The spindle checkpoint ensures accurate chromosome segregation and prevents aneuploidy. Unattached kinetochores during mitosis activate the checkpoint, which inhibits the anaphase-promoting complex or cyclosome (APC/C) and delays chromosome segregation.Citation1 The critical checkpoint protein Mad2 has multiple conformers, including the inactive open Mad2 (O-Mad2) and the active closed Mad2 (C-Mad2). At unattached kinetochores, the Mad1–C-Mad2 complex recruits cytosolic O-Mad2 and converts it to intermediate Mad2 (I-Mad2) (). I-Mad2 binds Cdc20 (the mitotic activator of APC/C) to form the Cdc20–C-Mad2 complex. Cdc20–C-Mad2 then associates with BubR1–Bub3 to form the mitotic checkpoint complex (MCC), which is an effective inhibitor of APC/C.
Figure 1. Activating p31comet through phosphorylation in Xenopus egg extracts. During checkpoint activation, Mad1–C-Mad2 at unattached kinetochores recruit O-Mad2 and converts it to I-Mad2, which binds Cdc20. Cdc20–C-Mad2 associates with BubR1–Bub3 to form MCC, which inhibits APC/C. During checkpoint inactivation, IKKβ phosphorylates p31comet and promotes its binding to C-Mad2, thereby blocking Mad2 activation and triggering MCC disassembly.
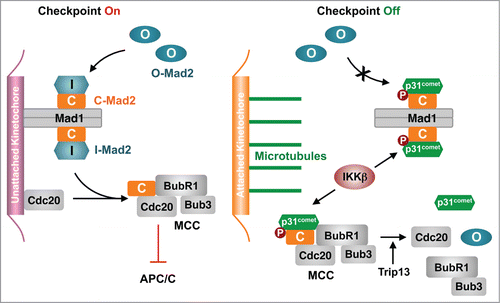
The Mad2-binding protein p31comet stimulates spindle checkpoint silencing and controls timely chromosome segregation.Citation1 It specifically binds to C-Mad2, but not to O-Mad2, and inhibits C-Mad2 in 2 ways.Citation2 First, p31comet binds Mad1–C-Mad2 and prevents conformational activation of O-Mad2. Second, p31comet weakens MCC by disrupting the BubR1–C-Mad2 interaction, and collaborates with the ATPase Trip13 to disassemble the Cdc20–C-Mad2 complex, leading to MCC disassembly and APC/C activation. Despite the functional importance of p31comet, how it is regulated during mitosis is poorly understood. The study of Mo et al. in this issue provides new insights into this important question.Citation3
Proteomic analysis revealed that p31comet is a phospho-protein during mitosis in human cells.Citation4 An earlier report showed that S102 phosphorylation of human p31comet inhibits its function and weakens its interaction with Mad2.Citation5 Using Xenopus egg extracts as the model system, Mo et al. have now demonstrated that the kinase IKKβ phosphorylates and activates p31comet during mitotic exit.Citation3
The Xenopus egg extract is a powerful cell-free system that recapitulates many mitotic events. Mature Xenopus eggs are arrested at metaphase by the cytostatic factor (CSF) Emi2/xErp1, an inhibitor of APC/CCdc20. The mitotic CSF extracts can turn added sperm nuclei into discrete condensed chromosomes, which nucleate the formation of bipolar spindles. Addition of Ca2+ triggers Emi2 degradation and mitotic exit. Microtubule depolymerization by nocodazole followed by Ca2+ addition to these extracts creates a mitotic arrest dependent on the spindle checkpoint.
Mo et al. first showed that p31comet promoted Ca2+-triggered mitotic exit in CSF extracts supplemented with sperm nuclei, as evidenced by delayed cyclin B degradation in p31comet-depleted extracts.Citation3 Co-depletion of Mad2 rescued the mitotic delay caused by p31comet depletion, indicating that p31comet promotes checkpoint inactivation through antagonizing Mad2 in these extracts, rather than through Emi2-dependent mechanisms. This finding further suggests that, in the absence of nocodazole, the spindle checkpoint is engaged in these reconstituted extracts permissible for spindle formation.
Mo et al. then showed that the level of p31comet phosphorylation was high during mitosis and decreased after mitotic exit.Citation3 They identified IKKβ as a kinase of p31comet. Depletion or chemical inhibition of IKKβ delayed mitotic exit. IKKβ phosphorylated S4, T6, and T179 of Xenopus p31comet. Recombinant purified phospho-mimicking p31comet EEE mutant bound more tightly to Mad2 in vitro. The phosphorylation-deficient p31comet AAA mutant was less efficient in promoting mitotic exit whereas p31comet EEE was more effective. Strikingly, p31comet EEE greatly reduced the amount of Mad2 bound to chromosomes, suggesting that p31comet phosphorylation was particularly important for blocking Mad2 activation at kinetochores. Thus, phosphorylation-enhanced binding of p31comet to Mad2 blocks Mad2 activation at kinetochores and promotes MCC disassembly in the cytosol, leading to APC/C activation and mitotic exit ().
S4 and T6 are located in the flexible N-terminal region of p31comet dispensable for Mad2 binding. It is not apparent how their phosphorylation might enhance p31comet binding to Mad2. On the other hand, our structural modeling reveals that T179 of p31comet is located in a loop close to the Mad2–p31comet interface. Intriguingly, 2 conserved arginines of Mad2, R129 and R133, lie in the vicinity of T179, and could conceivably engage in favorable electrostatic interactions with phospho-T179. T179 phosphorylation of Xenopus p31comet might underlie the increased affinity toward Mad2.
Unfortunately, T179 is not conserved in other vertebrate p31comet proteins.Citation3 Moreover, there are conflicting reports on the mitotic role of IKKβ in human cells.Citation6,7 Additional studies are needed to test whether IKKβ-mediated phosphorylation regulates p31comet in other species and during the somatic cell cycle. Regardless, the Mo et al. study provides the first evidence that phosphorylation can boost the function of p31comet during mitotic exit. It also raises the exciting possibility that upstream signals in the canonical, pro-survival NF-κB pathway might have non-canonical roles in regulating the spindle checkpoint and mitotic progression.
References
- Jia L, et al. Trends Biochem Sci 2013; 38:302-11; PMID:23598156; http://dx.doi.org/10.1016/j.tibs.2013.03.004
- Yang M, et al. Cell 2007; 131:744-55; PMID:18022368; http://dx.doi.org/10.1016/j.cell.2007.08.048
- Mo M, et al. Cell Cycle 2015: in press
- Hegemann B, et al. Sci Signal 2011; 4:rs12; PMID:22067460; http://dx.doi.org/10.1126/scisignal.2001993
- Date DA, et al. J Biol Chem 2014; 289:11367-73; PMID:24596092; http://dx.doi.org/10.1074/jbc.M113.520841
- Irelan JT, et al. Proc Natl Acad Sci U S A 2007; 104:16940-5; PMID:17939994; http://dx.doi.org/10.1073/pnas.0706493104
- Blazkova H, et al. Cell Cycle 2007; 6:2531-40; PMID:17704647; http://dx.doi.org/10.4161/cc.6.20.4807
