?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To investigate the impact of the effective radiation dose to immune cells (EDIC) and gross tumor volume (GTV) on lymphopenia and survival in patients with locally advanced esophageal squamous cell carcinoma (LAESCC). Between January 2013 and December 2020, 272 LAESCC patients were treated with definitive radiotherapy in two institutions. Based on radiation doses to the lungs, heart, and body region scanned, EDIC was calculated as an equal uniform dose to the total blood considering blood flow and fraction effect. The radiotherapy plan was used to calculate the GTVs. Lymphopenia was graded based on the lowest lymphocyte count during RT. The overall survival (OS), progress-free survival (PFS), and local recurrence-free survival (LRFS) were analyzed statistically. The lowest lymphocyte count was significantly correlated with EDIC (r= −0.389, p < .001) and GTV (r= −0.211, p < .001). Lymphopenia, EDIC, and GTV are risk factors for patients with ESCC. In a Kaplan-Meier analysis with EDIC and GTV as stratification factors, lymphopenia was not associated with OS in the EDIC>12.9 Gy group (p = .294)and EDIC ≤ 12.9 Gy group, and it was also not associated with OS in GTV>68.8 cm3 group (p = .242) and GTV ≤ 68.8 cm3 group(p = .165). GTV and EDIC had an impact on the relationship between lymphopenia and OS in patients with LAESCC undergoing definitive RT. Poorer OS, PFS, and LRFS are correlated with lymphopenia, higher EDIC, and larger GTV.
Introduction
The immune system is essential for preventing and curing cancer,Citation1,Citation2 and peripheral blood lymphocytes are important immune system constituents.Citation3,Citation4 Previous studies have discovered a relationship between radiation-related lymphopenia with poorer survival of various cancers, including esophageal carcinoma (ECC).Citation5–10 Therefore, finding impact factors of lymphopenia can help to improve efficacy and choose high-risk ECC patients for treatment optimization and follow-up management.
However, most previous studies concentrated on adenocarcinoma and included patients at various stages of the disease. Furthermore, the lymphopenia cutoff values varied across studies. These factors may have influenced the outcome. As a result, we conducted this study to investigate the lymphopenia criteria and see if GTV and EDIC can influence lymphopenia and poor outcomes in patients with locally advanced esophageal squamous cell carcinoma (LAESCC).
Materials and methods
Patients
Patients treated with definitive (chemo) radiotherapy (RT) at the radiation oncology department of the Second Affiliated Hospital and the Cancer Hospital of Guangxi Medical University between January 2013 and December 2020 were selected. 272 patients were included with stage II-IVA (American Joint Committee on Cancer, eighth edition, cM1, positive nonregional lymph nodes and irradiated during radiotherapy).
Treatment
Patients received a total radiation dose of 50 to 70.4 Gy in 25–33 fractions with elective nodal irradiation. Radiation was administered for 5–7 weeks using IMRT. All patients received definitive RT with or without chemotherapy. The chemotherapy regimens were nedaplatin/cisplatin and paclitaxel/docetaxel/5-fluorouracil.
Data collection
The following information was gathered from medical records: age, gender, performance status, smoking history, drinking history, heart disease history, pathology, staging, differentiation, tumor location, treatment modality, and lymphocyte count. And the RT plan provided data for radiation dose, gross tumor volume (GTV), mean lung dose (MLD), mean heart dose (MHD), and mean body dose (MBD).
Lymphocyte counts (cells × 109/L) were collected 1 week before the start of treatment, every week during RT, and 2 weeks after RT. Lymphopenia was graded based on the lowest lymphocyte count and the Common Terminology Criteria for Adverse Events, version 5.0. G4 nadir was defined as lymphocyte count<0.2 × 109/L.
The effective radiation dose to immune cells (EDIC) was calculated as follows using the model developed by Jin et al.Citation11and improved by Ladbury et al.Citation12:
Statistical analysis
The primary endpoint of this study was Overall survival (OS) and the secondary endpoint was progression-free survival (PFS) and locoregional recurrence-free survival (LRFS). The survival duration was calculated from the start of treatment to the corresponding event (loco-regional recurrence, tumor progression, death, or the last follow-up).
To compare continuous and categorical variables, the independent sample t-test or the χ2 test was used. According to receiving operating characteristic (ROC) survival curve analysis, the best cutoff value for EDIC and GTV was determined. The univariate and multivariate Cox regression models were used to investigate the relationship between survival and variables (inclusion criteria, p < .1). To examine correlations between variables, Spearman’s rank correlation coefficient was utilized. Spearman’s rank correlation coefficient was used to test correlations between variables. The tests were considered statistically significant if P < .05. All the p values were two-sided. SPSS 22.0 (IBM, Chicago, IL) and GraphPad Prism 9 were used for the statistical analyses.
Results
A total of 272 patients were analyzed. The patient characteristics are listed in . The median follow-up was 54 months (2–107), and 73.9% (201/272) died. A median dose of 61.5 Gy (range, 50–70.4) was administered to all patients. The 3-year OS, PFS, and LRFS rates were 24.6%, 21.3%, and 22.9%, respectively, with a median of 15, 12, and 13 months, respectively. According to the ROC curve, the median cutoff point for EDIC was 12.9 Gy (range, 2.4–19.2) and 68.8 cm3 (range,4.1–255.4) for GTV. In univariate analysis, lymphopenia, EDIC, GTV, and clinical stage are risk factors for OS, PFS, and LRFS (). In multivariate analysis, only EDIC and GTV are significant prognostic factors for OS, PFS, and LRFS ().
Table 1. Patient characteristics.
Table 2. Univariable analysis of clinical and dosimetric variables with outcomes.
Table 3. Multivariate analysis of clinical and dosimetric variables with outcomes.
Lymphocyte count
Before RT, the average lymphocyte count of 272 patients was 1.81(0.38–3.81 × 109/L), and after RT, it was 0.21(0.02–0.74 × 109/L)(p < 0.001). There were no patients with G4 nadir before RT, but 152 patients with G4 lymphopenia after RT (p < .001).
Patients who received concurrent chemotherapy (CRT group, n = 186) were compared to those without concurrent chemotherapy (RT group, n = 86). Before RT, the average lymphocyte count in the CRT group was 1.85 (0.38–3.58 × 109/L), while in the RT group it was 1.72 (0.53–3.81 × 109/L), with no statistically significant differences (p = .097). After RT, the average lymphocyte count in the CRT group was 0.19 (0.02–0.71 × 109/L), while in the RT group it was 0.23 (0.02–0.74 × 109/L), with no statistically significant differences (p = .053).
Lymphopenia and survival
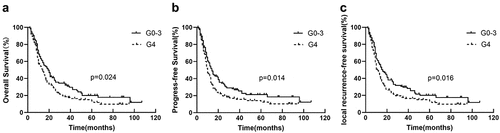
Compared to G4 nadir, patients with G0–3 nadir had a significantly better OS (19 vs. 14 months, p = .024) (), PFS (14 vs. 10 months, p = .014) (), and LRFS (16 vs. 11 months, p = .016) ().
Lymphopenia correlate with EDIC result in survival
The lowest lymphocyte count was correlated with EDIC (r = −0.389, p < .001). To investigate the relationship between lymphopenia and EDIC, the EDIC was divided into quartiles based on prevalence, and the percentage of G4 lymphopenia was 32.4% (2.4–10.3 Gy), 44.1% (10.3–12.7 Gy), 69.1% (12.7–15.3 Gy), and 77.9% (15.3–19.2 Gy), respectively (p < .001).
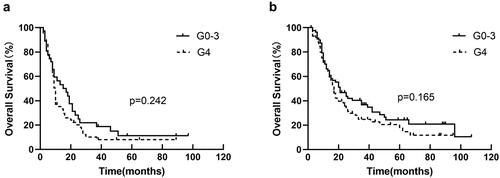
EDIC was used as a stratification factor in univariate analysis and Kaplan-Meier analysis to further investigate whether EDIC influences the prognostic role of lymphopenia on survival. After considering EDIC as a stratification factor, there was no discernible connection between lymphopenia and OS (HR = 1.184, p = .26) in univariate analysis. In Kaplan – Meier analysis, lymphopenia was not associated with OS in the EDIC>12.9 Gy group (p = .294) () and EDIC ≤12.9 Gy group (p = .637) ().Other survival statistics (PFS and LRFS) were list in .
Figure 2. Kaplan-Meier curves for OS by dichotomy (a) in EDRIC>12.9 Gy group, and (b) in EDIC ≤12.9 Gy group.
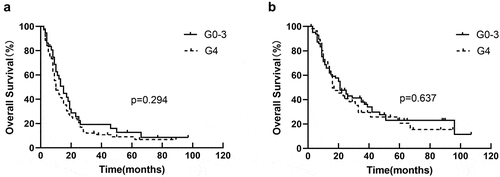
Table 4. Kaplan-Meier analysis of lymphopenia with outcomes.
Lymphopenia correlate with GTV result in survival
The lowest lymphocyte count was associated with GTV (r = −0.211, p < .001). To investigate the relationship between lymphopenia and GTV, the GTV was divided into quartiles based on prevalence, and the percentage of G4 lymphopenia was 41.2% (4.1–38.4 cm3), 51.5% (38.4–57.9 cm3), 63.2% (57.9–92.2 cm3), and 67.6% (92.2–255.4 cm3), respectively (p < .001).
There was no significant correlation between lymphopenia with OS after using GTV as a stratification factor (HR = 1.321, p = .055) in univariate analysis. In Kaplan – Meier analysis, lymphopenia was not associated with OS in GTV>68.8 cm3 group (p = .242) () and GTV ≤68.8 cm3 group (p = .165) (). Other survival statistics (PFS and LRFS) were list in .
Discussion
In our study, we found that definitive RT for LAESCC can reduce the lymphocyte count, and whether concurrent chemotherapy has no discernible effect on the lymphocyte count. We also discovered that lymphopenia was linked to poor survival, which was influenced by GTV and EDIC. After adjusting for GTV and EDIC, lymphopenia’s prognostic role during RT remained insignificant. The higher EDIC and larger GTV correlate with worse OS, PFS, and LRFS.
As we all know, RT is an important treatment for ECC.Citation13–15 According to growing clinical data, low absolute lymphocyte count (ALC) during RT is associated with poor survival of ECC. A retrospective studyCitation10 enrolled 504 patients with stage I-III ECC who received neoadjuvant or definitive CRT and discovered that G4 ALC nadir (defined as a nadir of < 200 cells/ml) was correlated with poor OS and disease-specific survival outcomes. The median OS in patients with G0–2 nadir and G4 nadir was significantly different (5.0 vs. 2.8 years, p = .027). It should be mentioned that the majority of the included patients were adenocarcinoma and received CRT with 50.4 Gy. To further investigate the role of ALC in patients with esophageal squamous cell carcinoma (ESCC) treated with definitive RT, Wang et al.Citation16 conducted a study of 189 patients with ESCC treated with definitive RT (50-68 Gy) combined with or without chemotherapy. Patients were divided into two groups based on the cut-off value of ALC nadir (defined as 0.38 × 103 cells/µl), and there were significant differences in OS (p < .001), PFS (p = .0048), and LRFS (p < .0053) in low ALC nadir group (≤0.38 × 103 cells/µl) and high ALC nadir group(>0.38 × 103 cells/µl). In our study, we also discovered that radiation-related lymphopenia was linked to poor outcomes in patients with LAESCC.
Although the mechanisms underlying the relationship between lymphopenia and the outcome of ECC treatment are still unknown. However, a variety of studies have focused on the identification of risk factors for treatment-related lymphopenia in ECC. Xu et al.Citation17 improved the model used to calculate EDIC for ECC patients who received CRT and investigated the relationship between EDIC and lymphopenia. Higher EDIC values (>4 Gy) were associated with G4 nadir during CRT (67.3% vs. 40.8%, p < .001), and EDIC score was a significant risk factor for severe lymphopenia. EDIC was also an independent prognostic factor for OS, PFS, and distant metastasis-free survival (DMFS). Another study draws the same conclusion. Cai et al.Citation18 found that the cutoff value of EDIC was 10.3 Gy according to the ROC curve, then 146 patients with ECC were divided into the EDIC ≥10.3 Gy group and EDIC<10.3 Gy group, and the final result showed that patients with EDIC ≥10.3 Gy had a significantly lower median OS (14.2 vs.39.6 months, p < .001), and PFS (28.1 vs.39.4 months, p = .024). It should be noted that they may disregard the impact of the other dosimetry parameters. There are certainly other studies focusing on this issue. Wang et al.Citation16 concluded that large PTVs affected a low ALC nadir during RT in the study mentioned above. In addition to PTVs, Xu et al.Citation19 showed that G4 nadir was related to other dosimetry parameters such as lung V10 and heart V10 in patients with ESCC treated with definitive CRT. And G0–3 nadir group was significantly associated with better OS (p < .001), PFS (p < .001), and DMFS (p = .008) than the G4 nadir group, but not with locoregional failure-free survival (LRFFS) (p = .202). Moreover, to look into the relationship between lymphopenia and radiation dose to marrow during CRT for ECC. Anderson et al.Citation20 calculated thoracic vertebra volume spared 5–40 Gy (TVS5–40) and found that TVS10-TVS40 was significantly associated with higher lymphocyte nadirs in 46 patients with ECC. Furthermore, Newman et al.Citation21 investigated the relationship between lymphopenia and vertebra volume in 54 patients with ECC, finding that absolute vertebral volume receiving 10 Gy > 289 cc, 20 Gy ≥ 270 cc, and 30 Gy ≥ 197 cc all correlated with absolute lymphocyte nadir. More samples may be required to support this conclusion. Besides that, mixed pathology and stages may have an impact on the results. In our study, we included 272 LAESCC patients and discovered that lymphopenia does not correlate with OS if we used GTV or EDIC as a stratification factor based on the GTV and EDIC cutoff values. Then we concluded that GTV and EDIC affect the relationship between lymphopenia and OS.
Based on our findings, we can propose specific strategies to improve the outcomes. To begin, radiotherapy techniques can be improved to reduce the radiation dose delivered to bone marrow, circulating lymphocyte cells, the lung, and the heart. In addition, we can also identify high-risk patients and modify their treatment, such as induction chemotherapy for GTV reduction. Our study also has several limitations. At first, this was a retrospective study with selective bias. Second, there were limitations in the EDIC model. Third, other factors may correlate with lymphocyte counts, such as infections or medications.
Conclusions
In conclusion, GTV and EDIC had an impact on the relationship between lymphopenia and OS in patients with LAESCC undergoing definitive RT. Poorer OS, PFS, and LRFS are correlated with lymphopenia, higher EDIC, and larger GTV.’
Author contributions
XZ and WL conceived and designed the study. HY, JW and QL collected the data. DL and QZ drafted the manuscript. All authors participated in the review and final approval of the submitted report.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Second Affiliated Hospital and the Cancer Hospital of Guangxi Medical University. Written informed consent for participation was not required for this study by the national legislation and the institutional requirements.
Abbreviations
| EDIC | = | The effective radiation dose to immune cells |
| GTV | = | Gross tumor volume |
| PTV | = | Planning target volume; |
| MLD | = | mean lung dose (MLD) |
| MHD | = | mean heart dose (MHD); |
| MBD | = | mean body dose (MBD) |
| LAESCC | = | Locally advanced esophageal squamous cell carcinoma |
| ECC | = | Esophageal carcinoma; |
| OS | = | The overall survival |
| PFS | = | progress-free survival |
| LRFS | = | Local recurrence-free survival |
| DMFS | = | distant metastasis-free survival |
| LRFFS | = | Locoregional failure-free survival |
| CI | = | Confidence interval; |
| CRT | = | Concurrent chemoradiotherapy |
| RT | = | Radiotherapy |
| IMRT | = | Intensity-modulated radiotherapy |
| ROC | = | receiving operating characteristic |
| ALC | = | Absolute lymphocyte count |
| TVS | = | Thoracic vertebra volume spared. |
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–6. doi:10.1038/ni1102-991.
- Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12(4):307–13. doi:10.1038/nrc3246.
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–68. doi:10.1084/jem.188.12.2357.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–89. doi:10.1038/nature10673.
- Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1(2):149–54. doi:10.2217/cns.12.14.
- Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, Hong DS, Komaki R, Welsh JW. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084–91. doi:10.1016/j.ijrobp.2014.04.025.
- Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, Brock M, Balmanoukian A, Ye X. Survival in patients with Severe Lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13(10):1225–31. doi:10.6004/jnccn.2015.0151.
- Cho O, Oh YT, Chun M, Noh OK, Hoe JS, Kim H. Minimum absolute lymphocyte count during radiotherapy as a new prognostic factor for nasopharyngeal cancer. Head Neck. 2016;38(Suppl S1):E1061–1067. doi:10.1002/hed.24158.
- Cho O, Oh YT, Chun M, Noh OK, Lee HW. Radiation-related lymphopenia as a new prognostic factor in limited-stage small cell lung cancer. Tumour Biol. 2016;37(1):971–78. doi:10.1007/s13277-015-3888-y.
- Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, Welsh J, Cox JD, Crane CH, Hsu CC, et al. Lymphocyte Nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):128–35. doi:10.1016/j.ijrobp.2017.05.037.
- Jin JY, Hu C, Xiao Y, Zhang H, Paulus R, Ellsworth SG, Schild SE, Bogart JA, Dobelbower MC, Kavadi VS, et al. Higher radiation dose to the immune cells correlates with worse tumor control and overall survival in patients with stage III NSCLC: a secondary analysis of RTOG0617. Cancers (Basel). 2021;13(24):6193. doi:10.3390/cancers13246193.
- Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2019;105(2):346–55. doi:10.1016/j.ijrobp.2019.05.064.
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation therapy oncology group. JAMA. 1999;281(17):1623–27. doi:10.1001/jama.281.17.1623.
- Minsky BBD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–74. doi:10.1200/JCO.2002.20.5.1167.
- Ajani JD, Bd TA, Chao J, Corvera C. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Esophageal and Esophagogastric Junction Cancers. Version 1; 2022. https://www.nccn.:org/professionals/physician_gls/pdf/esophageal.
- Wang X, Zhao Z, Wang P, Geng X, Zhu L, Li M. Low lymphocyte count is associated with radiotherapy parameters and affects the outcomes of esophageal squamous cell carcinoma patients. Front Oncol. 2020;10:997. doi:10.3389/fonc.2020.00997.
- Xu C, Jin JY, Zhang M, Liu A, Wang J, Mohan R, Kong F, Lin SH. The impact of the effective dose to immune cells on lymphopenia and survival of esophageal cancer after chemoradiotherapy. Radiother Oncol. 2020;146:180–86. doi:10.1016/j.radonc.2020.02.015.
- Cai S, Fan Y, Guo Q, Sun Y, Zhao P, Tian Y, Fan Q. Impact of radiation dose to circulating immune cells on tumor control and survival in esophageal cancer. Cancer Biother Radiopharm. 2021;38(6):380–87.
- Xu H, Lin M, Hu Y, Zhang L, Li Q, Zhu J, Wang S, Xi M. Lymphopenia during definitive chemoradiotherapy in esophageal squamous cell carcinoma: association with dosimetric parameters and patient outcomes. The Oncologist. 2021;26(3):e425–34. doi:10.1002/onco.13533.
- Anderson JL, Newman NB, Anderson C, Sherry AD, Yock AD, Osmundson EC. Mean cardiopulmonary dose and vertebral marrow dose differentially predict lineage-specific leukopenia kinetics during radiotherapy for esophageal cancer. Radiother Oncol. 2020;152:169–76. doi:10.1016/j.radonc.2019.12.008.
- Newman NB, Anderson JL, Sherry AD, Osmundson EC. Dosimetric analysis of lymphopenia during chemoradiotherapy for esophageal cancer. J Thorac Dis. 2020;12(5):2395–405. doi:10.21037/jtd.2020.03.93.