The primary cilium is a specialized dynamic signaling compartment that regulates cellular differentiation by the vertebrate hedgehog pathway. The microtubular axoneme of the primary cilium is built from the mother centriole (basal body) by a conserved process called intraflagellar transport (IFT).Citation1 IFT consist of trains of intraflagellar particles, biochemically characterized as IFT-A and IFT-B that are transported in the anterograde direction by kinesin II, and in the retrograde direction by dynein 2.Citation1 Mutations in IFT-A and dynein 2 result in accumulation of IFT-B at ciliary tips, and axonemal bulges, characteristic of disrupted retrograde transport inside cilia.Citation1 Defects in primary cilia and in trafficking of proteins to the cilia are associated with a heterogeneous group of newly described human diseases affecting multiple tissues known as “ciliopathies." One particular class of skeletal ciliopathies affects hedgehog-regulated bone and cartilage morphogenesis, and results in chondrodysplastic phenotypes. These include the short-rib polydactyly syndrome (SRPS), asphyxiating thoracic dystrophy or Jeune syndrome (JATD), and Sensenbrenner syndrome, and result from mutations mainly in IFT-ACitation2 or dynein 2.Citation3 Recently, two genes, WDR34 and WDR60, have been implicated in SRPS and JATD,Citation4,5 and could function as dynein intermediate chains in retrograde IFT.Citation6,7 A recent paper in Cell Cycle describes a candidate dynein light chain protein, TCTEX1D2, with a conserved domain similar to the C-terminus of the dynein light chain TCTEX1 subunit (), to be associated with WDR34/60, and the authors propose that TCTEX1D2 could be functioning as a light chain subunit in dynein complexes, particularly in relation to ciliogenesis.
Figure 1. Cartoon showing dynein motor subunits. LT, LL and RB represent dynein light chain subunits. TCTEX1D2 has a conserved domain similar to the C-terminus of the dynein light chain TCTEX1 subunit (LT), and could be functioning as a light chain subunit in dynein complexes, particularly in relation to ciliogenesis.
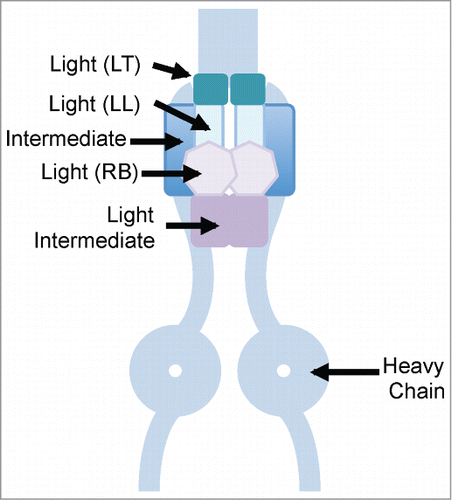
While identifying TCTEX1D2-binding proteins using tandem affinity purification and mass spectrometry, the authors discovered TCTEX1D2 to be associated with dynein complexes. Interestingly, in nonciliated cells, TCTEX1D2 was associated with the cytoplasmic dynein 1 heavy chain (DYNC1H1), whereas, in ciliated cells it was exclusively associated with the dynein 2 heavy chain (DYNC2H1). In both cases, TCTEX1D2 was associated with WDR34 and WDR60. Similar to WDR34 and WDR60, TCTEX1D2 was localized to the basal body, and the primary cilium. In addition, TCTEX1D2 was also localized to pericentriolar satellites around the basal body. The localization of TCTEX1D2 to the basal body was dependent on WDR60. While its unclear if TCTEX1D2 is involved in retrograde IFT in cilia, lack of this protein resulted in decreased ciliogenesis. Defects in IFT-A and dynein 2 characteristically results in accumulation of IFT-B proteins in ciliary tips, and is indicative of disrupted retrograde IFT. The Chlamydomonas ortholog of WDR60 (FAP163) has been shown to be present in the flagellar matrix and is actively trafficked by IFT, and knockdown of the Planaria ortholog results in severe defects in ciliary assembly with accumulated electron-dense material, characteristic of defective retrograde IFT.Citation7 In addition, the Chlamydomonas ortholog of WDR34 (FAP133) has been shown to associate with the LC8 dynein light chain and the IFT dynein heavy chain and light intermediate chain motor complex.Citation6 Surprisingly, fibroblasts from WDR60 patients do not show accumulation of IFT-B in ciliary tips,Citation5 and fibroblasts from WDR34 patients do not show defects in ciliogenesis,Citation4 possibly because of the hypomorphic nature of the alleles. Alternatively, defects in WDR34/WDR60/TCTEX1D2 may cause less severe accumulation of IFT trains in cilia.
Exome capture and parallel sequencing efforts in the past few years have resulted in the identification of multiple novel cilia/centrosome-associated genes in the complex ciliopathy disease spectrum. Proteomic analyses of proteins associated with cilia localized factors also provide a rich trove of information and missing links in identification of novel disease-associated genes. The identification of TCTEX1D2 as a dynein-associated protein in the current study strongly implies that defects in this protein could be associated with skeletal ciliopathies. The identification of TCTEX1D2 as a dynein subunit could also provide links to novel dynein-associated cargos important in ciliogenesis.
References
- Rosenbaum JL, Witman GB. Nat Rev Mol Cell Biol 2002; 3:813-25; PMID:12415299; http://dx.doi.org/10.1038/nrm952
- Bredrup C, et al. Am J Hum Genet 2011; 89:634-43; PMID:22019273; http://dx.doi.org/10.1016/j.ajhg.2011.10.001
- Schmidts M, et al. J Med Genet 2013; 50:309-23; PMID:23456818; http://dx.doi.org/10.1136/jmedgenet-2012-101284
- Schmidts M, et al. Am J Hum Genet 2013; 93:932-44; PMID:24183451; http://dx.doi.org/10.1016/j.ajhg.2013.10.003
- McInerney-Leo AM, et al. Am J Hum Genet 2013; 93:515-23; PMID:23910462; http://dx.doi.org/10.1016/j.ajhg.2013.06.022
- Rompolas P,et al. J Cell Sci 2007; 120:3653-65; PMID:17895364; http://dx.doi.org/10.1242/jcs.012773
- Patel-King RS,et al. Mol Biol Cell 2013; 24:2668-77; PMID:23864713; http://dx.doi.org/10.1091/mbc.E13-05-0266
