Abstract
The KRAS-variant is a biologically functional, microRNA binding site variant, which predicts increased cancer risk especially for women. Because external exposures, such as chemotherapy, differentially impact the effect of this mutation, we evaluated the association of estrogen exposures, breast cancer (BC) risk and tumor biology in women with the KRAS-variant. Women with BC (n = 1712), the subset with the KRAS-variant (n = 286) and KRAS-variant unaffected controls (n = 80) were evaluated, and hormonal exposures, KRAS-variant status, and pathology were compared. The impact of estrogen withdrawal on transformation of isogenic normal breast cell lines with or without the KRAS-variant was studied. Finally, the association and presentation characteristics of the KRAS-variant and multiple primary breast cancer (MPBC) were evaluated. KRAS-variant BC patients were more likely to have ovarian removal pre-BC diagnosis than non-variant BC patients (p = 0.033). In addition, KRAS-variant BC patients also appeared to have a lower estrogen state than KRAS-variant unaffected controls, with a lower BMI (P < 0.001). Finally, hormone replacement therapy (HRT) discontinuation in KRAS-variant patients was associated with a diagnosis of triple negative BC (P < 0.001). Biologically confirming our clinical findings, acute estrogen withdrawal led to oncogenic transformation in KRAS-variant positive isogenic cell lines. Finally, KRAS-variant BC patients had greater than an 11-fold increased risk of presenting with MPBC compared to non-variant patients (45.39% vs 6.78%, OR 11.44 [3.42–37.87], P < 0.001). Thus, estrogen withdrawal and a low estrogen state appear to increase BC risk and to predict aggressive tumor biology in women with the KRAS-variant, who are also significantly more likely to present with multiple primary breast cancer.
Introduction
MicroRNA (miRNA) binding site variants in the 3′ untranslated region (3′UTR) of important growth and survival genes are a recently discovered, novel class of functional, biologically active, germ-line mutations that are powerful biomarkers of cancer risk and treatment response.Citation1 One of the first mutations discovered in this class is the KRAS-variant, a let-7 binding site mutation in the 3′UTR of the KRAS oncogene.Citation2 This mutation predicts an increased risk of several cancers, including non-small cell lung cancer,Citation2 triple negative breast cancer (TNBC) in premenopausal womenCitation3 and ovarian cancer.Citation4-6 The KRAS-variant also predicts unique tumor biology, with tumors in KRAS-variant patients exhibiting a KRAS-addicted signature, as well as an estrogen negative, basal-like gene expression pattern.Citation3,5 Perhaps most powerful is the extensive evidence that the KRAS-variant is biologically functional, as exemplified by its role as a strong biomarker of response to cancer therapy. KRAS-variant patients with ovarian cancer or head and neck cancer are cisplatin resistant,Citation5,7 those with colon cancer or head and neck cancer exhibit cetuximab sensitivity,Citation7,8 and those with non-small cell lung cancer (NSCLC) are resistant to erlotinib but sensitive to sorafenib.Citation9 Cell line data further supports the unique response of the KRAS-variant to chemotherapy exposure.Citation8
Women with the KRAS-variant are also at a significantly increased risk of developing multiple primary cancers, including breast and ovarian cancer, as well as a third independent cancer in their lifetime.Citation6 Multiple primary cancer, although difficult to predict, is not rare, as one in 8 cancer patients will be diagnosed with a new primary cancer after their first cancer diagnosis (metachronous cancer), and one in 40 patients will be diagnosed with 2 cancers at the same time (synchronous cancer).Citation10 While it is hypothesized that some metachronous cancers are caused by primary cancer treatment, it is also thought that genetics may play a significant role in the development of both synchronous and metachronous cancers.Citation11 Multiple primary breast cancer (MPBC) is one of the most common forms of multiple primary cancer.Citation12 Currently known risk factors for MPBC include young age at first diagnosis;Citation13-15 first BC of lobular histology;Citation12,16,17 high BMI (>30) in pre-menopausal patients with a hormone-receptor negative first primary;Citation18 positive family history of BC;Citation19 and mutations in BRCA1, BRCA2Citation20 or CHEK2.Citation21 Most recently, the KRAS-variant was found to also be associated with MPBC in a small case series, as it was found in 57.1% (4/7) of patients who developed bilateral BC and ovarian cancer who were uninformative (BRCA negative).Citation6 Factors thought to decrease MPBC risk have also been identified, and include menarche after age 13, multiparity,Citation17 treatment with anti-hormonal agents or chemotherapyCitation22,23 and prophylactic surgical intervention.Citation24 These findings suggest that multiple primary BC risk can be impacted by estrogen alterations either before or after the first BC diagnosis.
In general, evidence suggests that elevated estrogen increases primary BC risk. Such evidence includes increased BC risk in women experiencing early menarche, late menopause, obesity (in post-menopausal patients), nulliparity, or advanced maternal age at first birth.Citation25 In addition, in vitro studies using the breast epithelial line MCF10A show that excess estrogen and its metabolites can lead to increased transformation, i.e. BC initiation.Citation26,27 However, estrogen is not a risk for BC for all women, as has become clear through clinical studies of HRT use.Citation28,29 Initially, the Million Women Study and Women's Health Initiative reported that current and/or prolonged use of HRT correlated with an increased risk of BC. Because these tumors tended to be lower grade, with over-representation of lobular or tubular subtypes compared to other ductal cancers,Citation30 it was hypothesized that HRT was causing cancers that otherwise would not have arisen. However, a follow-up WHI report found that there was actually no increased BC risk for patients assigned to estrogen-only preparations compared to placebo. In fact, after a median follow up of 11.8 years (IQR 9.1–12.9), post-menopausal use of estrogen alone was associated with a lower BC incidence than placeboCitation31 (HR 0.77 (CI 0.62–0.95, p 0.02). These findings suggest that there may be a group of women for whom estrogen is actually protective against BC development.
For women with the KRAS-variant, there is growing evidence that estrogen may differentially impact their overall cancer risk and tumor biology. This evidence includes: a higher risk of non-small cell lung cancer in women versus men with the KRAS-variant (unpublished data); ovarian cancer almost exclusively post-menopausally for women with the KRAS-variant;Citation6 an increased risk of estrogen receptor (ER) negative tumor development in KRAS-variant patients (TNBC and type II uterine cancer,Citation32) and; the finding that post-menopausal KRAS-variant BC patients with a history of HRT use are more likely to develop biologically aggressive BC.Citation33 Although it may seem unusual that an external exposure, such as estrogen, could impact the function of an inherited 3′UTR variant like the KRAS-variant, there is in fact strong evidence that such miRNA binding site mutations are “influenced” by external exposures.Citation34 This is believed to be through alterations in miRNAs, which are immediate responders to cellular stress, and which directly act through the 3′UTR sites affected by these mutations.Citation34
Based on the association of the KRAS-variant with cancer in women and the biological impact of external exposures on this type of mutation, we hypothesized that estrogen alterations could impact cancer risk and tumor biology for individuals with the KRAS-variant. Here, we test this hypothesis, with a large cohort of BC survivors and unaffected KRAS-variant controls. We further biologically confirm our findings by utilizing an isogenically matched normal breast epithelial cell line with, or without the KRAS-variant. Finally, we define the association of the KRAS-variant with MPBC in these patients. We show here for the first time that estrogen withdrawal appears to increase both BC risk and to predict aggressive tumor biology for women with the KRAS-variant. We furthermore find that KRAS-variant BC patients are at a significantly elevated risk for both synchronous and metachronous BC development, which is not explained by other known risk factors.
Methods
Study groups
A cohort of BC patients were invited (through the Susan Love Foundation) to join a study called “The KRAS-variant and hormones” (http://www.armyofwomen.org/current/view?grant_id=438). 1906 women responded to the invitation and completed questionaires regarding age at diagnosis; anthropomorphic measurements including weight and height; reproductive history including parity, age at first birth, use of contraceptives and hormone replacement therapy; and personal and familial cancer history. Participants signed a consent approved through the Yale University Human Investigation Committee (HIC), and were mailed a cheek swab or saliva kit (Oragene) for DNA testing, and requested to supply pathology reports for their BC(s). 1712 patients supplied DNA samples. Pathology reports were used in all cases of second primary BCs, where synchronous second primary BCs were either in the contralateral breast, or if in the same breast were classified as multi-centric on the pathology reports, with different pathologies. Metachronous BC was of different pathology if in the same breast and classified as a new primary, or in the contralateral breast.
Control samples were provided by the Human Genetics Sample Bank at the Ohio State University Medical Center (OSUMC). All controls were women who had the KRAS-variant but were unaffected by cancer at the time of testing (n = 80). The Columbus Area Controls Sample Bank is a collection of control samples for use in human genetics research that includes both donor's anonymized biological specimens and linked phenotypic data. The data and samples are collected under the protocol “Collection and Storage of Controls for Genetics Research Studies,” which is approved by the Biomedical Sciences Institutional Review Board at OSUMC. Recruitment takes place in OSUMC primary care and internal medicine clinics. If individuals agree to participate, they provide written informed consent, complete a questionnaire that includes demographic, medical and family history information, and donate a blood sample, which is used for genomic DNA extraction and the establishment of an EBV-transformed lymphoblastoid cell culture, cell pellet in Trizol, and plasma.
KRAS-variant testing
For all participants, DNA was extracted from buccal swabs or saliva according to the manufacturer's protocol (Oragene). Coded patient samples were genotyped for the KRAS-variant using a Taqman-based assay as previously described,Citation2 in the MiraDx CLIA certified laboratory, through MiraKind, a non-profit organization.
Isogenic cell line creation
We generated isogenic MCF10A lines with and without the KRAS-variant using the CompoZrTM custom designed zinc-finger nuclease (ZFN) targeted genome editing technology (Sigma-Aldrich),Citation35 per manufacturer's instructions. MCF10a cells are an immortalized, non-transformed mammary epithelial cell line derived from human fibrocystic mammary tissue and have a lack of tumorigenicity in nude mice and lack of anchorage independent growth.Citation36 A ZFN pair was designed and constructed to specifically target the KRAS 3′UTR. The donor construct containing the homology arms on either side of the KRAS-variant was generated by PCR amplifying a 2087 base pair region containing the KRAS-variant from genomic DNA with forward primer 5′ AGGACTCTGATTTTGAGGACATC 3′ and reverse primer 5′ AACATGCCCCACAAAGTTTC 3′ and cloning into the pGEM-T (Promega) cloning vector. The ZFN plasmids (500 ng) and the donor plasmid (2 ug) were transfected into 2 × 105 MCF10A cells by nucleofection, program T-024, according to manufacturer's instructions (Amaxa), in media containing 100 uM chloroquine. The media was changed after 4 hrs and the cells were incubated overnight and re-seeded as single cells into 24-well plates. After passage and DNA collection, clones were assessed for the presence of the KRAS-variant using an allele-specific primer and a PCR-based TaqMan assay using.Citation2 Secondary validation was carried out by allele-specific sequencing of TOPO TA® cloned, PCR amplified genomic DNA using forward primer 5′ AAGGCATACTAGTACAAGTGGTAATTT 3′ and reverse primer 5′ TAGGAGTAGTACAGTTCATGACAAAAA 3′, which hybridize to the KRAS locus outside of the region corresponding to the donor plasmid recombination site. In addition, 2 positive clones were authenticated using bi-allelic short tandem repeat (STR) analysis at 16 different genomic loci, yielding 32 diagnostic markers for confirmation (Genetica DNA Laboratories, Inc.). STR analysis confirmed that the MCF10AKRAS-variant−/− (Parental, WT) and the 2 MCF10AKRAS-variant+/− (MT) cell lines were (a) identical to the ATCCs STR profile and (b) identical to each other, except for the presence or absence of the KRAS-variant.
Cell line and anchorage independent growth assays
MCF10A (WT) and (MT) cells were cultured in regular DMEM/F12 medium (Invitrogen) as per the Brugge lab protocol.Citation37 Anchorage independent growth was assessed as described previously.Citation38 After thawing and growing cells until confluence in EGF supplemented media (20 ng/ml), cells were plated into conditions of study for 2 passages. For estrogen depletion experiments phenol red free DMEM/F12 medium (Invitrogen) and 5% charcoal-stripped horse serum (Thermo Fisher), were used, and Tamoxifen or estrogen was added to a final concentration of 1uM after the first passage, as appropriate. To plate, 100 μl of MCF10A (WT) or (MT) cells at a density of 400,000 cells/ml were mixed with 2ml of media for the condition under study containing 2 ml 0.7% noble agar (USB). 1ml of the cell mixture was added to 1ml of 1.0% noble agar in a well of a 6-well dish. Cells were fed twice weekly by layering on a 50:50 mixture of media with 0.7% agar for 2 weeks, followed by only media for 2–3 additional weeks. The number of colonies present in each of 10 microscope fields per well from a total of 3 wells per experiment was counted and is reported as an average of the 2 separate MT lines.
Statistics
Data was analyzed using the R environment for statistical computing and graphics. Continuous data was assessed for normality using Shapiro-Wilk test and parametric or non-parametric tests applied as appropriate. Student t tests were used to compare continuous variables that were normally distributed and Mann Whitney U test for non-normally distributed data. Categorical data was analyzed using 2 × 2 contingency tables (chi-square). In order to assess association between the likelihood of being diagnosed with a second primary BC and KRAS variants, we used logistic regression and quantified differential risk through odds ratios (OR). A similar analysis was replicated to associate the time from primary diagnosis to diagnosis with a second primary BC through the Cox proportional hazard model. Differential timing of second primary cancers was compared through hazard ratios (HR). In both modeling frameworks, when adjusting for potential confounders, we selected order and scope of interaction effects through the Bayesian Information Criterion (BIC). In the Cox proportional hazard model, the assumption of proportionality was assessed both visually by inspection of Kaplan-Meier survival curves and formally, through the analysis of Schoenfeld residuals (P > 0.10).
Results
KRAS-variant BC patients vs non-variant BC patients
We first evaluated history of estrogen exposure in BC patients with and without the KRAS-variant. Of the 1712 patients who supplied DNA samples, 17.4% (n = 298) had the KRAS-variant, and 70 (4.0%) had other known genetic mutations associated with increased BC risk, including BRCA1, BRCA2 and PTEN. In the 1642 women without other mutations, 286 (17.42%) had the KRAS-variant, and 1356 (82.58%) did not. We evaluated the association of self-reported estrogen exposures in these BC patients to determine if there were any differences for BC patients with vs. without the KRAS-variant. By univariate analysis, KRAS-variant BC patients were significantly more likely to have had an oophorectomy before their BC diagnosis (15.5% vs 10.7%, p = 0.024) and to be on HRT when diagnosed with BC (66.3% vs 54.4%, p = 0.034) than non-variant BC patients (Table S1). By multivariate analysis, KRAS-variant BC patients continued to be significantly more likely to have a history of ovarian removal (oophorectomy) pre-diagnosis (OR = 1.42, CI 1.03–1.42, p = 0.033) (). In addition, although KRAS-variant patients were not significantly more likely to have a family history of breast or ovarian cancer than non-variant BC patients (62.66% vs 64.01%, NS), they were significantly more likely to have a family history of a relative with multiple primary cancers than non-variant BC patients (4.98% vs 0.92%, P < 0.0001), in agreement with our prior findings of increased multiple primary cancer risk.Citation6
Table 1. KRAS-variant BC cases compared to non-variant BC cases. By a logistic regression model, with predictors included in the model assuming a linear additive structure, BC patients with the KRAS-variant were more likely to have had an oophorectomy compared to non-variant breast cancer patients
The association of HRT with BC subtype and grade
We next evaluated the association of hormone replacement therapy (HRT) use and tumor biology in women with the KRAS-variant. We grouped post-menopausally diagnosed BC patients into 3 HRT use groups based on their HRT use at the time of their diagnosis. These groups comprised “never users,” “current users” (women on HRT at the time of their BC diagnosis), or “past users” (women with a history of HRT preceding their BC diagnosis by at least 6 months). We then compared histologic BC tumor subtypes (ER/PR+, HER2+, or ER/PR/HER2- [triple negative]) and grade with these categories of HRT use for KRAS-variant (n = 133) vs non-variant BC patients (n = 612) with complete histologic tumor documentation.
Overall, there was no difference in tumor grade between KRAS-variant versus non-variant BC patients, but the TNBC tumor subtype was significantly more common in post-menopausal women with the KRAS-variant (13.9% vs 7.7%, p = 0.029). For non-variant BC patients, there were no differences in the proportion of women with each tumor subtype between the never, current or past HRT user groups. However, as reported previouslyCitation30 there was a trend for current or past HRT users to have lower grade breast tumors than never users, but this difference was not statistically significant in our study cohort. For KRAS-variant BC patients, past HRT users were significantly more likely to be diagnosed with TNBC than KRAS-variant never or current HRT users (35.5% [n = 11/31] vs 6.6% [6/91], P < 0.0001). In addition, compared to non-variant past HRT users, KRAS-variant past HRT users were significantly more likely to be diagnosed with TNBC (35.5% vs 7.3% [n = 11/151] P < 0.0001, ), and also to have significantly higher-grade tumors (2.33 vs 1.98, p = 0.029). In contrast, there were no statistically significant differences in tumor subtype or grade between KRAS-variant never or current HRT users.
Table 2. Histologic breast cancer subtype and history of hormone replacement therapy use. Tumor grade between all KRAS-variant vs. non-KRAS-variant BC patients was non-significant. KRAS-variant patients were significantly more likely to have triple negative breast cancers as a group (13.9% vs 7.7%, p = 0.029). KRAS-variant patients with a history of past HRT use were significantly more likely to have TNBC. There were no differences in cancer subtype by HRT use for non-variant patients
KRAS-variant BC patients vs unaffected KRAS-variant controls
We then evaluated if differences in hormonal exposures might impact BC risk in women with the KRAS-variant, by comparing hormonal exposures in KRAS-variant BC patients (n = 286) with a cohort of KRAS-variant cancer free unaffected controls (n = 80). In univariate analysis we found numerous significant differences, including factors associated with HRT use, pregnancy, OCP use and BMI (Table S2). By multivariate analysis we confirmed that KRAS-variant BC patients remained significantly more likely to have a lower BMI, and have fewer live births than KRAS-variant cancer free controls (). Of note, we found no difference in age of diagnosis vs age of enrollment between the BC patients and the controls.
Table 3. KRAS-variant BC cases compared to KRAS-variant controls. Women with breast cancer with the KRAS-variant by a binary logistic model were significantly more likely to have fewer live births, and to have a lower Body Mass Index (BMI)
KRAS-variant MCF10A cell lines and Transformation
To biologically confirm our clinical findings, that a low estrogen state and/or estrogen withdrawal may be associated with increased BC risk for women with the KRAS-variant, we created an isogenic MCF10a line, with (MCF10aKRASv+/−, MT1 and MT2) vs. without (MCF10aKRASv−/−, WT) the KRAS-variant. We found that KRAS mRNA was lower in the MT cells, but KRAS protein was fairly equivalent or slightly elevated (Fig. S1), consistent with prior reports in KRAS-variant-associated tissues.Citation39
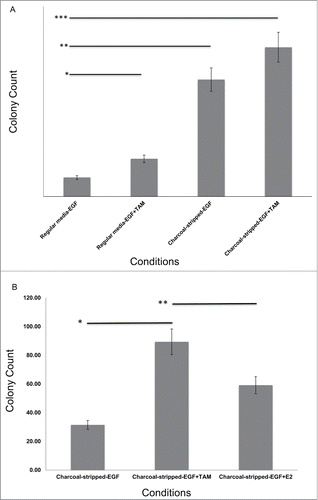
WT and MT lines were plated in soft agar to test for transformation, as measured by anchorage independent growth. There was no colony formation seen in the presence of Epidermal Growth Factor (EGF) during the course of the experiment for either the WT or MT lines, indicating that neither line, at baseline, was transformed (Fig. S2). However, when the cell lines were grown without EGF, as is standard to promote transformation, the MT lines exhibited low levels of colony formation by the fifth soft agar plating (10 +/− 2.24, P < 0.001). We next evaluated if estrogen withdrawal would enhance transformation, consistent with our clinical findings, by growing the cells in charcoal stripped serum, tamoxifen, or a combination of the two. We found for both MT lines a 2-fold increased colony formation rate when cells were grown in Tamoxifen (p = 0.002), a 6.2 fold increased colony formation in charcoal stripped media (P < 0.001), and a 7.9 fold increased colony formation with the combination (P < 0.001, ). Supporting that the impact of charcoal stripping on transformation was due to estrogen depletion, return of estrogen to the media resulted in decreased colonies for the MT cell lines (p = 0.018, ). These findings biologically confirm wide spread transformation in normal breast epithelium with acute estrogen withdrawal in breast cells with the KRAS-variant.
Figure 1. Transformation in MCF10AKRAS+/−(MT1 and MT2) epithelial breast cell lines. (A) Under EGF and estrogen withdrawal conditions, MT cells become transformed and develop colonies in an anchorage independent growth assay. Each sample represents 10 counted 10 × fields from 3 different experimental replicates, and is the average of the MT1 and MT2 lines. Experiments were repeated 3 times. Data is from Passage 2 into soft agar. Error bars represent SEM. TAM = tamoxifen, 10 ug/ml. *p = 0.002, **P < 0.001, ****P < 0.001 (B) MCF10A MT lines form colonies which are reduced when estrogen is returned to the media. Each sample represents 10 counted 10 × fields from 3 different experimental replicates, and is the average of the MT1 and MT2 lines. Experiments were repeated 3 times. Data is from Passage 3 into soft agar. Error bars represent SEM. TAM = tamoxifen, final concentration 1 uM. *P < 0.001, **p = 0.018
Multiple primary BC Risk in KRAS-variant BC patients
Based on our findings that estrogen withdrawal appears to increase wide spread breast cell transformation in KRAS-variant breast epithelial cells, we evaluated the association of the KRAS-variant with MPBC. We found that overall women with the KRAS-variant (GT or GG) did exhibit a 2.04-fold increase risk of having a second primary BC, compared to women without the variant (12.93% vs 6.78% with MPBC, P < 0.001). In addition, we found a genetic dose effect of the KRAS-variant, with women heterozygous (GT) for the variant exhibiting a 1.81-fold increased risk of having a second primary BC (11.64% with MPBC, p = 0.006), and women homozygous (GG) for the KRAS-variant having an 11.64-fold increase risk of having a second primary BC (45.39% with MPBC, P < 0.001) compared to non-variant BC patients ().
Table 4. Second breast cancer risk in KRAS-variant breast cancer patients. Women with the KRAS-variant are significantly more likely to be diagnosed with multiple primary breast cancer, including synchronous and metachronous second primary breast cancer. This is especially true for KRAS-variant homozygous (GG) patients
We next investigated if second BC for KRAS-variant patients primarily occurred at the same time as their first diagnosis (synchronous MPBC), or after their first diagnosis (metachronous MPBC). We found that women with the KRAS-variant had a 2.63-fold increased risk of being diagnosed with a synchronous second primary BC compared to non-variant BC patients (6.79% vs 2.70% with synchronous MPBC, p = 0.001). This was again most pronounced for women homozygous for the KRAS-variant, who had a 12.03-fold increased risk of having a synchronous second primary BC (25.02% with synchronous MPBC, p = 0.003) compared to non-variant patients. However, women with the KRAS-variant also continued to be at an elevated risk for a metachronous BC, with a 1.72-fold increased risk of developing a metachronous second primary tumor when compared to non-variant patients (8.05% vs 4.84% with metachronous MBPC, p = 0.05). This difference was again primarily explained by the large increased risk of metachronous BC for the homozygous KRAS-variant group, who had a 14.72-fold increased risk of developing a second primary BC after their first BC diagnosis (42.80% with metachronous MPBC, P < 0.001, , Fig. S3).
Multiple primary breast cancer risk and other risk factors
We next evaluated MPBC risk controlling for the extent of surgery and time of follow up. Controlling for extent of primary surgery, women with the KRAS-variant who had a lumpectomy or a unilateral mastectomy were significantly more likely to have a synchronous second primary tumor than non-variant patients (lumpectomy OR = 4.32, CI 1.15–16.40, p = 0.03; unilateral mastectomy OR = 18.42, CI 3.88–87.82, P < 0.001, Table S3A). In addition, controlling for number of years at risk, women with the KRAS-variant treated with a lumpectomy were significantly more likely to develop a second, metachronous primary BC (OR = 1.84, CI-1.03–3.27, p = 0.04) when compared to non-variant patients treated in the same manner (Table S3B). This was confirmed using a time to event analysis (p = 0.05). Of note, we found that KRAS-variant and non-variant patients did not significantly differ in their choice of lumpectomy, unilateral mastectomy or bilateral mastectomy at the time of diagnosis (Table S4A). As expected, women with either a unilateral or a bilateral mastectomy were more likely to have a diagnosis of a synchronous MPBC (Table S4B).
We next evaluated the association of lobular histology with the KRAS-variant and second BC risk. We found that the KRAS-variant was not associated with lobular histology, although in agreement with prior reports, in our cohort lobular histology alone was associated with increased rates of second primary BC, both synchronous and metachronous (Table S5A and B).
Finally, we controlled for lobular histology, extent of surgery and number of years at risk and evaluated the association of the KRAS-variant with MPBC. We found that women with the KRAS-variant treated with unilateral mastectomy were significantly more likely to have a synchronous second primary tumor regardless of lobular histology (OR = 40.75, CI = 4.98–339.72, P < 0.01). In addition, women with the KRAS-variant treated with lumpectomy with non-lobular histology continued to be significantly more likely to develop a second, metachronous primary BC (OR = 2.01, CI = 1.05–3.86, p = 0.04). Similar conclusions were found using a time to event analysis (HR = 2.011, p = 0.03)().
Table 5. Second breast cancer risk in KRAS-variant breast cancer patients controlling for lobular histology, extent of surgery and time. Women with the KRAS-variant continue to be at a significantly increased risk of synchronous and metachronous breast cancer when controlling for lobular histology, extent of surgery and time
To confirm that having the KRAS-variant was an independent predictor of MPBC, we performed a multivariate analysis using a logistic regression model, assuming that the predictors included in the model had a linear additive structure. We confirmed using this model that the KRAS-variant was an independent predictor of MPBC risk considering all other risk factors (OR = 2.26, CI 1.44–2.26, P < 0.001, Table S6).
Discussion
In this study we show for the first time that estrogen withdrawal increases breast cancer risk in women with the KRAS-variant, who are also significantly more likely to present with and develop multiple primary breast cancers. This finding was confirmed biologically in cell lines with the KRAS-variant compared to isogenic controls. BC risk appears to be increased by a low estrogen state in general, and abrupt estrogen withdrawal, as found with oophorectomy, discontinuation of HRT, or in our cell line assays, enhances transformation and appears to increase the risk of aggressive breast tumor biology. We find that women with the KRAS-variant are at greatest risk of presenting with multiple primary synchronous breast cancer, although also continue to be at risk of metachronous breast cancer development. These findings further highlight the unique paradigm of 3′UTR mutations, as well as give new insight into how this mutation could meaningfully subgroup patients to develop the best preventive approaches for breast cancer.
The role of estrogen withdrawal on BC risk for women with the KRAS-variant could be due to a relationship between the KRAS-variant, its downstream pathways and estrogen signaling, as there are known interaction between estrogen signaling and the RAS pathway. Alternatively, the relationship between estrogen and the KRAS-variant may instead be due to alterations in miRNA expression or regulation caused by this powerful hormone. In support of the later, we have previously shown that TNBC tumors from women with the KRAS-variant have significantly higher aromatase expression and ER Beta expression. Both of these genes are regulated by the miRNA let-7, which is known to be low in KRAS-variant associated tissues and tumors. One could speculate that sudden estrogen withdrawal disrupts these biological interactions in KRAS-variant tissues, ultimately leading to escape, independent signaling and growth, and oncogenesis. Extensive cell line work is ongoing to define the relationship with estrogen and the series of mechanistic events leading to cancer in individuals with the KRAS-variant. Regardless, our cell line findings confirm that breast cells with the KRAS-variant are transformed by estrogen withdrawal. In addition, our clinical findings that BC patients with the KRAS-variant are more likely to have an oophorectomy than non-variant patients, have a lower BMI, and thus lower circulating estrogen than controls, and that HRT discontinuation leads to aggressive tumor biology, supports the hypothesis that acute estrogen withdrawal alters breast cell biology for KRAS-variant individuals.
A genetic marker of increased risk of synchronous MPBC has not been previously identified. Other BC associated genetic mutations are generally considered to predict an increased risk of second, metachronous BC, likely due to the continued DNA damage-prone state of the tissues in these individuals. For women with the KRAS-variant, our findings here suggest instead a scenario where an “event” promotes cancer initiation, globally impacting their breast tissue. Based on our results, we hypothesize that the event could be some form of acute estrogen withdrawal, a hypothesis requiring further confirmation. As treatment for BC general involves acute estrogen withdrawal, through chemotherapy and/or anti-estrogen therapy, it seems possible that the continued risk of metachronous breast cancer in KRAS-variant patients may be partly a result of treatment for their first BC. Studies are currently on-going for women with the KRAS-variant to both better understand the first potential “causative event,” as well as to define the most efficacious, and safest, treatment strategies to avoid metachronous breast cancer.
Limitations of our clinical studies include self-reported lifestyle factors for our BC patients, which are prone to recall bias. However, our most critical findings, regarding tumor biology post-HRT, and second BC risk, were all confirmed with pathologic documentation. Another limitation of our study is that our population was not prospectively collected, allowing survivor bias for metachronous BC development. However, our cohorts in this study have identical length of follow up, and we controlled for time in our metachronous BC analysis. Also, as women with the KRAS-variant are significantly more likely to be diagnosed with premenopausal TNBC, which is the most deadly form of breast cancer, if anything, this bias should have decreased our ability to identify an association between metachronous BC and the KRAS-variant.
Perhaps most importantly, the findings from this study further highlight the critical importance of studying biologically functional 3′UTR miRNA binding site mutations in the appropriate cohorts. Unlike previously discovered mutations that impact DNA repair, 3′UTR mutations instead alter the appropriate cellular response to external factors. Since both lifestyle and environmental exposures will differ across populations, and represent external factors, increasing subject numbers as is standard by large consortia by combining patients of numerous ethnic backgrounds and cultures should be avoided in the study of 3′UTR mutations. Since such consortia have begun to study 3′UTR mutations, it should be recognized that their findings, or lack of findings, will be biased against finding the mutations that are perhaps the most important – those that could be managed by lifestyle modifications. Utilizing the correct cohorts to define the factors that can modify cancer risk in biologically functional 3′UTR mutations should be an extremely high priority in cancer prevention studies at this time.
Although the best estrogen management strategies for women with the KRAS-variant are yet to be defined, our findings do suggest that sudden estrogen withdrawal, such as that caused by oophorectomy or abrupt discontinuation of HRT, may increase breast cancer risk for these women. It also appears that women with the KRAS-variant are significantly at increased risk of MPBC, and at the time of their first BC diagnosis should be carefully evaluated for other synchronous primaries. While those at highest risk are women homozygous for the KRAS-variant, a relatively rare genotype (∼3% of the healthy population), it is important to note that the prevalence of homozygote KRAS-variant patients is still >10 fold higher than BRCA mutant individuals in the healthy population (∼0.25%). While the best way to integrate these findings into current BC management is an active area of discussion between both physicians and BC patients, this marker is a potentially vital additional tool to help guide both estrogen tailoring and BC management for women with the KRAS-variant, who comprise one in 5 newly diagnosed BC patients.
Disclosure of Potential Conflicts of Interest
FJS and JBW declare that they have financial interests in Mira Dx, a company that has licensed the KRAS-variant from Yale University.
1041694_supplemental_files.zip
Download Zip (2.2 MB)Acknowledgments
We thank Catherine Curran and Colm McVeigh for assistance with data input. We thank Don Lannin for input on our MPBC findings.
Funding
TPM was supported by the National Breast Cancer Research Institute. JBW, FJS and SYJ were supported by NIH CA157749.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
References
- Cipollini M, Landi S, Gemignani F. MicroRNA binding site polymorphisms as biomarkers in cancer management and research. Pharmacogenomics Pers Med 2014; 7:173-191; PMID:25114582
- Chin LJ, Ratner E, Leng S, Zhai R, Nallur S, Babar I, Muller RU, Straka E, Su L, Burki EA, et al. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res 2008; 68:8535-8540; PMID:18922928; http://dx.doi.org/10.1158/0008-5472.CAN-08-2129
- Paranjape T, Heneghan H, Lindner R, Keane FK, Hoffman A, Hollestelle A, Dorairaj J, Geyda K, Pelletier C, Nallur S, et al. A 3′-untranslated region KRAS variant and triple-negative breast cancer: a case-control and genetic analysis. Lancet Oncol 2011; 12:377-386; PMID:21435948; http://dx.doi.org/10.1016/S1470-2045(11)70044-4
- Ratner E, Lu L, Boeke M, Barnett R, Nallur S, Chin LJ, Pelletier C, Blitzblau R, Tassi R, Paranjape T, et al. A KRAS-variant in ovarian cancer acts as a genetic marker of cancer risk. Cancer Res 2010; 15:6509-6515; PMID: 20647319; http://dx.doi.org/10.1158/0008-5472.CAN-10-0689
- Ratner E, Keane FK, Lindner R, Tassi RA, Paranjape T, Glasgow M, Nallur S, Deng Y, Lu L, Steele L, et al. A KRAS variant is a biomarker of poor outcome, platinum chemotherapy resistance and a potential target for therapy in ovarian cancer. Oncogene 2012; 31:4559-4566; PMID:22139083; http://dx.doi.org/10.1038/onc.2011.539
- Pilarski R, Patel DA, Weitzel J, McVeigh T, Dorairaj JJ, Heneghan HM, Miller N, Weidhaas JB, Kerin MJ, McKenna M, et al. A KRAS-variant is associated with risk of developing double primary breast and ovarian cancer. PLos One 2012; 7:e37891; PMID:22662244; http://dx.doi.org/10.1371/journal.pone.0037891
- Chung CH, Lee JW, Slebos RJ, Howard JD, Perez J, Kang H, Fertig EJ, Considine M, Gilbert J, Murphy BA, et al. A 3′UTR KRAS variant is associated with cisplatin resistance in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2014; 25:2230-6.
- Saridaki Z, Weidhaas JB, Lenz H-J, Laurent-Puig P, Jacobs B, De Schutter J, De Roock W, Salzman DW, Zhang W, Yang D, et al. A let-7 microRNA-binding site polymophism is KRAS predicts improved outcome in metastatic colorectal cancer (mCRC) patients treated with salvage cetuximab/panitumumab monotherapy. Clin Cancer Res 2014; 20:4499-4510; PMID:25183481; http://dx.doi.org/10.1158/1078-0432.CCR-14-0348
- Weidhaas J, Kim ES, Herbst RS, Yu J, Slack F, Blumenschein GR, Tsao AS, Wistuba II, Lee JJ, Papadimitrakopoulou V, et al. The KRAS-variant and treatment response in BATTLE-1. J Clin Oncol 2014; 32(9S); abstr 8135
- Levi F, Randimbison L, Rafael B-M, Manuela M-C, Vecchia CL. Second primary cancers in the Vaud and Neuchatel Cancer Registries. Eur J Cancer Prev 2015; 24:150-4; PMID:25397586
- Bhatia S. Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer 2014; 121:648-63; PMID:25355167; http://dx.doi.org/10.1002/cncr.29096
- Howe HL, Weinstein R, Alvi R, Kohler B, Ellison JH. Women with multiple primary breast cancers diagnosed within a five year period, 1994–1998. Breast Cancer Res Treat 2005; 90:223-232; PMID:15830135; http://dx.doi.org/10.1007/s10549-004-4258-4
- Raymond JS, Hogue CJR. Multiple primary tumours in women following breast cancer, 1973–2000. Br J Cancer 2006; 94:1745-1750; PMID:16721370
- Marcu LG, Santos A, Bezak E. Risk of second primary cancer after breast cancer treatment. Eur J Cancer Care 2014; 23:51-64; PMID:23947545; http://dx.doi.org/10.1111/ecc.12109
- Kurian AW, McClure LA, John EM, Horn-Ross PL, Ford JM, Clarke CA. Second primary breast cancer occurrence according to hormone receptor status. J Natl Cancer Inst 2009; 101:1058-1065; PMID:19590058; http://dx.doi.org/10.1093/jnci/djp181
- Chen Y, Thompson W, Semenciw R, Mao Y. Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev 1999; 8:855-861; PMID:10548312
- Narod SA. Bilateral breast cancers. Nat Rev Clin Oncol 2014; 11:157-166; PMID:24492834; http://dx.doi.org/10.1038/nrclinonc.2014.3
- Brooks JD, John EM, Mellemkjær L, Reiner AS, Malone KE, Lynch CF, Figueiredo JC, Haile RW, Shore RE; WECARE Study Collaborative Group, et al. Body mass index and risk of second primary breast cancer: The WECARE Study. Breast Cancer Res Treat 2012; 131:571-580; PMID:21892703; http://dx.doi.org/10.1007/s10549-011-1743-4
- Reiner AS, John EM, Brooks JD, Lynch CF, Bernstein L, Mellemkjær L, Malone KE, Knight JA, Capanu M, Teraoka SN, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the women's environmental cancer and radiation epidemiology study. J Clin Oncol 2013; 31:433-439; PMID:23269995; http://dx.doi.org/10.1200/JCO.2012.43.2013
- Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, Xue S, Teraoka S, Bernstein L, Capanu M, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol 2010; 28:2404-2410; PMID:20368571; http://dx.doi.org/10.1200/JCO.2009.24.2495
- Broeks A, de Witte L, Nooijen A, Huseinovic A, Klijn JG, van Leeuwen FE, Russell NS, van't Veer LJ. Excess risk for contralateral breast cancer in CHEK2*1100delC germline mutation carriers. Breast Cancer Res Treat 2004; 83:91-93; PMID:14997059; http://dx.doi.org/10.1023/B:BREA.0000010697.49896.03
- Clarke M, Collins R, Darby S, Davies C, Evans V, Godwin J, Gray R, McGale P, Peto R, Wang Y, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687-1717; PMID:15894097; http://dx.doi.org/10.1016/S0140-6736(05)71056-4
- Alkner S, Bendahl PO, Ferno M, Nordenskjold B, Ryden L. Tamoxifen reduces the risk of contralateral breast cancer in premenopausal women: Results from a controlled randomised trial. Eur J Cancer 2009; 45:2496-2502; PMID:19535242; http://dx.doi.org/10.1016/j.ejca.2009.05.022
- Lostumbo L, Carbine N, Wallace J, Ezzo J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev 2004; 4:CD002748; PMID:15495033
- Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: A review of the literature. Breast Cancer Res Treat 2014; 144:1-10; PMID:24477977; http://dx.doi.org/10.1007/s10549-014-2852-7
- Liu S, Lin YC. Transofrmation of MCF-10A human breast epithelial cells by zeronal and estrodial-17β. Breast J 2004; 10:514-521; PMID:15569208; http://dx.doi.org/10.1111/j.1075-122X.2004.21410.x
- Wang J, Gildea JJ, Yue W. Aromatase overexpression induces malignant changes in estrogen receptor α negative MDF-10A cells. Oncogene 2013; 32:5233-5240; PMID:23178495; http://dx.doi.org/10.1038/onc.2012.558
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003; 362:419-427; PMID:12927427; http://dx.doi.org/10.1016/S0140-6736(03)14596-5
- Chlebowski RT, Manson JE, Anderson GL. Estrogen plus progestin and breast cancer indicence and mortality in the women's health initiative observational study. JNCI 2013; 105:526-535; PMID:23543779; http://dx.doi.org/10.1093/jnci/djt043
- Calle EE, Feigelson HS, Hildebrand JS, Teras LR, Thun MJ, Rodriguez C. Postmenopausal hormone use and breast cancer associations differ by hormone regimen and histologic subtype. Cancer 2009; 115:936-945; PMID:19156895; http://dx.doi.org/10.1002/cncr.24101
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004; 291:1701-1712; PMID:15082697; http://dx.doi.org/10.1001/jama.291.14.1701
- Lee L, Ratner E, Uduman M, Winter K, Boeke M, Greven KM, King S, Burke TW, Underhill K, Kim H, et al. The KRAS-variant and miRNA expression in RTOG Endometrial Cancer Clinical Trials 9708 and 9905. PLos One 2014; 9(4):e94167; PMID:24732316; http://dx.doi.org/10.1371/journal.pone.0094167
- Cerne J, Stegel V, Gersak K, Novakovic S. KRAS rs61764370 is associated with HER2-overexpressed and poorly-differentiated breast cancer in hormone replacement therapy users: a case control study. BMC Cancer 2012; 12:105-111; PMID:22436609; http://dx.doi.org/10.1186/1471-2407-12-105
- Salzman DW, Weidhaas JB. miRNAs in the spotlight: Making ‘silent’ mutations speak up. Nat Med 2011; 17:934-935; PMID:21818091; http://dx.doi.org/10.1038/nm0811-934
- Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 2005; 435:646-651; PMID:15806097; http://dx.doi.org/10.1038/nature03556
- Soule H, Maloney TM, Wolman SR, Peterson WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 1990; 50:6075-6086; PMID:1975513
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30:256-268; PMID:12798140; http://dx.doi.org/10.1016/S1046-2023(03)00032-X
- Sweasy J, Lang T, Starcevic D, Sun K, Lai C, Dimaio D, Dalal S. Expression of DNA polymerase {beta} cancer-associated variants in mouse cells results in cellular transformation. PNAS 2005; 102:14350-14355; PMID:16179390; http://dx.doi.org/10.1073/pnas.0505166102
- Grechukhina O, Petracco R, Popkhadze S, Massasa E, Paranjape T, Chan E, Flores I, Weidhaas JB, Taylor HS. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med 2012; 4:206-217; PMID:22307873; http://dx.doi.org/10.1002/emmm.201100200
