment. These cells, found in both primary and metastatic tumors, are involved in a range of functions, including extracellular matrix remodeling, metabolism modulation, angiogenesis, and the production of growth factors, cytokines, and chemokines.Citation77 They display a high degree of plasticity and versatility, adapting and responding to the dynamic TME.Citation78 The interactions between the stroma and tumor cells are related to resistance to ICIs. CAFs are implicated in creating an environment that impedes T-cell activity, contributing to the formation of “tumor deserts” that exclude immune cells. Furthermore, the patterns of gene expression related to CAFs are correlated with reduced T-cell infiltration and diminished responsiveness to widely used ICIs such as nivolumab.Citation79
The extracellular matrix built by CAFs limits T-cell movement, potentially hindering their tumor-killing functions. It has been reported that substances secreted by CAFs can weaken immune defenses against the tumor.Citation80 A study focusing on the role of CAFs in bladder cancer progression found that CAFs, activated by cancer-derived exosomes, induce EMT in cancer cells, thereby enhancing their growth, migration, and invasion. This process was primarily driven by the cytokine IL-6 secreted by CAFs, with the inhibition of IL-6 significantly reversing the EMT phenotype, highlighting its crucial role in bladder cancer progression.Citation81 In another study examining the impact of CAFs in urothelial bladder cancer (UBC), it was revealed that CAFs, through the overexpression of miR-146a-5p, promote cancer stemness and chemoresistance to drugs like gemcitabine and cisplatin. This process involves miR-146a-5p’s regulation of key signaling pathways in UBC cells, where increased levels of this microRNA in patient serum are correlated with advanced tumor stages and increased relapse risk, suggesting its potential as both a biomarker and therapeutic target in UBC.Citation82
Beyond CAFs, other stromal cells specific to certain tissues can also play a role in influencing resistance to ICIs. In the case of prostate cancer bone metastases, resistance to ICIs has been partially attributed to the release of TGF-β following bone degradation, which affects the numbers of T helper type 1 (Th1) cells.Citation83 Additionally, in brain metastases, phosphoSTAT3-positive astrocytes have been shown to suppress CD8+ T-cell activity and increase a subset of microglia/macrophages that facilitate immune evasion, potentially contributing to immunotherapy resistance.Citation84 In the context of GU cancers, these insights into the biology and function of CAFs are essential for developing more effective therapeutic strategies. The identification and precise characterization of different populations of CAFs and their tumor-promoting or restraining functions could lead to novel diagnostic and therapeutic approaches. As our understanding of the diversity and roles of these CAFs in cancer evolves, it offers new avenues for targeted therapies that could potentially improve outcomes in GU cancers.
Acquired resistance
In the context of acquired resistance, one of the broad categories of escape strategies that impact the response to anti-PD-1 therapies is the loss of heterozygosity in HLA genes. These genes are essential for distinguishing self from non-self and clearing pathogens. Notably, 40% of patients exhibiting loss of HLA heterozygosity also showed a consequent loss of neoantigen presentation, leading to immune evasion.Citation85 Moreover, cancer cells have developed multiple strategies for HLA downregulation, including genetic, epigenetic, post-transcriptional, and translational modifications, all aimed at evading detection by the immune system.Citation86 For example, in a case of metastatic melanoma, an initially effective response to pembrolizumab, an anti-PD-1 therapy, was eventually overcome by resistance due to a new truncating mutation in the B2M gene, crucial for the stability of MHC-I.Citation87 Further studies have reinforced the important role of these genetic factors in immune response, revealing that mismatch repair-deficient tumors that are resistant to anti-PD-1 therapy also contain mutations in B2M gene.Citation88
Epigenetic modifications have also been shown to play a role in resistance against ICIs. Alterations in the epigenetic landscape of tumor cells, such as those involving the Switch/Sucrose non-fermenting (SWI-SNF) chromatin remodeling complex, have been implicated in sensitizing tumors to ICIs. Epigenetic modulators can enhance the anti-tumor response of ICIs by increasing the production of chemokines crucial for attracting CD8+ T-cells to the tumor microenvironment.Citation89 Despite these findings, the exact role of the tumor’s epigenetic makeup in resistance, particularly in relation to T-cell exhaustion, remains unclear, necessitating more research in this area.Citation90 Mutations and epigenetic silencing within the interferon receptor signaling pathway have been shown to negate the anti-tumor effects of IFN-γ, demonstrating how the tumor can escape immune surveillance. These alterations can lead to diminished responses to the potent anti-cancer activity of IFN-γ.Citation91 Furthermore, genetic and epigenetic alterations that impair antigen presentation are also known to contribute to both primary and acquired resistance to ICIs, an effect observed irrespective of the TMB.Citation92 This highlights the complexity of resistance mechanisms, suggesting that a high TMB alone is not sufficient to ensure responsiveness to ICIs, as cancer cells can evade the immune system by reducing MHC expression through a combination of genetic and epigenetic mechanisms. These mechanisms include NF-kB pathway dysregulation, hypermethylation and deacetylation of the MHC promoter, interference by noncoding microRNAs, and suppression of chaperone protein expression, all contributing to reduced antigen presentation and immune detection.Citation93,Citation94
Recent epigenetic investigations of RCC have highlighted the significant role of Polybromo-1 (PBRM1) gene mutations in creating a TME that is less responsive to immune system interventions. Specifically, the loss of PBRM1 disrupts the interaction between the Brahma-related gene 1 (BRG1) and the IFN-γ receptor 2 (IFN-γ r2) promoter, leading to decreased STAT1 activation and IFN-γ target gene expression. This impacts IFNγ-STAT1 signaling pathways in both human and murine RCC cell lines, resulting in a decrease in immune response within the tumor and manifesting as resistance to ICIs. This resistance was parallel in clinical observations of nearly 700 ccRCC patients, showing poor response to ICIs and reduced immune cell infiltration in cases with PBRM1 mutations. Further research into human ccRCC has revealed a significant link between PBRM1 mutations and reduced expression of genes within the immunomodulatory signature. This pattern, affecting immune responses like IFN-γ and IFN-α, was consistent across various patient cohorts, reinforcing the notion that PBRM1 mutations on the immune dynamics of RCC and its responsiveness to immunotherapy.Citation95 These mutations not only disrupt crucial immune signaling pathways but also alter the tumor’s immune environment, influencing the tumor’s response to immunotherapy.
Tregs, characterized by expression of CD4, CD25, and the transcription factor FoxP3, play a pivotal role in immune regulation within the TME. They help maintain self-tolerance by restricting the activity of CD4+ and CD8+ T-cells through various mechanisms, including the secretion of immunosuppressive cytokines such as IL-2, IL-10, IL-35, and TGF-β.Citation96 The presence of Tregs is associated with a dampening of the anti-tumor response, and the ratio of effector T-cells (Teff) to Tregs has been suggested as a predicting factor for the efficacy of ICIs.Citation97 An increased Teff-to-Treg ratio has been correlated with improved responses to these treatments. Conversely, resistance to ICIs may develop due to compensatory Treg proliferation or the upregulation of alternative checkpoint molecules on these cells.Citation98 A study involving bladder cancer patients who underwent radical cystectomy revealed a higher frequency of CD4+ Tregs, identified by high expression of CD4, CD25, FOXP3, and low/neg CD127 expression, in tumor-draining lymph nodes in cases of advanced-stage disease.Citation99 These findings suggest that Tregs may hinder anti-tumor immune responses, highlighting their potential impact on the effectiveness of immunotherapy in bladder cancer treatment.
MDSCs have been identified as key players in creating an immunosuppressive environment in the TME. They impair the functions of Teff and NK cells through mechanisms involving substances such as arginase 1, inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), and peroxynitrite.Citation100 Furthermore, MDSCs influence the maturation of Tregs and drive macrophages toward a suppressive phenotype, contributing to tumor angiogenesis, invasion, and metastasis.Citation101 Clinical data indicate a correlation between MDSCs presence and poorer cancer outcomes, including diminished responses to immunotherapies.Citation102
Experimental studies in rodent cancer models have revealed that inhibiting macrophage PI3Kγ activity enhances the response to ICIs, reduces tumor size, and improves survival.Citation103 This improvement is associated with heightened pro-inflammatory signaling and decreased suppressive factors within tumors. Furthermore, inhibiting PI3Kγ leads to the upregulation of immune activation markers in macrophages, pinpointing PI3Kγ as a pivotal regulator of macrophage function in cancer.Citation104
Investigations into the dynamic nature of TAMs have revealed a wide range of functional states, notably the classic pro-inflammatory, tumor-combative M1 macrophages and the anti-inflammatory, tumor-promoting M2 macrophages, which serve opposing roles. M1 macrophages are known for their role in activating anti-tumor immunity through inflammatory cytokines, whereas M2 macrophages are implicated in down-regulating immune responses by releasing cytokines that suppress CD8+ T-cell activity, recruit Tregs, and facilitate the evasion of tumor cells from immune surveillance. M2 macrophages also often exhibit surface molecules like PD-L1 which further contribute to their ability to suppress immune responses.Citation105,Citation106 Various studies have shown that disrupting the M2 macrophage pathway and guiding these cells toward an M1 phenotype can potentially improve the efficacy of ICIs.Citation107 In addition, an imbalance marked by a lower ratio of adaptive immune responses to pro-tumorigenic signals within phagocytic myeloid cells has been associated with a lack of response to PD-L1 blockade therapy, particularly in cancer types such as urothelial cancer.Citation108
In solid tumors, such as prostate cancer, a significant portion of the tumor mass consists of macrophages, particularly TAMs, which predominantly exhibit M2-like characteristics. These TAMs play a crucial role in various cancer stages, from the initial development to metastasis, by influencing the tumor’s malignant properties. A higher presence of TAMs in prostate cancer correlates with more severe clinicopathological features, making them a key focus for new treatments. Specifically, in advanced stages like metastatic castration-resistant prostate cancer, an increase in M2-TAMs is linked to a higher likelihood of cancer recurrence and increased mortality rates. This trend highlights the pivotal role of macrophage behavior in determining cancer outcomes. Notably, the prevalence of M2-TAMs progressively increases from healthy prostate tissue to high-stage prostate cancer, and their high concentration is closely associated with elevated Gleason scores, indicating poorer survival rates and a higher chance of recurrence following hormone therapy.Citation109 This emerging evidence underscores the significance of macrophage plasticity in influencing the outcomes of immunotherapies.
Within the spectrum of acquired resistance, genetic and cellular changes in cancer cells are particularly critical, as they can profoundly alter the tumor’s interaction with therapeutic agents and the immune system. Cancer cells can evade immune detection and become resistant to treatments like ICIs by acquiring mutations that alter the antigens targeted by immune cells.Citation110,Citation111 As mentioned above, mutations affecting the IFN-γ pathway, the aging-associated immune function decline, the overall tumor mutational burden, and mutations in specific tumor suppressor/DNA damage repair genes (KDM6A, PTEN, STAG2, TP53, ATM, and BRCA2) have been associated with suboptimal responses in GU cancers.Citation112,Citation113 Additionally, the behavior of TAMs and host CD8+ T-cells is crucial to the effectiveness of immunotherapy. Further investigation into epigenetics and the cellular interactions between immune and tumor cells is crucial to tailoring current treatments and identifying novel therapeutic targets.
Approaches to predicting and overcoming immunotherapy resistance
As discussed above, primary resistance involves patient factors that are present and often quantified prior to initiating therapy. One such widely utilized example is the percentage of PD-L1 expression on tumor cells, intended to predict the efficacy of PD-L1 inhibition, though outcomes vary. Additional pathways, proteins, and cytokines of interest for predicting the efficacy of immunotherapies include MAPK, PTEN, WNT, IL-8, and IFN. Zhang, et. al. recently discussed a prognostic risk model of MAPK pathway-related proteins in ccRCC.Citation114 Using RNA sequencing data and transcriptome analyzes, they identified numerous MAPK-related genes with abnormal expression in ccRCC patients and subsequently were able to risk-stratify patients and identify those patients at high risk for severe and progressive disease based on their data. An additional study in urothelial carcinoma by Bekele, et. al. demonstrated that RAF1 amplification may increase susceptibility to MAPK-directed treatments.Citation115 Liu et. al. investigated the loss of PTEN association with therapeutics, finding that PTEN-deficient cells demonstrated hypersensitivity to rapalogs such as everolimus and temsirolimus.Citation116 demonstrates the role of PTEN in the TME. This suggests the importance of expanding pre-treatment genetic and biomarker profiling to identify alterations that may influence therapy responsiveness. Genetic profiling could also uncover susceptibilities to combination therapies that are not previously considered.
In the context of cytokines, several trials, including analyzes from the CheckMate025 and IMMotion150 trial cohorts that analyzed RCC patient samples following treatment with ICIs (both combination or single agent), reported poorer outcomes for individuals with higher pre-treatment plasma levels of IL-8. Elevated initial plasma IL-8 levels may refine patient selection for ICIs, particularly for those with a high T-cell effector signature score. This score reflects CD8+ T-cell activity and is associated with improved outcomes of ICIs in RCC and urothelial cancer. Patients with both a high T-cell effector score and low IL-8 levels tend to benefit the most from ICIs.Citation48
IFN-γ has additionally been explored in the context of GU tumors. Sakatani et al.‘s study on advanced urothelial cancer patients found that high IFN-γ expression in the TME significantly correlates with improved PFS following pembrolizumab treatment, highlighting IFN-γ’s prognostic relevance in response to ICIs.Citation117 In addition, a study involving 234 urinary bladder cancer patients demonstrated that a high IFN-γ gene signature is inversely related to mortality risk.Citation118 Particularly, those with the highest IFN-γ gene signature tertile had a substantially reduced mortality risk, indicative of better survival outcomes. These insights into IFN-γ’s impact on GU tumors emphasize the importance of understanding its complex role in tumor progression and immunotherapy response, including its interaction with key pathways like the JAK-STAT signaling cascade.Citation62
As previously mentioned, the gut microbiome has been studied to identify baseline characteristics that might identify patients as either more or less susceptible to ICIs. A phase I trial involving nivolumab and ipilimumab, with or without the live bacterial supplement CBM588 (bacterium spp Clostridium butyricum), showed a trend toward improved outcomes. This suggests that manipulation of the gut microbiome could enhance the response to ICIs.Citation119 Similarly, a randomized trial found that dietary interventions with a probiotic containing Bifidobacterium spp. in patients receiving VEGF tyrosine kinase inhibitors (VEGF-TKIs) successfully modulated the microbiota levels of this bacteria and led to some clinical benefit in the treatment group.Citation120 In RCC patients receiving ICIs, greater gut microbiome diversity was associated with more positive treatment outcomes and heightened clinical benefit.Citation121
Immune profiling of patients may also hold significance in the context of previously discussed immunomodulation across various cancer types. For example, studies involving ovarian cancer have shown that an increased presence of Tregs is often associated with unfavorable prognoses.Citation122 Current research is exploring strategies such as reducing Treg numbers and modifying checkpoints with antibodies to enhance the presence of tumor-reactive tumor-infiltrating lymphocytes (TILs), thereby potentially inhibiting cancer progression. The therapeutic goal is to shift the balance toward Teff, thereby amplifying the immune system’s ability to target and destroy tumor cells. Interestingly, a high baseline level of FoxP3+ Tregs within a tumor does not necessarily predict poor outcomes.Citation98,Citation123 In some cases, these Tregs co-exist with active immune responses, and their presence has been associated with improved clinical outcomes following anti-CTLA-4 therapy.Citation123 Ongoing research is focused on fully understanding the role of Tregs and their impact on the efficacy of immunotherapies in different cancer settings.
In a similar vein, the concurrent administration of a PI3Kγ inhibitor with anti-PD-1 therapy significantly elevated the success rate of tumor rejection and increased survival in animal studies related to bladder cancer.Citation104 Investigators analyzed MDSCs in 113 bladder cancer patients and found that these patients had higher numbers of MDSCs compared to healthy volunteers, with MDSC counts correlating with clinical grade, stage, and poor prognosis.Citation124 The study also revealed that lower serum IL-6 levels in bladder cancer patients were associated with increased MDSC formation, suggesting that targeting IL-6 signaling could be a potential treatment strategy. This highlights MDSCs as a possible prognostic marker in bladder cancer.
An additional study focusing on the role of MDSCs in cisplatin-resistant bladder cancer revealed that these cells, particularly the monocytic subtype, are more prevalent in resistant tumors. It was demonstrated that therapies targeting MDSCs, when used in combination with PD-L1 inhibitors, significantly reduced tumor size and increased CD + 8 T-cell activity, suggesting a promising approach for treating cisplatin-resistant bladder cancers.Citation125 Furthermore, a systematic review and meta-analysis, which included 16 studies with 1864 patients, found that a higher frequency of MDSCs was associated with shorter OS and poor disease-free survival/recurrence-free survival after treatment.Citation126
Therapeutic approaches such as Chimeric antigen receptor T (CAR T) cells and Bi-specific T cell engagers (BiTE) have been developed as an option for resistant malignancies associated with minimal response to ICIs. These more novel agents utilize the patient’s own immune cells which are extracted and bioengineered to identify, bind to, and eliminate cancer cells more effectively.Citation127 Most relevant to our discussion in this review are the currently described methods of resistance observed in CAR T and BiTE therapies. Shen et al. describe how loss of CD58 influences resistance via down-regulation of the extrinsic apoptotic pathway, and how inhibiting PI3K expression may potentiate the efficacy of BiTE therapy.Citation128 Interestingly, the group also demonstrated that loss of IFN-γ signaling, while typically associated with resistance to ICIs, was associated with greater efficacy of BiTE therapy.Citation128,Citation129 This relationship demonstrates the dynamic role of IFN-γ signaling in T-cell based therapies. Studies have also identified that co-expression of CCR8 and TGF-β receptor 2 on CAR T cells improves their ability to overcome innate immune suppression by T regulatory cells.Citation130 Currently, these treatments are reserved for patients who have undergone multiple lines of chemotherapy and demonstrated treatment failure, and as such further research is needed to study the impact of chemotherapy-driven immune alteration on the efficacy of CAR T and BiTE therapy, as well as the contextual role of the TME on the modality of treatment.
Conclusion
Immunotherapy has proven its effectiveness in treating various GU malignancies, particularly in bladder and kidney cancers. Despite the increasing use of ICIs in combination with each other, chemotherapy, and other targeted therapies, a significant number of patients still experience progression on treatment. As discussed, there are multiple mechanisms of immunotherapy resistance that require further investigation, including genetic profiling and real-time assessment of the dynamic changes of biomarker expression and mutations during therapy. While the gut microbiome has been clearly shown to impact immune responsiveness, lack of consistent data with similar species across cancer types emphasizes the need for further research to better understand this complex relationship. Additionally, challenges persist in identifying reliable prognostic indicators of response to ICI and the role of immunomodulation and immune profiling in guiding the selection of treatment regimens. Correlation with mechanisms of resistance observed in more novel cellular-based therapies may also provide insight into better understanding the mechanisms we describe here. Although immunotherapy is generally well tolerated, novel ICIs are currently being developed to enhance therapeutic benefits for patients who have shown resistance to current immunotherapy regimens, addressing unmet needs in the treatment landscape.
Disclosure statement
M.A. Bilen has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, EMD Serono, SeaGen, and Sanofi and has received grants to his institution from Merck, Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, SeaGen, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Exelixis, Nikang, Loxo Oncology, Ambrx, Regeneron, Acrivon Therapeutics, Amgen, Genome & Company, AAA, Peloton Therapeutics, and Pfizer for work performed as outside of the current study.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, MAB. The data are not publicly available due to production of illustrations by an author and protection of independent work.
Additional information
Funding
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–15. doi:10.3322/caac.21763.
- SEER*Explorer. An interactive website for SEER cancer statistics. 2023.
- Moreira M, Pobel C, Epaillard N, Simonaggio A, Oudard S, Vano Y-A. Resistance to cancer immunotherapy in metastatic renal cell carcinoma. Cancer Drug Resistance. 2020;3(3):454–471. doi:10.20517/cdr.2020.16.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515(7528):568–571. doi:10.1038/nature13954.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi:10.1126/scitranslmed.aad7118.
- Shan J, Han D, Shen C, Lei Q, Zhang Y. Mechanism and strategies of immunotherapy resistance in colorectal cancer. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.1016646.
- Said SS, Ibrahim WN. Cancer resistance to immunotherapy: comprehensive insights with future perspectives. Pharmaceutics. 2023;15(4):1143. doi:10.3390/pharmaceutics15041143.
- Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, Chen JQ, Li HS, Watowich SS, Yang Y, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013; 19(2):393–403. doi:10.1158/1078-0432.CCR-12-1626.
- Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, Bordbar D, Shan D, Samanamud J, Mahajan A, et al. Author correction: immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019; 25(6):1022. doi:10.1038/s41591-019-0449-8.
- Miricescu D, Balan D, Tulin A, Stiru O, Vacaroiu I, Mihai D, Popa C, Papacocea R, Enyedi M, Sorin N, et al. PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (review). Exp Ther Med. 2021; 21(5):540. doi:10.3892/etm.2021.9972.
- Zhang P, Li J, Wang Z, Zhao L, Qiu J, Xu Y, Wu G, Xia Q. Establishment of a new prognostic risk model of MAPK pathway-related molecules in kidney renal clear cell carcinoma based on genomes and transcriptomes analysis. Front Oncol. 2023;13:1077309. doi:10.3389/fonc.2023.1077309.
- Mashhadi R, Pourmand G, Mehrsai A, Pakdel S, Dialameh H, Ahmadi A, Salem S, Salimi E, Mahboubi R. Effect of PTEN gene mutations and environmental risk factors on the progression and prognosis of bladder cancer. Iran J Public Health. 2014;43(1):56–61.
- Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, Lotan TL. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15(4):222–234. doi:10.1038/nrurol.2018.9.
- Koushyar S, Meniel VS, Phesse TJ, Pearson HB. Exploring the wnt pathway as a therapeutic target for prostate cancer. Biomolecules. 2022;12(2):309. doi:10.3390/biom12020309.
- Murillo-Garzón V, Kypta R. WNT signalling in prostate cancer. Nat Rev Urol. 2017;14(11):683–696. doi:10.1038/nrurol.2017.144.
- Wang C, Chen Q, Xu H. Wnt/β-catenin signal transduction pathway in prostate cancer and associated drug resistance. Discov Onc. 2021;12(1):40. doi:10.1007/s12672-021-00433-6.
- Wu G, Weng W, Xia P, Yan S, Zhong C, Xie L, Xie Y, Fan G. Wnt signalling pathway in bladder cancer. Cell Signal. 2021;79:109886. doi:10.1016/j.cellsig.2020.109886.
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi:10.1038/nature14404.
- Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF, et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016; 30(2):324–336. doi:10.1016/j.ccell.2016.06.003.
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31(1):563–604. doi:10.1146/annurev-immunol-020711-074950.
- Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 2019;25(10):3074–3083. doi:10.1158/1078-0432.CCR-18-1942.
- Taylor BC, Balko JM. Mechanisms of MHC-I downregulation and role in immunotherapy response. Front Immunol. 2022;13:844866. doi:10.3389/fimmu.2022.844866.
- D’Urso CM, Wang ZG, Cao Y, Tatake R, Zeff RA, Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J Clin Invest. 1991;87(1):284–292. doi:10.1172/JCI114984.
- Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017; 7(12):1420–1435. doi:10.1158/2159-8290.CD-17-0593.
- Burns JA, Weiner AB, Catalona WJ, Li EV, Schaeffer EM, Hanauer SB, Strong S, Burns J, Hussain MHA, Kundu SD, et al. Inflammatory bowel disease and the risk of prostate cancer. Eur Urol. 2019; 75(5):846–852. doi:10.1016/j.eururo.2018.11.039.
- Wilson JC, Furlano RI, Jick SS, Meier CR. A population-based study examining the risk of malignancy in patients diagnosed with inflammatory bowel disease. J Gastroenterol. 2016;51(11):1050–1062. doi:10.1007/s00535-016-1199-8.
- Golombos DM, Ayangbesan A, O’Malley P, Lewicki P, Barlow L, Barbieri CE, Chan C, DuLong C, Abu-Ali G, Huttenhower C, et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Urology. 2018; 111:122–128. doi:10.1016/j.urology.2017.08.039.
- Huang P-Y, Yang Y-C, Wang C-I, Hsiao P-W, Chiang H-I, Chen T-W. Increase in akkermansiaceae in gut microbiota of prostate cancer-bearing mice. Int J Mol Sci. 2021;22(17):9626. doi:10.3390/ijms22179626.
- Pernigoni N, Zagato E, Calcinotto A, Troiani M, Mestre RP, Calì B, Attanasio G, Troisi J, Minini M, Mosole S, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science. 2021; 374(6564):216–224. doi:10.1126/science.abf8403.
- Sfanos KS, Markowski MC, Peiffer LB, Ernst SE, White JR, Pienta KJ, Antonarakis ES, Ross AE. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018;21(4):539–548. doi:10.1038/s41391-018-0061-x.
- Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–5337. doi:10.1016/j.cell.2021.09.020.
- Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPM, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015; 350(6264):1079–1084. doi:10.1126/science.aad1329.
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018; 359(6371):91–97. doi:10.1126/science.aan3706.
- Kalaora S, Nagler A, Nejman D, Alon M, Barbolin C, Barnea E, Ketelaars SLC, Cheng K, Vervier K, Shental N, et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature. 2021; 592(7852):138–143. doi:10.1038/s41586-021-03368-8.
- Sears CL, Pardoll DM. The intestinal microbiome influences checkpoint blockade. Nat Med. 2018;24(3):254–255. doi:10.1038/nm.4511.
- Meza L, Feng M, Lee K, Sperandio R, Pal SK. The gut microbiome and metastatic renal cell carcinoma. J Clin Med. 2023;12(4):1502. doi:10.3390/jcm12041502.
- Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, Segata N, Desnoyer A, Pietrantonio F, Ferrere G, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020; 78(2):195–206. doi:10.1016/j.eururo.2020.04.044.
- Goubet A-G, Rouanne M, Derosa L, Kroemer G, Zitvogel L. From mucosal infection to successful cancer immunotherapy. Nat Rev Urol. 2023;20(11):682–700. doi:10.1038/s41585-023-00784-5.
- Pernigoni N, Guo C, Gallagher L, Yuan W, Colucci M, Troiani M, Liu L, Maraccani L, Guccini I, Migliorini D, et al. The potential role of the microbiota in prostate cancer pathogenesis and treatment. Nat Rev Urol. 2023; 20(12):706–718. doi:10.1038/s41585-023-00795-2.
- Daud AI, Wolchok JD, Robert C, Hwu W-J, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. Programmed death-ligand 1 expression and response to the anti–programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016; 34(34):4102–4109. doi:10.1200/JCO.2016.67.2477.
- Yin H, Harrison TA, Thomas SS, Sather CL, Koehne AL, Malen RC, Reedy AM, Wurscher MA, Hsu L, Phipps AI, et al. T cell-inflamed gene expression profile is associated with favorable disease-specific survival in non-hypermutated microsatellite-stable colorectal cancer patients. Cancer Med. 2023; 12(6):6583–6593. doi:10.1002/cam4.5429.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015; 372(21):2018–2028. doi:10.1056/NEJMoa1501824.
- Yuan C, Liu Z, Yu Q, Wang X, Bian M, Yu Z, Yu J. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-50898-3.
- Lagos GG, Izar B, Rizvi NA. Beyond tumor PD-L1: emerging genomic biomarkers for checkpoint inhibitor immunotherapy. Am Soc Clin Oncol Educ Book. 2020;40(40):e47–e57. doi:10.1200/EDBK_289967.
- Richard C, Fumet J-D, Chevrier S, Derangère V, Ledys F, Lagrange A, Favier L, Coudert B, Arnould L, Truntzer C, et al. Exome analysis reveals genomic markers associated with better efficacy of nivolumab in lung cancer patients. Clin Cancer Res. 2019; 25(3):957–966. doi:10.1158/1078-0432.CCR-18-1940.
- Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer. 2018;6(1):157. doi:10.1186/s40425-018-0479-7.
- Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6(1):35. doi:10.1186/s40425-018-0342-x.
- Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang S-P, Walsh AM, Baxi V, Pandya D, Baradet T, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020; 26(5):688–692. doi:10.1038/s41591-020-0856-x.
- Hirsch L, Flippot R, Escudier B, Albiges L. Immunomodulatory roles of VEGF pathway inhibitors in renal cell carcinoma. Drugs. 2020;80(12):1169–1181. doi:10.1007/s40265-020-01327-7.
- Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11(8):1000–1017. doi:10.2174/138945010791591395.
- Han Z, Dong Y, Lu J, Yang F, Zheng Y, Yang H. Role of hypoxia in inhibiting dendritic cells by VEGF signaling in tumor microenvironments: mechanism and application. Am J Cancer Res. 2021;11(8):3777–3793.
- Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: perspectives for therapeutic implications. Med Oncol. 2019;37(1). doi:10.1007/s12032-019-1329-2.
- Cho H, Kaelin WG. Targeting HIF2 in clear cell renal cell carcinoma. Cold Spring Harb Symp Quant Biol. 2016;81:113–121. doi:10.1101/sqb.2016.81.030833.
- Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates Vascular Endothelial Growth Factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFκB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284(10):6038–6042. doi:10.1074/jbc.C800207200.
- Kim J-H. Interleukin-8 in the tumor immune niche: lessons from comparative oncology. Adv Exp Med Biol. 2020;1240:25–33.
- Rizzo M, Varnier L, Pezzicoli G, Pirovano M, Cosmai L, Porta C. IL-8 and its role as a potential biomarker of resistance to anti-angiogenic agents and immune checkpoint inhibitors in metastatic renal cell carcinoma. Front Oncol. 2022;12:990568. doi:10.3389/fonc.2022.990568.
- Najjar YG, Rayman P, Jia X, Pavicic PG, Rini BI, Tannenbaum C, Ko J, Haywood S, Cohen P, Hamilton T, et al. Myeloid-derived suppressor cell subset accumulation in renal cell carcinoma parenchyma is associated with intratumoral expression of IL1β, IL8, CXCL5, and Mip-1α. Clin Cancer Res. 2017; 23(9):2346–2355. doi:10.1158/1078-0432.CCR-15-1823.
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33(5):949–955. doi:10.1093/carcin/bgs123.
- Yuen KC, Liu L-F, Gupta V, Madireddi S, Keerthivasan S, Li C, Rishipathak D, Williams P, Kadel EE, Koeppen H, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020; 26(5):693–698. doi:10.1038/s41591-020-0860-1.
- Takeda K, Nakayama M, Hayakawa Y, Kojima Y, Ikeda H, Imai N, Ogasawara K, Okumura K, Thomas DM, Smyth MJ, et al. IFN-γ is required for cytotoxic T cell-dependent cancer genome immunoediting. Nat Commun. 2017; 8(1):14607. doi:10.1038/ncomms14607.
- Kolodziejski T, Klein A, Nowacki M. Lymphography in the evaluation of various radical operations. Pol Tyg Lek. 1969;24(45):1727–1729.
- Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. 2020;8(1):49. doi:10.1186/s40364-020-00228-x.
- Cerezo-Wallis D, Contreras-Alcalde M, Troulé K, Catena X, Mucientes C, Calvo TG, Cañón E, Tejedo C, Pennacchi PC, Hogan S, et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat Med. 2020; 26(12):1865–1877. doi:10.1038/s41591-020-1073-3.
- Lu J, Xu W-H, Zhang H, Ye D. Editorial: the mechanism of tumor evolution and microenvironmental changes of genitourinary oncology in clinical diagnosis and treatment. Front Oncol. 2023;13:1272984. doi:10.3389/fonc.2023.1272984.
- Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34. doi:10.1038/s41571-020-0403-1.
- Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, Sevillano M, Palomo-Ponce S, Tauriello DVF, Byrom D, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015; 47(4):320–329. doi:10.1038/ng.3225.
- Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DMA, Haibe-Kains B, Karn T, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015; 75(21):4494–4503. doi:10.1158/0008-5472.CAN-14-3569.
- Cao W, Ramakrishnan R, Tuyrin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Min Zhang L, Klein-Seetharaman J, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014; 192(6):2920–2931. doi:10.4049/jimmunol.1302801.
- Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, Delgoffe GM. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45(2):374–388. doi:10.1016/j.immuni.2016.07.009.
- Ye L-Y, Chen W, Bai X-L, Xu X-Y, Zhang Q, Xia X-F, Sun X, Li G-G, Hu Q-D, Fu Q-H, et al. Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016; 76(4):818–830. doi:10.1158/0008-5472.CAN-15-0977.
- Henze A-T, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–3679. doi:10.1172/JCI84427.
- Rechsteiner MP, von Teichman A, Nowicka A, Sulser T, Schraml P, Moch H. VHL gene mutations and their effects on hypoxia inducible factor HIFα: identification of potential driver and passenger mutations. Cancer Res. 2011;71(16):5500–5511. doi:10.1158/0008-5472.CAN-11-0757.
- Zhang J, Zhang Q. VHL and hypoxia signaling: beyond HIF in cancer. Biomedicines. 2018;6(1):35. doi:10.3390/biomedicines6010035.
- Labrousse-Arias D, Martínez-Alonso E, Corral-Escariz M, Bienes-Martínez R, Berridy J, Serrano-Oviedo L, Conde E, García-Bermejo M-L, Giménez-Bachs JM, Salinas-Sánchez AS, et al. VHL promotes immune response against renal cell carcinoma via NF-κB–dependent regulation of VCAM-1. J Cell Biol. 2017; 216(3):835–847. doi:10.1083/jcb.201608024.
- Brauch H, Weirich G, Brieger J, Glavac D, Rödl H, Eichinger M, Feurer M, Weidt E, Puranakanitstha C, Neuhaus C, et al. VHL alterations in human clear cell renal cell carcinoma: association with advanced tumor stage and a novel hot spot mutation. Cancer Res. 2000;60(7):1942–1948.
- Yao M, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002; 94(20):1569–1575. doi:10.1093/jnci/94.20.1569.
- Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18(12):792–804. doi:10.1038/s41571-021-00546-5.
- Ligorio M, Sil S, Malagon-Lopez J, Nieman LT, Misale S, Di Pilato M, Ebright RY, Karabacak MN, Kulkarni AS, Liu A, et al. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell. 2019; 178(1):160–175.e27. doi:10.1016/j.cell.2019.05.012.
- Chang SS. Re: EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. J Urol. 2019;202:458. doi:10.1097/01.JU.0000569196.28580.f2.
- Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013; 110(50):20212–20217. doi:10.1073/pnas.1320318110.
- Goulet CR, Champagne A, Bernard G, Vandal D, Chabaud S, Pouliot F, Bolduc S. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer. 2019;19(1):137. doi:10.1186/s12885-019-5353-6.
- Zhuang J, Shen L, Li M, Sun J, Hao J, Li J, Zhu Z, Ge S, Zhang D, Guo H, et al. Cancer-associated fibroblast–derived miR-146a-5p generates a niche that promotes bladder cancer stemness and chemoresistance. Cancer Res. 2023; 83(10):1611–1627. doi:10.1158/0008-5472.CAN-22-2213.
- Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, Sharma P. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell. 2019;179(5):1177–1190.e13. doi:10.1016/j.cell.2019.10.029.
- Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martínez L, Martínez-Saez E, Ramón y Cajal S, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018; 24(7):1024–1035. doi:10.1038/s41591-018-0044-4.
- McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P, Herrero J, et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell. 2017; 171(6):1259–1271.e11. doi:10.1016/j.cell.2017.10.001.
- O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–167. doi:10.1038/s41571-018-0142-8.
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016; 375(9):819–829. doi:10.1056/NEJMoa1604958.
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357(6349):409–413. doi:10.1126/science.aan6733.
- Ramelet AA, Walker-Nasir E. One daily application of oxiconazole cream is sufficient for treating dermatomycoses. Dermatologica. 1987;175(6):293–295. doi:10.1159/000248837.
- Marwitz S, Scheufele S, Perner S, Reck M, Ammerpohl O, Goldmann T. Epigenetic modifications of the immune-checkpoint genes CTLA4 and PDCD1 in non-small cell lung cancer results in increased expression. Clin Epigenetics. 2017;9(1):51. doi:10.1186/s13148-017-0354-2.
- Piper M, Kluger H, Ruppin E, Hu-Lieskovan S. Immune resistance mechanisms and the road to personalized immunotherapy. Am Soc Clin Oncol Educ Book. 2023;43(43):e390290. doi:10.1200/EDBK_390290.
- Snahnicanova Z, Kasubova I, Kalman M, Grendar M, Mikolajcik P, Gabonova E, Laca L, Caprnda M, Rodrigo L, Ciccocioppo R, et al. Genetic and epigenetic analysis of the beta-2-microglobulin gene in microsatellite instable colorectal cancer. Clin Exp Med. 2020; 20(1):87–95. doi:10.1007/s10238-019-00601-7.
- Lorenzi S, Forloni M, Cifaldi L, Antonucci C, Citti A, Boldrini R, Pezzullo M, Castellano A, Russo V, van der Bruggen P, et al. IRF1 and NF-kB restore MHC class I-restricted tumor antigen processing and presentation to cytotoxic T cells in aggressive neuroblastoma. PLoS One. 2012; 7(10):e46928. doi:10.1371/journal.pone.0046928.
- Mari L, Hoefnagel SJM, Zito D, van de Meent M, van Endert P, Calpe S, Sancho Serra MDC, Heemskerk MHM, van Laarhoven HWM, Hulshof MCCM, et al. microRNA 125a regulates MHC-I expression on esophageal adenocarcinoma cells, associated with suppression of antitumor immune response and poor outcomes of patients. Gastroenterology. 2018; 155(3):784–798. doi:10.1053/j.gastro.2018.06.030.
- Liu X-D, Kong W, Peterson CB, McGrail DJ, Hoang A, Zhang X, Lam T, Pilie PG, Zhu H, Beckermann KE, et al. PBRM1 loss defines a nonimmunogenic tumor phenotype associated with checkpoint inhibitor resistance in renal carcinoma. Nat Commun. 2020; 11(1):2135. doi:10.1038/s41467-020-15959-6.
- Saleh R, Elkord E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. 2019;457:168–179. doi:10.1016/j.canlet.2019.05.003.
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med. 2013; 210(9):1695–1710. doi:10.1084/jem.20130579.
- Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gómez H, Bastholt L, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011; 9(1):204. doi:10.1186/1479-5876-9-204.
- Ariafar A, Vahidi Y, Fakhimi M, Asadollahpour A, Erfani N, Faghih Z. Prognostic significance of CD4-positive regulatory T cells in tumor draining lymph nodes from patients with bladder cancer. Heliyon. 2020;6(12):e05556. doi:10.1016/j.heliyon.2020.e05556.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi:10.1038/nri2506.
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–983. doi:10.4049/jimmunol.179.2.977.
- Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011; 118(8):2254–2265. doi:10.1182/blood-2010-12-325753.
- De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016; 539(7629):443–447. doi:10.1038/nature20554.
- Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016; 539(7629):437–442. doi:10.1038/nature19834.
- Xue J, Schmidt S, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel T, Emde M, Schmidleithner L, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014; 40(2):274–288. doi:10.1016/j.immuni.2014.01.006.
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017; 545(7655):495–499. doi:10.1038/nature22396.
- Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2(8):578–588. doi:10.1038/s41551-018-0236-8.
- Wang L, Sfakianos JP, Beaumont KG, Akturk G, Horowitz A, Sebra RP, Farkas AM, Gnjatic S, Hake A, Izadmehr S, et al. Myeloid cell–associated resistance to PD-1/PD-L1 blockade in urothelial cancer revealed through bulk and single-cell RNA sequencing. Clin Cancer Res. 2021; 27(15):4287–4300. doi:10.1158/1078-0432.CCR-20-4574.
- Han C, Deng Y, Xu W, Liu Z, Wang T, Wang S, Liu J, Liu X. The roles of tumor-associated macrophages in prostate cancer. J Oncol. 2022;2022:1–20. doi:10.1155/2022/8580043.
- Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to Anti-CTLA-4 therapy. Cell. 2016; 167(2):397–404.e9. doi:10.1016/j.cell.2016.08.069.
- Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284(1):1–5. doi:10.1111/nyas.12105.
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017; 7(2):188–201. doi:10.1158/2159-8290.CD-16-1223.
- Koti M, Bivalacqua T, Black PC, Cathomen T, Galsky MD, Gulley JL, Ingersoll MA, Kamat AM, Kassouf W, Siemens DR, et al. Adaptive immunity in genitourinary cancers. Eur Urol Oncol. 2023; 6(3):263–272. doi:10.1016/j.euo.2023.03.002.
- Cai Z, Chen J, Yu Z, Li H, Liu Z, Deng D, Liu J, Chen C, Zhang C, Ou Z, et al. BCAT2 shapes a noninflamed tumor microenvironment and induces resistance to Anti-PD-1/PD-L1 immunotherapy by negatively regulating proinflammatory chemokines and anticancer immunity. Adv Sci. 2023;10(8). doi:10.1002/advs.202207155.
- Lee YC, Lam H-M, Rosser C, Theodorescu D, Parks WC, Chan KS. The dynamic roles of the bladder tumour microenvironment. Nat Rev Urol. 2022;19(9):515–533. doi:10.1038/s41585-022-00608-y.
- Yu A, Hu J, Fu L, Huang G, Deng D, Zhang M, Wang Y, Shu G, Jing L, Li H, et al. Bladder cancer intrinsic LRFN2 drives anticancer immunotherapy resistance by attenuating CD8 + T cell infiltration and functional transition. J Immunother Cancer. 2023; 11(10):7230. doi:10.1136/jitc-2023-007230.
- Sakatani T, Kita Y, Fujimoto M, Sano T, Hamada A, Nakamura K, Takada H, Goto T, Sawada A, Akamatsu S, et al. IFN-Gamma expression in the tumor microenvironment and CD8-positive tumor-infiltrating lymphocytes as prognostic markers in urothelial cancer patients receiving pembrolizumab. Cancers Basel. 2022; 14(2):263. doi:10.3390/cancers14020263.
- Gillezeau C, Movva N, van Gerwen M, Rabon-Stith K, Shire N, Brohawn PZ, Taioli E, Fryzek J. Interferon gamma expression and mortality in unselected cohorts of urothelial bladder cancer patients. PLoS One. 2022;17(8):e0271339. doi:10.1371/journal.pone.0271339.
- Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022; 28(4):704–712. doi:10.1038/s41591-022-01694-6.
- Dizman N, Hsu J, Bergerot PG, Gillece JD, Folkerts M, Reining L, Trent J, Highlander SK, Pal SK. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med. 2021;10(1):79–86. doi:10.1002/cam4.3569.
- Salgia NJ, Bergerot PG, Maia MC, Dizman N, Hsu J, Gillece JD, Folkerts M, Reining L, Trent J, Highlander SK, et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving Anti–PD-1 immune checkpoint inhibitors. Eur Urol. 2020; 78(4):498–502. doi:10.1016/j.eururo.2020.07.011.
- Santoiemma PP, Powell DJ. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16(6):807–820. doi:10.1080/15384047.2015.1040960.
- Kavanagh B, O’Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112(4):1175–1183. doi:10.1182/blood-2007-11-125435.
- Yang G, Shen W, Zhang Y, Liu M, Zhang L, Liu Q, Lu HH, Bo J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget. 2017;8(24):38378–38388. doi:10.18632/oncotarget.16386.
- Takeyama Y, Kato M, Tamada S, Azuma Y, Shimizu Y, Iguchi T, Yamasaki T, Gi M, Wanibuchi H, Nakatani T, et al. Myeloid-derived suppressor cells are essential partners for immune checkpoint inhibitors in the treatment of cisplatin-resistant bladder cancer. Cancer Lett. 2020; 479:89–99. doi:10.1016/j.canlet.2020.03.013.
- Ai L, Mu S, Wang Y, Wang H, Cai L, Li W, Hu Y. Prognostic role of myeloid-derived suppressor cells in cancers: a systematic review and meta-analysis. BMC Cancer. 2018;18(1):1220. doi:10.1186/s12885-018-5086-y.
- Slaney CY, Wang P, Darcy PK, Kershaw MH. Cars versus BiTEs: a comparison between T cell–redirection strategies for cancer treatment. Cancer Discov. 2018;8(8):924–934. doi:10.1158/2159-8290.CD-18-0297.
- Shen Y, Eng JS, Fajardo F, Liang L, Li C, Collins P, Tedesco D, Nolan-Stevaux O. Cancer cell-intrinsic resistance to BiTE therapy is mediated by loss of CD58 costimulation and modulation of the extrinsic apoptotic pathway. J Immunother Cancer. 2022;10(3):e004348. doi:10.1136/jitc-2021-004348.
- Belmontes B, Sawant DV, Zhong W, Tan H, Kaul A, Aeffner F, O’Brien SA, Chun M, Noubade R, Eng J, et al. Immunotherapy combinations overcome resistance to bispecific T cell engager treatment in T cell–cold solid tumors. Sci Transl Med. 2021;13(608). doi:10.1126/scitranslmed.abd1524.
- Salas-Benito D, Berger TR, Maus MV. Stalled CARs: mechanisms of resistance to CAR T cell therapies. Annu Rev Cancer Biol. 2023;7(1):23–42. doi:10.1146/annurev-cancerbio-061421-012235.