Abstract
The tissue inhibitor of metalloproteinase (TIMP) family, including TIMP-2, regulates the activity of multifunctional metalloproteinases in pathogenesis of melanoma. The Wnt/β-catenin pathway is constitutively activated and plays a critical role in melanoma progression. However, the relationship between TIMP-2 expression and β-catenin activity is still unclear. We hypothesize that TIMP-2 over expression inhibits the activation of the Wnt/β-catenin pathway in melanoma cells. Protein expression, distribution, and transcriptional activity of β-catenin were assayed in established stable melanoma cell lines: parental A2058 expressing, A2058 T2-1 over-expressing (T2-1), and A2058 T2R-7 under-expressing (T2R-7) TIMP-2. Compared to T2-1 cells at the basal level, T2R-7 showed significantly lower amount protein and weaker immunofluorescence staining of β-catenin. This regulation is through posttranslational level via ubiquitination. Functionally, proliferation and cell growth were lower in T2R-7 compared to A2058 and T2-1. Lithium treatment was used to mimics activation of the Wnt/β-catenin pathway. In T2R-7 cells under-expressing TIMP2, lithium significantly increased total β-catenin, nuclear β-catenin, and its downstream protein phosphor-c-Myc (S62). Nuclear β-catenin staining was enhanced in T2R-7. Beta-catenin transcriptional activity and cell proliferation were also increased significantly. Axins inhibit β-catenin pathway via GSK-3 β. We further found the ratio of p-GSK-3 β (S9) to β-catenin and protein levels of Axins were significantly lower, whereas downstream Wnt 11 was high in T2R-7 treated with lithium. Collectively, the high level of TIMP-2 protein inhibits the activation of the Wnt/β-catenin pathway, thus suppressing proliferation. Insights in the molecular mechanisms of TIMP-2 may provide promising opportunities for anti-proliferative therapeutic intervention.
Abbreviations
| APC | = | Adenomatous polyposis coli |
| GSK-3b | = | Glycogen synthase kinase 3b |
| MMPs | = | Metalloproteinases |
| TCF/LEF | = | T-cell factor/L-lymphoid enhancer factor |
| TIMP | = | Tissue inhibitor of metalloproteinases |
Introduction
Tissue inhibitor of matrix metalloproteinase (TIMP) family, including TIMP-1, 2, 3, and 4, naturally inhibits the activity of matrix metalloproteinases (MMPs).Citation1,2 MMP-2 expression is associated with the progression of melanoma.Citation3 TIMP-2 can inhibit active MMP-2 as well as promote the activation of pro-MMP-2 by MT1-MMP.Citation4,5 TIMP-2 can act as an endogenous inhibitor of both angiogenesis and tumor growth, via effects dependent or independent of MMP inhibition. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis.Citation6 TIMP-2 modulates critical signaling pathways, such as the NF-κB pathway,Citation7,8 vascular endothelial growth factor pathway,Citation9 p38 MAPKs pathway,Citation10 insulin-like growth factor,Citation11,12 etc. A recent study reports that TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/β-catenin complex expression in A549 lung cancer cells.Citation13
Beta-catenin is a multi-functional protein.Citation14 As an adhesion protein, β-catenin is associated with α-catenin and E-cadherin which plays a role in tumor suppressor gene.Citation15 As a transcriptional factor, β-catenin acts as a key transcriptional co-activator and transmits extracellular signals for the activation of target genes in the canonical Wnt pathway,Citation16,17 which plays an important role in embryonic development and neoplasia.Citation18 Under normal conditions, β-catenin associates with Axin and adenomatous polyposis coli (APC), a negative regulator of β -catenin. Beta-catenin is constitutively phosphorylated by glycogen synthase kinase 3β (GSK-3β), ubiquitinated, and degraded by the proteasome, thus maintaining appropriate cytoplasmic levels.Citation19 Without the constitutive β-catenin degradation, cytoplasmic levels rise and allow β-catenin to accumulate in the nucleus. Once in the nucleus, β-catenin binds to the transcription factor T-cell factor/L-lymphoid enhancer factor (TCF/LEF) and activates multiple target genes, such as c-Myc, which promotes cell proliferation, and cancer progression.
Melanoma is the fifth leading cancer in males and the seventh in females in the United States.Citation20 Constitutive activation of the Wnt/β-catenin signaling pathway is often observed in melanoma.Citation21,22 TIMP-2 expression modulates human melanoma cell adhesion and motility.Citation23 However, it is not clear how TIMP-2 directly contributes the activity of Wnt/β-catenin pathway. In this study, we employed stable melanoma cell lines: parental A2058 expressing, A2058 T2-1 over-expressing (T2-1), and A2058 T2R-7 (T2R-7) under-expressing TIMP-2Citation24,7 to investigate how TIMP-2 regulates Wnt/β-catenin pathway. We examined the signaling pathway of β-catenin at different levels, including total and phosphorylated of β-catenin, ubiquitinated β-catenin, cellular distribution, transcriptional activity, target genes, and cell growth. To mimic the enhanced Wnt/β-catenin micro-environment in melanoma, we treated these cells with lithium. We found that under activated states induced by lithium, TIMP-2 under- expression promoted β-catenin protein levels by activation of Wnt 11 and inhibition of GSK-3β, thus leading to enhance proliferation. On the contrary, TIMP-2 overexpression inhibited Wnt 11 and p-c-Myc levels to decrease tumor cells proliferation. Insights in the molecular mechanisms of TIMP-2 in cancer cells will provide promising opportunities for therapeutic intervention.
Results
TIMP-2 under-expression decreases total β-catenin
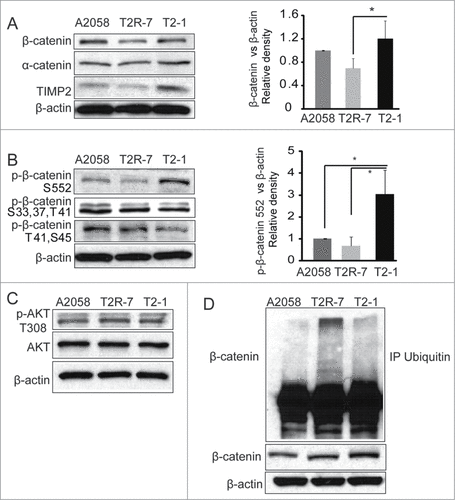
We first determined the expression level of β-catenin in the A2058, T2R-7 and T2-1 cell lines at the basal level. The western blot results showed that TIMP-2 over-expression increased the level of β-catenin, compared to that in T2R-7 cells. The densitometry of β-catenin v.s. β-actin was significantly higher in T2-1 cells than that in T2R-7 cells (). Beta-catenin is associated with the adhension protein α-catenin. We detected the α-catenin protein level in these cells. In contrast, α-catenin was not altered by TIMP-2 level. Interestingly, real-time PCR did not showed any difference of β-catenin at the mRNA level among 3 cell lines (data not shown).
Figure 1. TIMP-2 affects the total, phosphorylated, and ubiquitinated β-catenin in A2058 cells. (A) β-catenin protein expression in human melanoma cell lines: parental A2058 expressing, A2058T2R-7 (T2R-7) under-expressing and A2058T2-1 (T2-1) over-expressing TIMP-2.Densitometry of β-catenin. (B) Immunoblot of phospho-β-catenin in A2058, T2R-7 and T2-1 cells. Densitometry of p-β-catenin (ser 552). (C) Immunoblot of total and phosphor-AKT in A2058, T2R-7 and T2-1 cells.Data are expressed as mean ± SD. #P< 0.05. n= 3 separate experiments. (D) Representative ubiquitination of β-catenin in cells treated with 20μM MG262 for 4 hours. Using anti-β-catenin antibody pulled down, detected with anti-total ubiquitin antibody. β-catenin and actin showed input.
TIMP-2 overexpression elevates the post-translational modification of β-catenin
Beta-catenin activity is regulated by posttranslational modification. The increased level of phosphorylated β-catenin (p-β-catenin) indicates the high activity of β-catenin pathway. We further determined the p-β-catenin expression by Western blot. Interestingly, p-β-catenin (Ser552) increased in T2-1 cells compared with those in the A2058 and T2R-7 cells (). But there were no significant differences of phosphylated and total AKT (). Neither did β-catenin upstream regulators GSK-3β, Axin 1 (data not shown) among the 3 A2058 cell lines. To explore the potential mechanism of TIMP-2 increasing β-catenin, we detected ubiquitination of β-catenin. Our data showed that down-expressing TIMP-2 increased the ubiquitination of β-catenin in T2R-7 cells (). Taken together, our data suggested that TIMP-2 expression elevates β-catenin at the phosphorylation and ubiquitination level in cells at the basal level.
TIMP-2 overexpression affects distribution of β-catenin
We further examined the distribution of β-catenin by immunofluorescence staining. Beta-catenin (red staining) showed the strongest immunofluorescence in circum frontal rings around each cell at sites of cell-cell contact in T2-1 with TIMP-2 over-expression, weaker staining in T2R-7 with TIMP-2 under-expression (). We can also see small amount of β-catenin existing in nuclear area (green arrow) in T2-1 cells. The immunostaining of β-catenin was consistent with the Western blot result ().
Figure 2. Distribution of β-catenin and cell growth and proliferation in A2058, T2R-7, and T2-1 cell lines. (A) Distribution of β-catenin by immunostaining. Blue: DAPI; Red: β-catenin. Green arrow shows small amount of β-catenin at nuclear area. (B) Cell growth curve. (C) Cell proliferation measured by the MTT. Data are expressed as mean ± SD. #P< 0.05, n= 3 separate experiments.
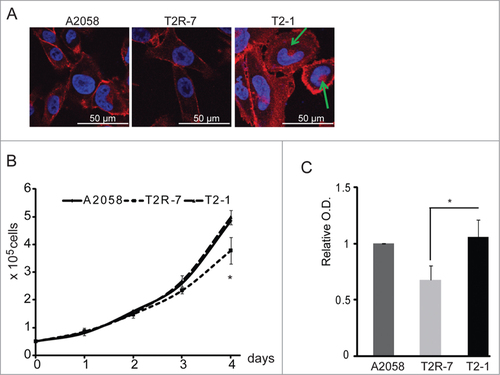
TIMP-2 under-expression inhibits cell growth
Cell growth is one of the biological effects regulated by the Wnt/β-catenin pathway. We then determined whether the TIMP-2 expression influences cell growth. As showed here, without any stimulation, TIMP-2 under-expressing T2R-7 cells had significantly lower growth rate compared to the parental A2058 and T2-1 cells (). Cell proliferation was further measured by MTT assay. The optical density (O.D.) was significant lower in T2R-7 cells (). We further assessed the transcriptional activity of β-catenin by luciferase reporter assay in the parental A2058, T2-1, and T2R-7 cells. Cells were transfected with a plasmid containing either the wild-type (WT) TCF binding site (pGL3-OT) or a defective TCF binding site (pGL3-OF) upstream of the SV40 promoter and luciferase reporter gene.Citation25 There were no significant signals detected in the cells. Taken together, these data indicate that TIMP-2 under-expression is able to inhibit proliferation in the basal level without any stimulation to activate the β-catenin activity.
E-cadherin expression is enhanced by TIMP-2
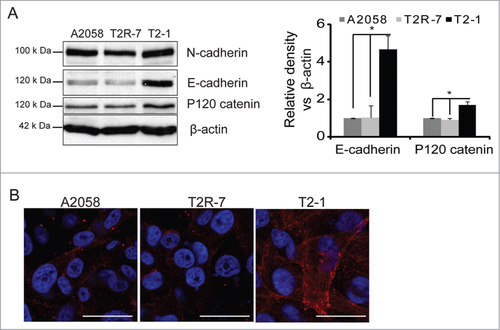
Beta-catenin is also one of the adhesion proteins including E-cadherin, α-catenin and P120 catenin, etc, which play an essential role in the tumorigenesis of melanoma. We also assessed the expression level of cadherin in cells with different levels of TIMP-2. Cadherin including epithelial (E), neural (N), placental (P)-cadherin is a family cell surface single-pass transmembrane protein that controls critical events in cell-cell adhesion, recognition and tissue development. E-cadherin plays a role of tumor suppressor gene. N-cadherin is expressed in highly invasive tumor cell lacking E-cadherin. It is known that there is a shift of E-cadherin to N-cadherin in the melanoma cells. We found that the expression level of E-cadherin was significantly increased in the T2-1 over expressing TIMP-2. Meanwhile, another adhesion protein, P120 catenin was increased in T2-1 cells. There is no significant difference in the expression of α-catenin () and N-cadherin (). We then further examined the distribution of E-cadherin by immunofluorescence staining. E-cadherin showed the strongest immunofluorescence in the membrane of T2-1 cells, weaker staining in A2058 and T2R-7 cells (), which was consistent with the immunoblot result of the E-cadherin.
Figure 3. TIMP-2 and E-cadherin. (A) E-cadherin and N-cadherin protein expression in human melanoma cell lines. Densitometry of E-cadherin v.s. β-actin was measured. Data are expressed as mean ± SD. #P< 0.05. n= 3 separate experiments. (B) Distribution of E-cadherin by immunostaining. Blue: DAPI; Red: E-cadherin.
TIMP-2 under expression enhances β-catenin protein induced by lithium
Constitutive activation of the Wnt/β-catenin signaling pathway is often observed in melanoma,Citation21 which may cause immune suppression and resistance in human melanoma cells.Citation26 β-catenin levels control tumor differentiation in Braf-activated Pten-deficient melanoma mouse model.Citation27 Lithium is capable of increasing β-catenin levels possibly through the inhibition GSK-3β, therefore mimicking the effects of Wnt signaling activation. Lithium treatment stabilizes cytosolic β-catenin, which translocates to the nucleus, binds to T-cell factor/L-lymphoid enhancer factor (TCF/LEF), and stimulates target genes such as c-Myc.Citation28-30 To mimic the activation of Wnt signaling pathway in melanoma, we treated cells with 20 mM lithium chloride for 30 h. We then detected protein levels of β-catenin and found β-catenin was significantly increased in T2R-7 with under-expressed TIMP-2. C-Myc is downstream of β-catenin pathway. We also found that p-c-Myc was increased in T2R-7 compared to A2058 and T2-1 cells (). However, we did not find difference of c-Myc or clyclin-D1 among 3 cell lines.
Figure 4. TIMP-2 inhibits β-catenin induced by lithium chloride. (A) Immunoblot of β-catenin and p-c-Myc (Ser62) in A2058 (A), A2058T2R-7 (R), and A2058T2-1 (T) cell lines treated with 20 mM lithium chloride (LiCl) 30h. Densitometry of β-catenin and p-c-Myc in cells treated with LiCl. (B) Western blot analysis of β-catenin levels in cytosolic (Cy) and nuclear (Nu) extracts isolated from cells. SP1 serves as a nuclear protein loading control. Densitometry of nuclear β-catenin. Data are reported as mean ± SD of 3 independent experiments. (C) Distribution of β-catenin by immunostaining in A, R and T cells treated with 20mM LiCl for 30h. Blue: DAPI; Red: β-catenin. (D) TIMP-2 inhibited Wnt/T cell factor (TCF) responsive reporter in A, R, T cell lines treated with 20mMLiCl 30h.Luciferase activity was normalized to the internal control, set A2058 as 1. (E) Cell Proliferation measured by the MTT.
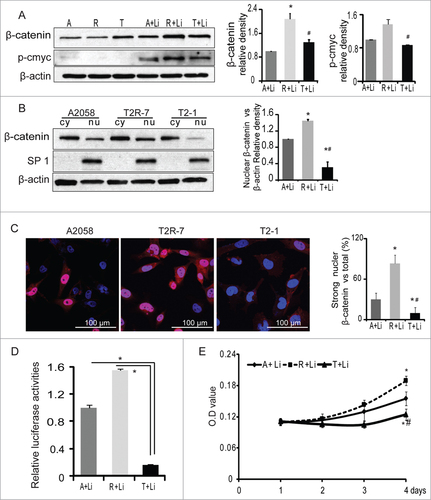
TIMP-2 decreases translocation of β-catenin from cytoplasm to nuclear induced by lithium
To confirm the distribution of β-catenin, we performed fractionation analysis. Our data showed that nuclear β-catenin protein was significantly lower in T2-1 cells than that in the parental A2058 and T2R-7 cells. The nuclear β-catenin level in A2058 showed a decreasing trend compared to T2R-7 (). We further examined the distribution of β-catenin by immunofluorescence staining. Our data shows that the nuclear fluorescence of β-catenin (red staining) in T2R-7 was enhanced compared to those in T2-1 with TIMP-2 overexpression (). These data indicates that TIMP-2 inhibited lithium induced translocation of β-catenin from cytoplasm to nucleus in cells with activated β-catenin signaling.
Transcriptional activity of β-catenin induced by lithium is inhibited by TIMP-2
Once in the nucleus, β-catenin binds to the transcriptional factors of TCF family to form specific DNA binding complexes. We then sought to ascertain whether TIMP-2 affected the transcriptional activity of β-catenin when cells were stimulated by lithium. Our luciferase reporter data showed that the transcriptional activity of β-catenin was significantly (8 folds) increased in T2R-7 compared to the T2-1 cells. In contract, in the parental A2058, T2-1, and T2R-7 cells without lithium treatment, there were no significant signals detected in the cells (). These data clearly showed that TIMP-2 overexpression significantly decreased lithium induced transcriptional activity of β-catenin.
TIMP-2 inhibits cell proliferation induced by lithium
To explore the role of TIMP2 on the biological effects of Wnt/β-catenin pathway, we then examined cell proliferation by MTT assay in A2058, T2R-7 and T2-1 cells with lithium treatment. Our data show that absorbance was highest in the TIMP-2 under-expressed T2R-7 cells, whereas, in TIMP-2 overexpressing cells it was lowest (). These data indicate that TIMP-2 over-expression inhibited lithium-induced proliferation.
TIMP-2 inhibits protein and mRNA levels of Wnt 11 induced by lithium
Lithium has been shown to be a direct, reversible inhibitor of GSK-3.Citation31 GSK-3 phosphorylates the amino-terminal region of β-catenin, targeting it for ubiquitination and degradation by proteasome.Citation32 We detected total and phosphorylated protein level of GSK-3β through Western blot. There were no significant differences of P-GSK-3β (Y216) and total GSK-3β among A2058, T2R-7 and T2-1 cells. P-GSK-3β (Y216) correlates with an increase of GSK-3β kinase activity.Citation33 In contrast, p-GSK-3β (S9) correlates with the inhibition of its kinase activity.Citation34 We found that the ratio of p-GSK-3β (S9) to β-catenin was significantly lower in T2R-7 with under-expressing TIMP-2 (). Axins inhibit β-catenin pathway and are regulated by GSK-3 β.Citation35 We found that the protein levels of Axin 1 and Axin 2 increased in T2-1 compared to T2R-7 cells (). The data indicate TIMP-2 under-expressing enhanced lithium-associated accumulation of β-catenin through GSK-3β.
Figure 5. TIMP-2 inhibits protein and mRNA level of Wnt11 induced by LiCl. (A) Immunoblot of phospho-GSK3β, Axins in the cells. Densitometry of p-GSK3β (Ser9), Axin1 and Axin2. (B) Immunoblot of Wnt 11 in the LiCl treated A2058 (A), A2058 T2R-7 (R), and A2058 T2-1 (T) cell lines. Densitometry of Wnt 11 in cells treated with LiCl. (C) TIMP-2 inhibits mRNA levels of Wnt 11 and Wnt 6 in A, R, T treated with LiCl. Data are expressed as mean ± SD. n= 3 separate experiments. # compared to A2058, P< 0.05; # compared to T2R-7, P<0.05. (D) The working model of TIMP-2 regulation of β-catenin activity in melanoma cells with or without lithium stimulation.
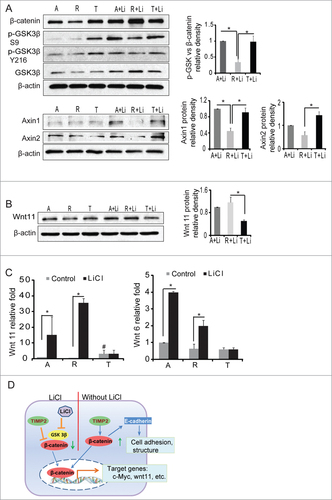
Wnt 11 is a direct target of the canonical β-catenin pathway.Citation36 Wnt11 transcripts were robustly induced upon treatment with lithium chloride in mouse myoblast C2C12 cells.Citation36 We measured mRNA levels of Wnts. The data show lithium increases mRNA levels of Wnt 11 in A2058 and T2R-7 cells. There was no significant change of Wnt11 mRNA levels in T2-1 with TIMP-2 overexpression cells after lithium treatment. The response of mRNA of Wnt 6 was similar to that of Wnt 11 (). Furthermore, our protein gel blots show that TIMP-2 inhibits the increase of Wnt 11 protein level in T2-1 cells treated with lithium ().
Discussion
In the current study, we have used stable melanoma cell lines, parental A2058, T2R-7 under-expressing and T2-1 over-expressing TIMP-2, to determine the TIMP-2 regulation of β-catenin activity. Without stimulation, TIMP-2 under-expression led to a decrease of β-catenin at the basal level in melanoma cells. With lithium treatment, which mimics the enhanced Wnt/β-catenin microenvironment in melanoma, TIMP-2 over-expression inhibits the increased protein expression and translocation of β-catenin, thus inhibiting the transcriptional activity of β-catenin and its consequences on increasing target genes and cell proliferation. Our data indicate that TIMP-2 plays an essential role in the homeostasis of β-catenin, thus inhibiting cancer cell proliferation when cells have an active β-catenin state ().
TIMP-2 affects extracellular matrix accumulation, which plays a crucial role in the regulation of cell proliferation through various pathways. Overexpression of TIMP-2 increases the proliferation of human choriocarcinoma cells through the mitogen-activated protein kinase (MAPK) signaling pathway.Citation37 TIMP-2 reduced tumor growth in diffuse-type gastric cancer cell lines through the transforming growth factor β (TGF-β) pathway.Citation38 Our data suggest that TIMP-2 over-expression is able to enhance proliferation in human melanoma A2058 cells at the basal level when β-catenin activity is relative low. It is consistent with previous studies which show that TIMP-2 overexpression protects melanoma cells from apoptosis.Citation1,6,7 TIMP-2 was shown to stimulate proliferation in human cells, including osteosarcoma cells, fibroblasts, and A549 lung adenocarcinoma cells.Citation39 We would like to put emphasis on the stage of cells with high β-catenin activity. Our data clearly demonstrate that TIMP-2 under-expression was not able to suppress the activation of β-catenin signaling, whereas over-expressing TIMP-2 inhibited β-catenin activity.
Our results of cadherin are consistent with recent studies which show that TIMP-2 over expression increased E-cadherin in human lung carcinoma A549 cells.Citation13,40 The C-terminus of E-cadherin binds directly to β-catenin to stabilize cell-cell adhesion. The distribution of β-catenin and E-cadherin are in cytoplasm and membrane of the cells. Meanwhile, p120 catenin, another adhesion junction protein, was increased under TIMP-2 overexpression. The regulation of assembly and disassembly of the cadherin-catenin complex underlie the dynamics of the adhesive interactions between cells during tissue development and cancer metastasis.Citation41,42 Cancer metastasis may include morphological changes characterized by epithelial-to-mesenchymal transition with down-regulation of E-cadherin and induction of N-cadherin, release of β-catenin from junctional complexes.Citation43 The loss of E-cadherin may result in a reduction in N-cadherin-mediated adhesiveness and facilitate the migration of tumor cells across the junctions. Rearrangements and defects of E-cadherin adhesions were found in many carcinomas.Citation40,44 Furthermore, E-cadherin-positive clones displayed a decrease of invasive abilities in bronchial tumor cells.Citation45 Silencing of N-cadherin can inhibit cell proliferation in human melanoma cell.Citation46
In the current study, we showed that TIMP-2 overexpression inhibited protein level of β-catenin and its translocation from cytoplasm to nuclear, and cell proliferation induced by lithium. Depends on the sites of phosphorylation, β-catenin can be activated or degraded. Phosphorylation mediated by AKT at a c-terminal serine residue (S552) can promote β-catenin transcriptional activity.Citation47 Beta-catenin phosphorylated by GSK3 at T41, S37 and S33, and recognized by the E3-ligase component, β-TrCP, for ultimate ubiquitination and destruction by proteasome.Citation48-50 Without lithium, TIMP2 increased p-β-catenin at S552, but had no effects on S33, S37 and other sites. Meanwhile, TIMP-2 didn't affect the protein amount of total and phosphorylated AKT. Fraction assay showed no nuclear β-catenin changed in the cell lines. We speculate that TIMP-2 may regulate cell proliferation through other pathways, in addition to the β-catenin signaling. For future study, we will illustrate the mechanism through investigation of other pathways, such as MAPK, TGF-β, etc.
TIMP-2 has been widely used as a therapeutic transgene in various animal models. TIMP-2 can significantly prolong survival time and decrease tumor size in ovarian cancer model.Citation51 Several other animal studies also showed that TIMP-2 can suppress tumor growth, migration and metastasis in vitroCitation23 and in various cancer models, such as, prostate cancer,Citation52 pancreatic cancer,Citation53 breast adenocarcinoma,Citation54 Colon Carcinoma,Citation55 melanoma,Citation56 etc. The roles of TIMP-2 on MMP-2, MMP-9 and E-cadherin may explain its inhibition tumor migration and metastasis. Our current findings illustrate the mechanism of TIMP-2 reduction of tumor growth in melanoma cells.
Altogether, we demonstrated that TIMP-2 inhibits the Wnt/β-catenin pathway, thus inhibiting proliferation of melanoma cells with high activity of-β-catenin. Insights in the molecular mechanisms of TIMP-2 in cancer will provide promising opportunities for therapeutic intervention.
Materials and Methods
Cell culture and treatment
Human melanoma A2058, T2-1, and T2R-7 cells were maintained in DMEM supplemented with 10% FBS and penicillin-streptomycin as previously described.Citation24 Equal number of cells was plated in 6-well plates. After growing for 24 h, cells were harvested for assays. For lithium treatment, cells were treated with 20 mM lithium chloride (Fisher Scientific, Pittsburgh, PA) for 30 h. For growth curve, cells were plated in triplicate in 6-well plates at 5 × 104 cells/well. The cells were trypsinized and counted at various time points after plating by trypan blue staining using a hemocytometer. For MTT assays, cells were plated in triplicate in 96-well plates at 1 × 104 cells/well with 200 μl medium. At various time points, each well was incubated with 50 μl MTT solution (1 mg/ml in PBS) at 37°C for 4 h. To resolve formazan crystals, 200 μl of DMSO was added to each well. The absorbance was measured at 570 nm on a microtiter plate ELISA reader while DMSO was used as blank.
Cell fractionation
Cells were planted in 6 cm dishes for 24h, then washed with HBSS 3 times and lysed. The cytoplasmic and nuclear proteins were extracted using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific, Rockford, IL, USA).
Immunoblotting
Cells were rinsed twice in ice-cold HBSS, lysed in protein loading buffer (50 mMTris, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol), and sonicated. Equal amount of proteins or equal volumes of total cultured cell lysates were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted with primary antibodies: anti-β-catenin, GSK-3β, E-cadherin (BD Transduction, San Jose, CA, USA), p-β-catenin (Ser33/37/Thr41), p-β-catenin(Thr41/Ser45), p-β-catenin (Ser552), TIMP-2, Axin1 (Cell Signaling Technology, Billerica, MA, USA), SP-1, P120 catenin, Cyclin D1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), α-catenin, N-cadherin (Life Technologies, Grand Island, NY), Wnt11, p-c-Myc (Abcam, Cambridge, MA) or β-actin (Sigma-Aldrich) antibodies and visualized by ECL (Thermo Scientific, Rockford, IL, USA) as previously described.Citation57,58
Immunoprecipitation
Cells were rinsed twice in ice-cold HBSS then lysed in cold immunoprecipitation buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris·HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, pH 8.0, 0.2 mM sodium orthovanadate) containing protease inhibitor cocktail (Boehringer Mannheim, Roche, Indianapolis, IN, USA). Samples were pre-cleared with protein A-agarose (Invitrogen). Pre-cleared lysates were then incubated with 2 μg of anti- β-catenin antibody overnight at 4°C. A 50% slurry of Protein A-agarose was added to the lysate and incubated for 1h with agitation at 4°C and then washed with cold immunoprecipitation buffer. The pellet was resuspended in protein loading buffer and centrifuged, boiled for 5 min, separated by SDS-PAGE, and transferred onto a nitrocellulose membrane. Membrane blots were probed with anti-ubiquitin antibody (UG9510, Enzo Life).
Immunofluorescence
Cells were grown in the Lab-Tek chambered coverglass system (Thermo Scientific), with/without 20 mM lithium chloride treatment 30 h. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Tritonx-100, incubated with anti-E-cadherin, anti-β-catenin antibody at 4°C overnight after blocking with 5% bovine serum albumin. Samples were then incubated with goat anti-mouse AlexaFluor 594 and DAPI (Life technologies) for 1 hour at room temperature. Slides were mounted with SlowFade (Life technologies), followed by a coverslip, and the edges were sealed to prevent drying. Specimens were examined with a Zeiss laser scanning microscope 710 (Carl Zeiss Oberkochen, Germany).
Quantitative real-time PCR analysis
Total RNA was extracted from epithelial cell monolayers using TRIzol reagent (Life technologies). The RNA integrity was verified by electrophoresis gel. RNA reverse transcription was done using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's directions. The RT cDNA reaction products were subjected to quantitative real-time PCR using the CFX96 Real-Time system C1000 Touch thermal cycler (Bio-Rad) and iTaq Universal SYBR green supermix(Bio-Rad) according to the manufacturer's directions with primers (). All real-time PCR reactions were performed in triplicate. Percent expression was calculated as the ratio of the normalized value of each sample to that of the corresponding control cells.
Table 1. Real-Time PCR Primers.
Luciferase reporter assay
TOPFlash luciferase reporter (Millipore, Billerica, MA) containing the TCF/LEF consensus sequence was used to assay β-catenin dependent promoter activity, with control FOPFlash reporter (Millipore) containing mutated TCF binding sites. Cells were transfected with TOPFlash and FOPFlash using Lipofectamine (Life technologies) in 24-well plates in triplicates. To normalize transfection efficiency, cells were co-transfected with internal control reporter Renilla luciferase plasmid (pRL-TK; Promega, Madison, WI, USA). 24h after transfection, cells were incubated with 20 mM lithium chloride 30 h, luciferase activity was determined using the dual luciferase reporter assay system (Promega) with a Sirius single tube luminometer (Berthold, Pforzheim, Germany). The luciferase activity was normalized with Renilla luciferase activity, and the activity was expressed as relative units as previously described.Citation57
Statistical analysis
Data were expressed as mean ± SD. Differences between 2 groups were analyzed by Student's t-test. More than 2 groups were analyzed by analysis of variance (ANOVA) followed by the Tukey-Kramer test, P values less than or equal to 0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
Conceived and designed the experiments: SW. Performed the experiments: YX, SW. Analyzed the data: SW. Wrote the paper: YX, SW. All authors read and approved the final manuscript.
Acknowledgments
We want to thank Dr. Jun Sun for providing her resources, cell lines, and extremely insightful advice on the manuscript. We want to thank Lester Liu for his excellent technical support and Dapeng Jin for critical editing of this manuscript.
References
- Sun J. Matrix metalloproteinases and tissue inhibitor of metalloproteinases are essential for the inflammatory response in cancer cells. J Signal Transduct 2010; 2010:985132; PMID:21152266; http://dx.doi.org/10.1155/2010/985132
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochimica Biophysica Acta 2000; 1477:267–83; PMID:10708863; http://dx.doi.org/10.1016/S0167-4838(99)00279-4
- Vaisanen AH, Kallioinen M, Turpeenniemi-Hujanen T. Comparison of the prognostic value of matrix metalloproteinases 2 and 9 in cutaneous melanoma. Hum Pathol 2008; 39:377–85; PMID:18187184; http://dx.doi.org/10.1016/j.humpath.2007.06.021
- Hernandez-Barrantes S, Shimura Y, Soloway PD, Sang QA, Fridman R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem Biophys Res Commun 2001; 281:126–30; PMID:11178970; http://dx.doi.org/10.1006/bbrc.2001.4323
- Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R. Regulation of membrane type-matrix metalloproteinases. Seminar Cancer Biol 2002; 12:131–8; PMID:12027585; http://dx.doi.org/10.1006/scbi.2001.0421
- Valente P, Fassina G, Melchiori A, Masiello L, Cilli M, Vacca A, Onisto M, Santi L, Stetler-Stevenson WG, Albini A. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer 1998; 75:246–53; PMID:9462715; http://dx.doi.org/10.1002/(SICI)1097-0215(19980119)75:2%3c246::AID-IJC13%3e3.0.CO;2-B
- Sun J, Stetler-Stevenson WG. Overexpression of tissue inhibitors of metalloproteinase 2 up-regulates NF-kappaB activity in melanoma cells. J Mol Signal 2009; 4:4; PMID:19627587; http://dx.doi.org/10.1186/1750-2187-4-4
- Lizarraga F, Maldonado V, Melendez-Zajgla J. Tissue inhibitor of metalloproteinases-2 growth-stimulatory activity is mediated by nuclear factor-kappa B in A549 lung epithelial cells. Int J Biochem Cell Biol 2004; 36:1655–63; PMID:15147743; http://dx.doi.org/10.1016/j.biocel.2004.02.004
- Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev 2008; 27:57–66; PMID:18058195; http://dx.doi.org/10.1007/s10555-007-9105-8
- Munshi HG, Wu YI, Mukhopadhyay S, Ottaviano AJ, Sassano A, Koblinski JE, Platanias LC, Stack MS. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-beta1-induced pericellular collagenolysis. J Biol Chem 2004; 279:39042–50; PMID:15247230; http://dx.doi.org/10.1074/jbc.M404958200
- Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 2003; 114:171–80; PMID:12887919; http://dx.doi.org/10.1016/S0092-8674(03)00551-8
- Fernandez CA, Roy R, Lee S, Yang J, Panigrahy D, Van Vliet KJ, Moses MA. The anti-angiogenic peptide, loop 6, binds insulin-like growth factor-1 receptor. J Biol Chem 2010; 285:41886–95; PMID:20940305; http://dx.doi.org/10.1074/jbc.M110.166439
- Bourboulia D, Han H, Jensen-Taubman S, Gavil N, Isaac B, Wei B, Neckers L, Stetler-Stevenson WG. TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/β-catenin complex expression in A549 lung cancer cells. Oncotarget 2013; 4:163–73; PMID:23847723
- Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J 2012; 31:2714–36; PMID:22617422; http://dx.doi.org/10.1038/emboj.2012.150
- Pecina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 2003; 3:17; PMID:14613514; http://dx.doi.org/10.1186/1475-2867-3-17
- Kim W, Kim M, Jho EH. Wnt/β-catenin signalling: from plasma membrane to nucleus. Biochemical J 2013; 450:9–21; PMID:23343194; http://dx.doi.org/10.1042/BJ20121284
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149:1192–205; PMID:22682243; http://dx.doi.org/10.1016/j.cell.2012.05.012
- Morin PJ. β-catenin signaling and cancer. BioEssays 1999; 21:1021–30; PMID:10580987; http://dx.doi.org/10.1002/(SICI)1521-1878(199912)22:1%3c1021::AID-BIES6%3e3.0.CO;2-P
- Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B. APC is essential for targeting phosphorylated β-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell 2008; 32:652–61; PMID:19061640; http://dx.doi.org/10.1016/j.molcel.2008.10.023
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA 2013; 63:11–30; PMID:23335087; http://dx.doi.org/10.3322/caac.21166
- Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Frontiers Biosci 2006; 11:733–42; PMID:16146765; http://dx.doi.org/10.2741/1831
- Sinnberg T, Menzel M, Ewerth D, Sauer B, Schwarz M, Schaller M, Garbe C, Schittek B. β-Catenin signaling increases during melanoma progression and promotes tumor cell survival and chemoresistance. PloS One 2011; 6:e23429; PMID:21858114; http://dx.doi.org/10.1371/journal.pone.0023429
- Ray JM, Stetler-Stevenson WG. TIMP-2 expression modulates human melanoma cell adhesion and motility. Annal New York Acad Sci 1994; 732:233–47; PMID:7978796; http://dx.doi.org/10.1111/j.1749-6632.1994.tb24739.x
- Ray JM, Stetler-Stevenson WG. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J 1995; 14:908–17; PMID:7534227
- Sun J, Hobert ME, Rao AS, Neish AS, Madara JL. Bacterial activation of β-catenin signaling in human epithelia. Am J Physiol 2004; 287:G220–7; PMID:14764450
- Yaguchi T, Goto Y, Kido K, Mochimaru H, Sakurai T, Tsukamoto N, Kudo-Saito C, Fujita T, Sumimoto H, Kawakami Y. Immune suppression and resistance mediated by constitutive activation of Wnt/β-catenin signaling in human melanoma cells. J Immunol 2012; 189:2110–7; PMID:22815287; http://dx.doi.org/10.4049/jimmunol.1102282
- Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D, Rimm DL, McMahon M, et al. β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 2011; 20:741–54; PMID:22172720; http://dx.doi.org/10.1016/j.ccr.2011.10.030
- Rao AS, Kremenevskaja N, Resch J, Brabant G. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/β-catenin signalling. Europ J Endocrinol 2005; 153:929–38; PMID:16322400; http://dx.doi.org/10.1530/eje.1.02038
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 1997; 185:82–91; PMID:9169052; http://dx.doi.org/10.1006/dbio.1997.8552
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S America 1996; 93:8455–9; PMID:8710892; http://dx.doi.org/10.1073/pnas.93.16.8455
- Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trend Pharmacol Sci 2003; 24:441–3; PMID:12967765; http://dx.doi.org/10.1016/S0165-6147(03)00206-2
- Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T. Stabilization of β-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci U S A 2003; 100:4610–5; PMID:12668767; http://dx.doi.org/10.1073/pnas.0835895100
- Fang X, Yu SX, Lu Y, Bast RC, Jr., Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A 2000; 97:11960–5; PMID:11035810; http://dx.doi.org/10.1073/pnas.220413597
- Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 β in intact cells via serine 9 phosphorylation. Biochem J 1994; 303 (Pt 3):701–4; PMID:7980435
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 2002; 22:1172–83; PMID:11809808; http://dx.doi.org/10.1128/MCB.22.4.1172-1183.2002
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet 2007; 39:1225–34; PMID:17767158; http://dx.doi.org/10.1038/ng2112
- Thang NM, Kumasawa K, Tsutsui T, Nakamura H, Masaki H, Ono T, Kimura T. Overexpression of endogenous TIMP-2 increases the proliferation of BeWo choriocarcinoma cells through the MAPK-signaling pathway. Reprod Sci 2013; 20:1184–92; PMID:23427184; http://dx.doi.org/10.1177/1933719113477485
- Johansson E, Komuro A, Iwata C, Hagiwara A, Fuse Y, Watanabe A, Morishita Y, Aburatani H, Funa K, Kano MR, et al. Exogenous introduction of tissue inhibitor of metalloproteinase 2 reduces accelerated growth of TGF-β-disrupted diffuse-type gastric carcinoma. Cancer Sci 2010; 101:2398–403; PMID:20718757; http://dx.doi.org/10.1111/j.1349-7006.2010.01688.x
- Nemeth JA, Rafe A, Steiner M, Goolsby CL. TIMP-2 growth-stimulatory activity: a concentration- and cell type-specific response in the presence of insulin. Exp Cell Res 1996; 224:110–5; PMID:8612674; http://dx.doi.org/10.1006/excr.1996.0117
- Giehl K, Menke A. Microenvironmental regulation of E-cadherin-mediated adherens junctions. Frontiers Bioscience 2008; 13:3975–85; PMID:18508491; http://dx.doi.org/10.2741/2985
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol 2000; 148:399–404; PMID:10662767; http://dx.doi.org/10.1083/jcb.148.3.399
- Takeichi M, Abe K. Synaptic contact dynamics controlled by cadherin and catenins. Trend Cell Biol 2005; 15:216–21; PMID:15817378; http://dx.doi.org/10.1016/j.tcb.2005.02.002
- Shah GV, Muralidharan A, Gokulgandhi M, Soan K, Thomas S. Cadherin switching and activation of β-catenin signaling underlie proinvasive actions of calcitonin-calcitonin receptor axis in prostate cancer. J Biol Chem 2009; 284:1018–30; PMID:19001380; http://dx.doi.org/10.1074/jbc.M807823200
- Mareel M, Boterberg T, Noe V, Van Hoorde L, Vermeulen S, Bruyneel E, Bracke M. E-cadherin/catenin/cytoskeleton complex: a regulator of cancer invasion. J Cell Physiol 1997; 173:271–4; PMID:9365535; http://dx.doi.org/10.1002/(SICI)1097-4652(199711)173:2%3c271::AID-JCP34%3e3.0.CO;2-G
- Nawrocki-Raby B, Gilles C, Polette M, Martinella-Catusse C, Bonnet N, Puchelle E, Foidart JM, Van Roy F, Birembaut P. E-Cadherin mediates MMP downregulation in highly invasive bronchial tumor cells. Am J Pathol 2003; 163:653–61; PMID:12875984; http://dx.doi.org/10.1016/S0002-9440(10)63692-9
- Ciolczyk-Wierzbicka D, Gil D, Laidler P. The inhibition of cell proliferation using silencing of N-cadherin gene by siRNA process in human melanoma cell lines. Curr Med Chem 2012; 19:145–51; PMID:22300088; http://dx.doi.org/10.2174/092986712803414006
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem 2007; 282:11221–9; PMID:17287208; http://dx.doi.org/10.1074/jbc.M611871200
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002; 108:837–47; PMID:11955436; http://dx.doi.org/10.1016/S0092-8674(02)00685-2
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997; 16:3797–804; PMID:9233789; http://dx.doi.org/10.1093/emboj/16.13.3797
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol 1999; 9:207–10; PMID:10074433; http://dx.doi.org/10.1016/S0960-9822(99)80091-8
- Yang SW, Chanda D, Cody JJ, Rivera AA, Waehler R, Siegal GP, Douglas JT, Ponnazhagan S. Conditionally replicating adenovirus expressing TIMP2 increases survival in a mouse model of disseminated ovarian cancer. PloS One 2011; 6:e25131; PMID:22022379; http://dx.doi.org/10.1371/journal.pone.0025131
- Deng X, He G, Levine A, Cao Y, Mullins C. Adenovirus-mediated expression of TIMP-1 and TIMP-2 in bone inhibits osteolytic degradation by human prostate cancer. Int J Cancer 2008; 122:209–18; PMID:17847032; http://dx.doi.org/10.1002/ijc.23053
- Rigg AS, Lemoine NR. Adenoviral delivery of TIMP1 or TIMP2 can modify the invasive behavior of pancreatic cancer and can have a significant antitumor effect in vivo. Cancer Gene Ther 2001; 8:869–78; PMID:11773977; http://dx.doi.org/10.1038/sj.cgt.7700387
- Li H, Lindenmeyer F, Grenet C, Opolon P, Menashi S, Soria C, Yeh P, Perricaudet M, Lu H. AdTIMP-2 inhibits tumor growth, angiogenesis, and metastasis, and prolongs survival in mice. Hum Gene Ther 2001; 12:515–26; PMID:11268284; http://dx.doi.org/10.1089/104303401300042429
- Brand K, Baker AH, Perez-Canto A, Possling A, Sacharjat M, Geheeb M, Arnold W. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res 2000; 60:5723–30; PMID:11059766
- Vincent L, Varet J, Pille JY, Bompais H, Opolon P, Maksimenko A, Malvy C, Mirshahi M, Lu H, Vannier JP, et al. Efficacy of dendrimer-mediated angiostatin and TIMP-2 gene delivery on inhibition of tumor growth and angiogenesis: in vitro and in vivo studies. Int J Cancer 2003; 105:419–29; PMID:12704680; http://dx.doi.org/10.1002/ijc.11105
- Sun J, Hobert ME, Duan Y, Rao AS, He TC, Chang EB, Madara JL. Crosstalk between NF-kappaB and β-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol 2005; 289:G129–37; PMID:15790758
- Mustafi R, Cerda S, Chumsangsri A, Fichera A, Bissonnette M. Protein Kinase-zeta inhibits collagen I-dependent and anchorage-independent growth and enhances apoptosis of human Caco-2 cells. Mol Cancer Res 2006; 4:683–94; PMID:16940160; http://dx.doi.org/10.1158/1541-7786.MCR-06-0057
