ABSTRACT
Foot-and-mouth disease is a highly contagious viral disease of cloven-hoofed animals that is caused by foot-and-mouth disease virus (FMDV). To replicate efficiently in vivo, FMDV has evolved methods to circumvent host antiviral defense mechanisms, including those induced by interferons (IFNs). Previous research has focused on the effect of FMDV Lpro and 3Cpro on type I IFNs. In this study, FMDV VP3 was found to inhibit type II IFN signaling pathways. The overexpression of FMDV VP3 inhibited the IFN-γ-triggered phosphorylation of STAT1 at Tyr701 and the subsequent expression of downstream genes. Mechanistically, FMDV VP3 interacted with JAK1/2 and inhibited the tyrosine phosphorylation, dimerization and nuclear accumulation of STAT1. FMDV VP3 also disrupted the assembly of the JAK1 complex and degraded JAK1 but not JAK2 via a lysosomal pathway. Taken together, the results reveal a novel mechanism used by which FMDV VP3 counteracts the type II IFN signaling pathways.
Introduction
Foot-and-mouth disease virus (FMDV) is a single-stranded positive-sense RNA virus, and the etiological agent of foot-and-mouth disease (FMD). FMD is a highly contagious viral disease that affects domestic and wild cloven-hoofed animals. Outbreaks of FMD are economically devastating.Citation1 There are seven immunologically distinct serotypes of FMDV: O, A, C, Asia 1, and South African Territories (SAT) type 1 (SAT1), SAT2, and SAT3. The FMDV genome contains approximately 8,200 nt that are translated into a polyprotein. The polyprotein is post-translationally processed by virus-encoded proteases into four structural proteins (VP1, VP2, VP3, and VP4) and eight non-structural proteins (Lpro, 2A, 2B, 2C, 3A, 3B, 3Cpro, and 3D).Citation2 FMDV induces a variety of host innate immune responses, which likely contributes to viral pathogenesis and/or influence the final outcome of viral infection in vitro and in vivo.Citation3 The innate immune response of the host is the first line of defense against viral infection until specific protection is established by the adaptive immune system. However, FMDV has evolved to counteract the host innate immune response.Citation4-6
Interferons (IFNs) are recognized as an essential component of the innate immune response.Citation7 IFNs, including IFN-α (IFN-α), IFN-β (IFN-β) and IFN -gamma (IFN-γ), are known to be important for triggering a robust host response to viral infection.Citation8,9 The antiviral functions of IFNs are initiated through the binding of these proteins to their cognate receptors.Citation7,10-12 The binding of IFN-γ to IFN-γ receptor 1 (IFNGR1) and IFNGR2 leads to the activation of Janus kinase 1 (JAK1) and JAK2 respectively. This binding occurs after receptor dimerization and tyrosine phosphorylation, which in turn leads to the phosphorylation of STAT1.Citation13 STAT1 homodimers then form, migrate to the nucleus, and bind to a DNA element, thereby specifically inducing transcription of IFN-γ target genes, such as IFN regulatory factor 1 (IRF1).Citation14
FMDV or some FMDV proteins has been shown to antagonize the host innate response in mammalian cells by preventing the translationalCitation15 or transcriptionalCitation16,17 regulation of host proteins. The viral proteases Lpro and 3Cpro have been found to antagonize the host innate immune response.Citation11-13 The FMDV leader proteinase (Lpro) plays a role in preventing IFN-α/β protein synthesis by reducing the levels of IFN-β mRNA and IFN-stimulated gene products.Citation15 In addition, Lpro prevents double-stranded RNA-induced type I IFN expression by decreasing interferon regulatory factor 3/7 (IRF-3/7) protein levelsCitation16,17 and affects NF-κB activity by mediating the degradation of p65/RelA.Citation18 Furthermore, Lpro is able to block IFN-β induction by cleaving ubiquitin moieties from critical signaling proteins in the type I IFN signaling pathway and/or by abrogating the deubiquitinating activity of enzymes.Citation19,20 The 3Cpro protease has been shown to cleave the NF-κB essential modulator NEMO and impair its ability to activate downstream type I IFNs. It was recently reported that 3Cpro antagonizes the type I IFN signaling pathway by blocking STAT1/STAT2 nuclear translocation.Citation21
FMDV VP1-induced suppression of type I IFNs is mediated by interactions with sorcin that appear to regulate the cellular response to viral infections.Citation22 However, whether other FMDV proteins are involved in inhibiting type II IFN signaling remains unclear, and the associated mechanisms are unknown. This paper provides the first evidence that FMDV VP3 inhibits the IFN-γ-JAK-STAT1 signaling pathway, which highlights a novel mechanism by which FMDV antagonizes host immune responses and provides a theoretical basis for improving vaccine efficacy.
Results
FMDV VP3 inhibits IFN-γ-triggered signaling
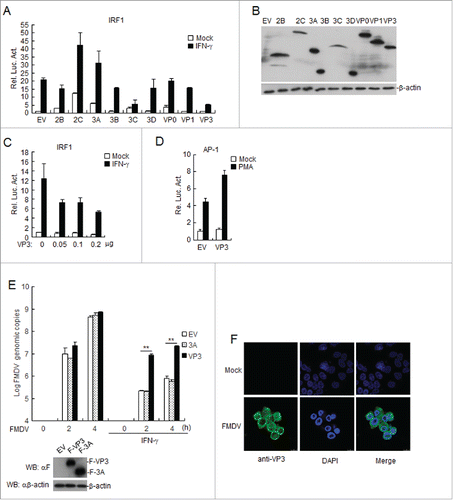
To investigate whether FMDV participates in the IFN-γ-triggered signaling pathway, we screened for FMDV proteins that can regulate IFN-γ-triggered IRF1 promoter activation by using reporter assays. As shown in , O-type FMDV VP3 (VP3) was identified as an inhibitor of IFN-γ-triggered IRF1 activation, but FMDV 2C and 3A increased IFN-γ-triggered IRF1 activation. shows the expression of the FMDV proteins, as indicated by western blotting. Further experiments indicated that the overexpression of VP3 inhibited the IFN-γ-triggered activation of the IRF1 promoter in a dose-dependent manner (). In contrast, VP3 did not inhibit phorbol-12-myristate-13-acetate-triggered activation of the activator protein 1 (AP-1) promoter (). To further validate the effects of VP3 overexpression on FMDV replication via the IFN-γ signaling pathway, stable BHK21 cell lines expressing FMDV 3A or VP3 were treated with IFN-γor left untreated. The results showed that VP3 but not 3A increased the FMDV genome copy number in cells treated with IFN-γ (). To examine the localization of VP3 in FMDV-infected cells, BHK21 cells were infected with O-type FMDV or left uninfected for 8 h. Confocal images of cells immunostained with anti-VP3 antibodies showed that VP3 localizes to the cytosol and nucleus in FMDV-infected cells (). These data suggest that VP3 specifically inhibits IFN-γ-triggered IRF1 activation.
Figure 1. FMDV VP3 negatively regulates the IFN-γ-triggered signaling pathway. (A-B) Effects of the overexpression of FMDV proteins on IFN-γ -triggered IRF1 promoter activation. HEK293T cells (5×104) were transfected with the IRF1 reporter (0.1 μg), pRL-TK (as an internal control; 10 ng), and the indicated expression plasmids (0.1 μg). Twenty hours after transfection, the cells were treated with IFN-γ (50 ng/ml) or left uninfected for 12 h before luciferase assays were performed. The expression of FMDV proteins was analyzed by western blotting in B. (C) Dose-dependent effects of FMDV VP3 on the IFN-γ-triggered activation of the IRF1 promoter. The experiments were performed as described in A. (D) Effects of overexpression of FMDV VP3 on PMA-triggered AP-1 promoter activation. HEK293T cells (5×104) were transfected with the AP-1 reporter (0.1 μg), 10 ng of pRL-TK (as an internal control) and the indicated expression (0.1 μg) plasmids. Twenty hours after transfection, the cells were treated with phorbol 12-myristate 13-acetate (50 ng/ml) or left untreated for 12 h before luciferase assays were performed. (E) Increased copies of the FMDV genome in VP3-transfected BHK-21 cells. BHK-21 cells stably expressing VP3 or 3A were treated with IFN-γ (100 ng/ml) or left untreated for 1 h and infected with FMDV at the indicated times. FMDV genome copies were assessed using a real time quantitative RT-PCR assay. The values are expressed as the mean ± SD of three independent experiments. Protein expression was analyzed by protein gel blotting before treatment with IFN-γ . (F) VP3 localizes to the cytoplasm and nucleus in FMDV-infected BHK21 cells. BHK21 cells were infected with O-type FMDV (MOI=0.1) or left uninfected. The cells were fixed at 8 h post-infection (hpi) and subjected to an indirect immunofluorescence assay to detect the VP3 protein (green). The position of the nucleus is indicated by DAPI (blue) staining in the merged image. Rel. Luc. Act.: relative luciferase activity, EV: empty vector.
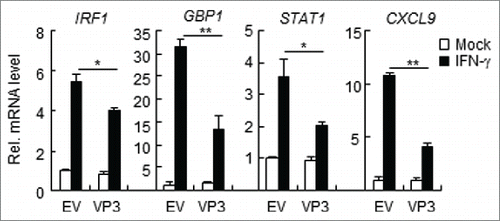
VP3 interferes with the IFN-γ-triggered induction of downstream genes
IFN-γ regulates the immune response to viruses, intracellular pathogens, and tumors by modulating cell proliferation, migration, invasion, and vesicle trafficking processes.Citation23 The large GTPase guanylate binding protein 1 (GBP-1) is a cellular protein that is induced by IFN-γ.Citation24,25 IFN-γ is crucial for host defense against intracellular pathogens and can induce the binding of STAT1 to the IRF1 promoter in host cells.Citation26 Large quantities of chemokine mRNAs, such as CXCL9 and CXCL-10, require IFN-γ for their expression.Citation17 To determine whether FMDV VP3 interferes with the downstream gene transcription induced by IFN-γ, HEK293T cells were transfected with pCAGGS-Flag-VP3 and then stimulated with IFN-γ. The mRNA levels of IRF1, GBP-1, STAT1, and CXCL9 were assessed via RT-PCR. The results demonstrated that VP3 inhibits the mRNA synthesis of IRF1, GBP-1, STAT1, and CXCL9 ().
Figure 2. FMDV VP3 inhibits the IFN-γ-triggered induction of downstream genes. HEK293T cells were transfected with a plasmid encoding VP3 or an empty vector (EV) (0.5 μg) for 24 h. The cells were then treated with IFN-γ (100 ng/ml) for 3 h, and the IRF1, GBP1, STAT1, CXCL9, and GAPDH genes were evaluated using a relative quantitative RT-PCR assay. The values are presented as the mean ± SD of three independent experiments.
FMDV VP3 inhibits the IFN-γ-induced phosphorylation, dimerization and nuclear accumulation of STAT1
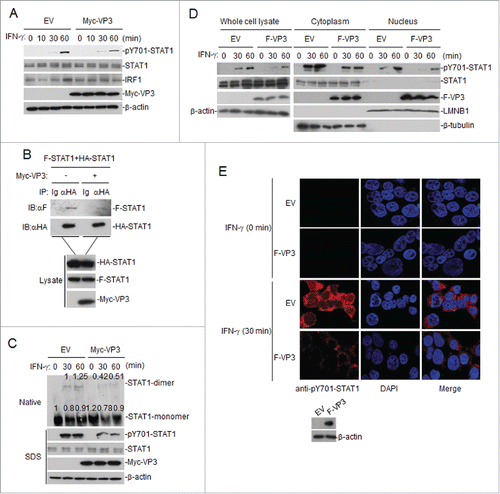
The binding of IFN-γ to its cognate receptors, IFN-γRα and IFN-γRβ, leads to the activation of JAKs. These JAKs then phosphorylate IFN-γRα and a specific group of STAT1 proteins.Citation27,28 These phosphorylated STATs undergo dimerization and enter the nucleus. The phosphorylation-dependent activation of STATs is critical for IFN-inducible antiviral responses.Citation29 We investigated whether VP3 interferes with the phosphorylation of STAT1. VP3-expressing cells were subjected to protein gel blotting using an antibody specific for phosphorylated STAT1 (STAT1-Y701). The results showed that VP3 inhibited the phosphorylation of STAT1 after IFN-γ stimulation (). In co-immunoprecipitation experiments, the overexpression of VP3 was found to inhibit the dimerization of STAT1 (). Furthermore, the overexpression of VP3 could also inhibit IFN-γ-triggered endogenous STAT1 dimerization (). To investigate the potential mechanism of IFN-γ signaling pathway inhibition by VP3, we used western blotting to examine the p-STAT1 expression levels in cytoplasmic and nuclear extracts from a HEK293T cell line stably expressing VP3. The results showed that the p-STAT1 protein was reduced in the cytoplasm and nucleus, while the amount of STAT1 was not altered (). As we expected, the nuclear p-STAT1 protein was reduced in IFN-γ-treated HEK293T cells stably expressing the VP3 protein, as indicated by immunofluorescence (). These data demonstrate that VP3 inhibits IFN-γ-triggered STAT1 phosphorylation, dimerization and nuclear accumulation.
Figure 3. FMDV VP3 inhibits the phosphorylation, dimerization and nuclear accumulation of STAT1 induced by IFN-γ. (A) Western blotting was used to assess the effects of VP3 on the phosphorylation of STAT1 after IFN-γ (100 ng/ml) stimulation. HEK293T cells were transfected with pCAGGS-Myc-VP3 for 24 h and were left untreated or treated with IFN-γ at the indicated time points. Western blotting was used to analyze pY701-STAT1, STAT1, IRF1 or Myc-VP3 expression in the cell lysates. (B) Western blotting was also used to examine the effects of VP3 on the dimerization of STAT1. HEK293T cells were transfected with 4 μg of the Myc-VP3 plasmid (+) or an empty vector (−), 5 μg of HA-STAT1 and 5 μg of Flag-STAT1. Co-immunoprecipitation was performed with anti-HA (F) or control IgG (Ig) antibodies. Immunoblotting analysis was performed with anti-Flag antibody (αF) (upper panels). The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with antibodies specific for HA, Flag and Myc (lower panels). (C) The effects of VP3 on the dimerization of STAT1 after IFN-γ stimulation. HEK293T cells were transfected with pCAGGS-Myc-VP3 (4 μg) for 24 h and were left untreated or treated with IFN-γ (100 ng/ml) at the indicated time points. Western blotting was used to analyze pY701-STAT1, STAT1 and Myc-VP3 expression in the cell lysates via SDS-PAGE, and STAT1 monomer and dimer expression in the cell lysates was assessed via Native-PAGE. Densitometry analysis of the original western blots was performed using the Image J software. (D) FMDV VP3 blocks the nuclear accumulation of phosphorylated STAT1. HEK293T cells stably expressing VP3 were treated with IFN-γ (100 ng/ml) at the indicated time points. The cytoplasmic and nuclear proteins were extracted using the CelLytic nuclear extraction kit (catalog no. Nxtract; Sigma-Aldrich). Western blotting was used to analyze pY701-STAT1, STAT1 and F-VP3 expression in the cytoplasm, nucleus and whole-cell lysate. (E) FMDV VP3 blocks the nuclear accumulation of phosphorylated STAT1, as indicated by confocal immunofluorescence microscopy. HEK293T cells stably expressing VP3 were treated with IFN-γ (100 ng/ml) at the indicated time points. The cells were fixed and subjected to an indirect immunofluorescence assay to detect the phosphorylated STAT1 protein (red). The cell nucleus was counterstained with DAPI. The nuclear accumulation of phosphorylated STAT1 was observed using a Leica SP2 confocal system (Leica Microsystems). Protein expression was analyzed by protein gel blotting before treatment with IFN-γ. EV, empty vector.
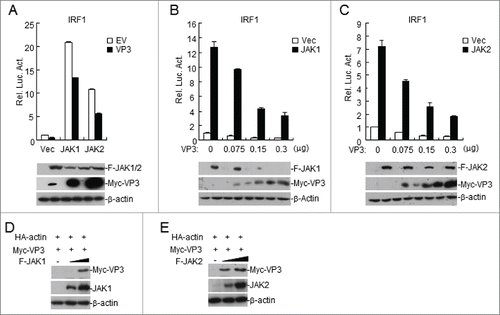
FMDV VP3 targets at JAKs level to regulate IFN-γ-triggered signaling
The JAK/STAT cascade is an essential signaling pathway in IFN-γ-induced immune responses.Citation30 Because JAK1 and JAK2 are two critical tyrosine kinases that directly phosphorylate STAT1 at Tyr701 and the overexpression of VP3 inhibited the IFN-γ-induced Tyr701 phosphorylation of STAT1, we next determined whether VP3 mediates IFN-γ signaling through JAKs. The effects of VP3 overexpression on JAK-mediated IRF1 promoter activation were examined using reporter assays. As shown in , overexpression of VP3 inhibited JAK1- or JAK2-mediated activation of the IRF1 promoter. Consistent with these findings, VP3 inhibited the JAK1- or JAK2-mediated activation of the IRF1 promoter in a dose-dependent manner (). It is very surprising that the amount of the VP3 protein differed significantly between empty vector-transfected cells and JAK1- or JAK2-transfected cells (). To examine the potential effects of JAK1 or JAK2 on VP3 expression, HEK293T cells were co-transfected with VP3 and JAK1 or JAK2. The results demonstrated that VP3 levels increased with increasing JAK1 or JAK2 expression (). These data demonstrated that FMDV VP3 targets JAKs to regulate IFN-γ-triggered signaling.
Figure 4. FMDV VP3 targets JAKs to regulate IFN-γ-triggered signaling. (A) Luciferase assays were used to assess how FMDV VP3 affects IRF1 activation through various signaling components. HEK293T cells were transfected with 100 ng of the IRF1 reporter, VP3 (100 ng), 10 ng of pRL-TK (as an internal control), and the indicated proteins (100 ng). Luciferase assays were performed 24 h after transfection. Western blotting was used to analyze JAK1, JAK2, and VP3 expression levels. (B-C) Dose-dependent effects of VP3 on JAK1- and JAK2-triggered activation of the IRF1 promoter. HEK293T cells were transfected with 100 ng of the IRF1 reporter, VP3, 10 ng of pRL-TK (as an internal control), and JAK1 or JAK2 (100 ng). Luciferase assays were performed 24 h after transfection. Western blotting was used to analyze JAK1, JAK2, and VP3 expression levels. (D-E) JAK1 and JAK2 increase the expression of the FMDV VP3 protein. HEK293T cells were co-transfected with increased amounts of JAK1 or JAK2 (0, 0.5, and 1.0 μg) and a constant quantity of VP3 (50 ng). At 24 h post-transfection, the cell lysate was analyzed by western blotting. The values are presented as the mean ± SD of three independent experiments.
FMDV VP3 interacts with JAK1 and JAK2
We next examined the association between VP3 and JAKs. As shown in , VP3 interacted with JAK1 and JAK2 but not with STAT1 in transient transfection and co-immunoprecipitation assays. Endogenous co-immunoprecipitation experiments indicated that FMDV VP3 interacted with endogenous JAK1 ().
Figure 5. FMDV VP3 interacts with JAK1 and JAK2. (A) Interaction between FMDV VP3 and JAK1 or JAK2 in a mammalian overexpression system. The HEK293T cells (2×106) were co-transfected with 4 μg of the FMDV Myc-Vp3 plasmid and plasmids expressing Flag-JAK1 (8 μg), Flag-JAK2 (8 μg) or Flag-STAT1 (5 μg). Co-immunoprecipitation was performed with anti-Flag (F) or control IgG (lg) antibodies. Immunoblotting analysis was performed with anti-Myc-HRP (αMyc) (upper panels). The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with antibodies specific for Flag and Myc (lower panels). (B) FMDV VP3 interacts with endogenous JAK1. The HEK293T cells (1.6×107) were co-transfected with 20 μg of the FMDV Flag-VP3 (F-VP3) plasmid for 24 hours. Co-immunoprecipitation was then performed with anti-JAK1 or control IgG (lg) antibodies. Immunoblotting analysis was performed with anti-JAK1 or anti-Flag-HRP (αF) (upper panels) antibodies. The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with anti-JAK1 and anti-Flag (lower panels). (C) VP3 affects JAK1-STAT1 interactions. HEK293T cells were transfected with plasmids encoding Myc-VP3 (4 μg), Flag-JAK1 (8 μg), and HA-STAT1 (5 μg) for 24 h. Co-immunoprecipitation was then performed with anti-Flag (F) or control IgG (lg) antibodies. Immunoblotting analysis was performed with anti-HA or anti-Flag (upper panels) antibodies. The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with anti-Flag, anti-HA, and anti-Myc (lower panels). (D) VP3 does not affect JAK2-STAT1 interactions. The experiments were performed as described in C.
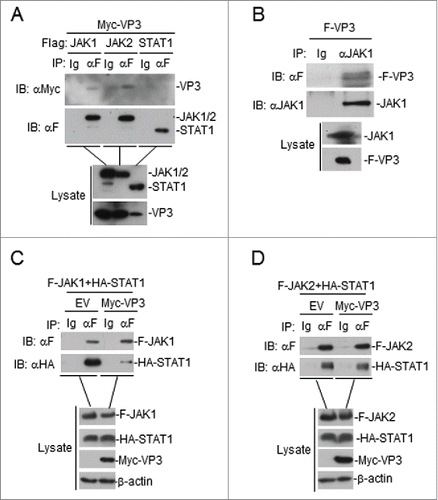
A previous study showed that JAK1 and JAK2 form a complex with STAT1.Citation31 The effects of FMDV VP3 on the formation of the JAK1-STAT1 and JAK2-STAT1 complexes were investigated. As shown in , the overexpression of VP3 disrupted the assembly of the JAK1-STAT1 complex but not the JAK2-STAT1 complex. Interestingly, the expression level of JAK1 in lysates was reduced when VP3 was present, but the JAK2 protein level was unchanged ().
FMDV VP3 adversely affects JAK1 protein levels
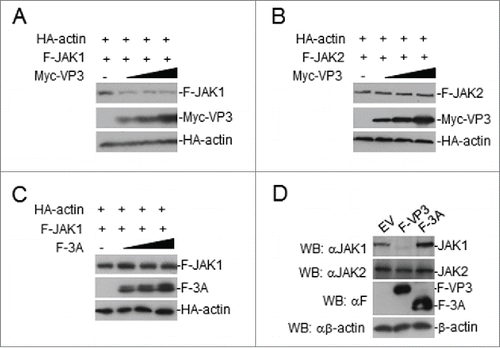
To examine the inhibitory effects of FMDV VP3 on JAK1 protein levels, the cells were transfected with JAK1 or JAK2 and VP3 in a dose-dependent manner. The results showed that the VP3 protein reduced the expression level of the JAK1 protein, but JAK2 levels were unaffected (). Moreover, the FMDV 3A protein had no effect on the expression of JAK1 (). Furthermore, we examined the potential effects of VP3 or 3A on the expression of endogenous JAK1 and JAK2. Endogenous JAK1 but not JAK2 was reduced in a HEK293T cell line stably expressing VP3, but endogenous JAK1 and JAK2 were unchanged in a HEK293T cell line stably expressing 3A (). Taken together, we conclude that FMDV VP3 adversely affects JAK1 protein levels.
Figure 6. FMDV VP3 adversely affects JAK1 protein levels. (A-B) Dose-dependent effects of VP3 on JAK1 and JAK2. HEK293T cells were co-transfected with increased amounts of Myc-VP3 (0, 0.5, 1.0, and 2.0 μg) and JAK1 or JAK2 (200 ng). At 24 hours post-transfection, the cell lysate was analyzed by protein gel blotting. (C) Dose-dependent effects of FMDV 3A on JAK1. HEK293T cells were co-transfected with increased amounts of Flag-3A (0, 0.5, 1.0, and 2.0 μg) and a constant quantity of JAK1 (200 ng). At 24 hours post-transfection, the cell lysate was analyzed by western blotting. (D) Effects of FMDV VP3 on endogenous JAK1 and JAK2 expression. HEK293T cells stably expressing VP3 or 3A were lysed and analyzed by protein gel blotting. EV or minus: empty vector.
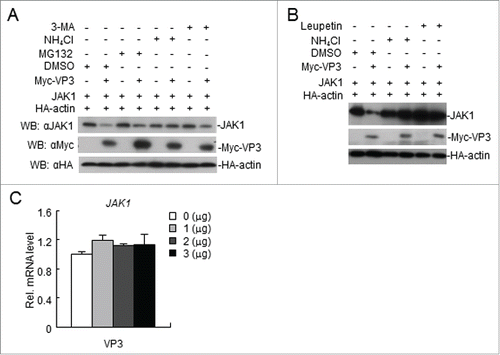
FMDV VP3 degrades JAK1 via a lysosomal pathway
We showed that FMDV VP3 mediates a reduction in JAK1 levels, possibly via degradation. We sought to determine the pathways by which VP3 degrades JAK1. Following transfection with VP3, the cells were treated with a proteasome inhibitor (MG-132), an autophagy inhibitor (3-MA), or a lysosomal inhibitor (NH4Cl and Leupeptin). The lysosomal inhibitor NH4Cl and Leupeptin blocked JAK1 degradation (). The RT-PCR data showed that the abundance of JAK1 mRNAs was not altered by VP3 at various doses (). Collectively, these results suggest that FMDV VP3 degrades JAK1 via a lysosomal pathway.
Figure 7. FMDV VP3 degrades JAK1 via a lysosomal pathway. (A-B) FMDV VP3 degrades JAK1 via a lysosomal pathway. HEK293T cells were transfected with VP3 (1.0 μg) and JAK1 (150 ng) for 18 h and then treated with dimethyl sulfoxide (DMSO), MG-132 (20 μM), 3-MA (0.5 mg/ml), NH4Cl (20 mM) or Leupeptin (400 μg/ml) for 6 h. The cell lysate was analyzed by western blotting. (C) Dose-dependent effects of VP3 on JAK1 mRNA expression levels. HEK293T cells were transfected with increasing amounts of FMDV VP3 for 24 h. The JAK1 gene was evaluated using a relative quantitative RT-PCR assay. The values are presented as the mean ± SD of three independent experiments.
Discussion
The activation of the innate immune response through the detection of viral replication products in the cell leads to the expression of hundreds of antiviral genes that control the spread of infection.Citation32 Animal viruses employ a broad range of anti-immune strategies, thereby enabling them to replicate efficiently. The rapid identification of host evasion mechanisms that are exploited by emerging pathogens is essential for the development of effective treatment strategies.Citation33 The IFN system plays a pivotal role in the antiviral response, as it is able to control most viral infections in the absence of adaptive immunity.Citation34
To survive in the host, many viruses have evolved mechanisms to counteract the host IFN response. Previous studies have shown that the expression of FMDV proteins can inhibit the production of IFNs and the IFN signaling pathways.Citation22,35-37 In the present study, we have shown that the structural protein VP3 of FMDV plays an important role in inhibiting IFN-γ-induced responses. Previous reports have focused on the functions of FMDV VP3 with respect to its involvement in the assembly of the virus particle and its influence on virulence.Citation38 VP3 is not the sole antigenic protein of FMDV, but it contains the main site that is recognized during infection.Citation38 In the current study, we showed that VP3 not only has the ability to inhibit IFN-γ-induced responses but also disrupts the assembly of the JAK1 complex and degrades JAK1 via a lysosomal pathway. Interestingly, we found that JAK1 and JAK2 can increase the expression of FMDV VP3, but the mechanism that underlies this effect requires further investigation. In addition, we demonstrated that FMDV VP3 inhibits the phosphorylation and dimerization of STAT1 and blocks the nuclear accumulation of phosphorylated STAT1. These results reveal for the first time that FMDV has evolved a novel mechanism by which the VP3 structural protein can counteract innate immune signaling, complementing the known FMDV-mediated immune evasion mechanisms.
Many viruses or viral proteins can inhibit the IFN-activated JAK-STAT signaling pathway. Rubulavirus V proteins (SV5 and mumps) inhibit IFNs by degrading STAT1.Citation39,40 The Sendai virus C protein induces STAT1 degradation and inhibits STAT1 phosphorylation.Citation41,42 The Measles virus V protein does not degrade STATs or prevent STAT phosphorylation; however, that protein does block the nuclear import of IFN-induced STAT1 and STAT2.Citation43,44 The V proteins of Nipah virus and Hendra virus block IFN-α/β and IFN-γ by preventing the phosphorylation and nuclear accumulation of STAT1.Citation45,46 In addition, the Henipavirus V proteins target STAT proteins by inducing the formation of high-molecular weight STAT-containing complexes that are localized in the cytoplasm.Citation33 The FMDV 3Cpro protein inhibits the JAK-STAT signaling pathway by blocking the nuclear translocation of STAT1/STAT2.Citation21 We showed that FMDV VP3 does not induce the downregulation of STAT1, but it prevents IFN-γ-induced STAT1-activated tyrosine phosphorylation and blocks the nuclear accumulation of phosphorylated STAT1.
Many strategies are used by pathogens to avoid host immune responses, but inhibition of the IFN-γ signaling pathway has not been observed for FMDV. This unique mechanism of IFN-mediated evasion could account for the emergence of FMDV as an animal pathogen. The ability to interfere with host defense response mechanisms is essential for viral pathogenesis. Our study has revealed a potential target for pharmaceutical intervention in FMD. Based on our study, the modification of the FMDV VP3 gene using a reverse genetics system might result in an attenuated FMDV that could be used for the production of vaccines. Given that the VP3 protein is an inhibitor of IFN-γ induction and a component of the FMDV capsid and virus-like particles (VLPs), this protein could be employed in FMD vaccines. The seed strain and VLPs of the FMD vaccine could be improved by mutating its functional sites. This approach would eliminate or reduce the inhibition of the host innate immune response that is conferred by VP3. Further studies are required to determine whether the inhibition of IFN-γ induction impacts the efficacy of a VP3-based vaccine.
In summary, we found that FMDV VP3 degrades JAK1, reduces the phosphorylation of IFN-γ-induced STAT1, and blocks the nuclear accumulation of phosphorylated STAT1. Taking these findings together, this study provides insight into the functions of FMDV structural proteins as inhibitors of IFN signaling pathways. We believe that these novel findings also provide a theoretical basis for the development of a highly efficacious FMD vaccine.
Materials and methods
Cells, viruses and chemicals
The human embryonic kidney 293T (HEK293T) and baby hamster kidney (BHK-21) cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 100 U/ml of penicillin-streptomycin at 37°C/5% CO2. IFN-γ (PeproTech, Rocky Hill, NJ, USA) was used for IFN stimulation. Type O FMDV was propagated in BHK-21 cells, and the supernatants of infected cells were clarified and stored at −80 °C.
Antibodies
Mouse monoclonal antibodies specific for Flag, hemagglutinin (HA), Myc, β-actin and lamin B were purchased from Sigma-Aldrich (Sigma-Aldrich). Rabbit polyclonal antibodies specific for STAT1, phosphorylated STAT1 (pY701-STAT1), and IRF1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and antibodies specific for VP3, JAK1 and JAK2 were produced in our laboratory.
Cloning of FMDV VP3 and other genes
The complete open reading frames of FMDV proteins were generated from cDNAs from BHK-21 cells infected with the type O FMDV strain Tibet/CHA/99 using reverse transcription polymerase chain reaction (RT-PCR). The amplicons were cloned into pCAGGS. The O-type FMDV 3A and VP3 genes were amplified by PCR and cloned into pMSCV. The human JAK1, JAK2 and STAT1 genes were amplified by PCR and cloned into pRK-Flag and pRK-HA. The primers used to amplify the genes are listed in .
Table 1. Primers used in this study.
Transfection and reporter gene assays
HEK293T cells (5×104) in 48-well plates were transfected with 100 ng of reporter plasmid, 10 ng of pRL-TK (Promega) (as an internal control), and 100 ng of the indicated plasmids. At 24 hours post-transfection (hpt), the cells were treated with IFN-γ (50 ng/ml) or left untreated for 12 h, and the whole-cell extracts were prepared for the analysis of dual-luciferase activities. The activities of the reporter genes, including firefly luciferase and Renilla luciferase, were determined using a dual-luciferase reporter 1000 assay system (Promega) according to the manufacturer's instructions. Three independent experiments were carried out in duplicate.
Co-immunoprecipitation and immunoblotting analyses
HEK293T cells (2×106) were transfected for 24 h and lysed in 1 ml of lysis buffer (20 mM Tris pH7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10 mg/ml aprotinin, 10 mg/ml leupeptin, and 1 mM PMSF). For each sample, 0.4 ml of lysate was incubated with 0.5 μg of antibody or control IgG and 30 μl of protein G-Sepharose in 20% ethanol (GE Healthcare) for 2 h. The sepharose beads were washed three times with 1 ml of lysis buffer containing 500 mM NaCl. The precipitates were analyzed by immunoblotting. For endogenous co-immunoprecipitation experiments, HEK293T cells (1.6×107) were transfected with 20 μg of the Flag-VP3 plasmid for 24 h. Co-immunoprecipitation and immunoblotting experiments were performed as described above.
Confocal microscopy
The BHK21 cells were left uninfected or infected with FMDV (MOI = 0.1) for 8 h. The cells were fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.1% Triton X-100 for 15 min. The cells were incubated with anti-VP3 for 2 h. The cells were then incubated with an anti-mouse IgG (whole molecule)–FITC antibody produced in goats (F2012, Sigma, USA). The cells were stained with 4′, 6′-diamidino-2-phenylindole and observed with a Leica confocal microscope under a 100× oil objective.
HEK293T cells stably expressing the VP3 protein were treated with IFN-γ (100 ng/ml) or left untreated for 30 min. The experiments were performed as described above.
Native polyacrylamide gel electrophoresis (PAGE)
Native PAGE and STAT1 dimerization assays were carried out as described previously.Citation47 Briefly, the lysates were mixed with 2×sample buffer (0.125 mM Tris-HCl pH 6.8, 30% glycerol, and 2% deoxycholate), loaded onto native polyacrylamide gels, and subjected to electrophoresis (25 mA for 1 h) on ice.
Viral infection and treatment
The BHK21 cell lines stably expressing the 3A or VP3 proteins were left untreated or treated with IFN-γ (100 ng/ml) for 1 h. The cells were infected with O type FMDV (MOI = 0.1) at the indicated times. After 2 h, the viral inoculum was removed and the infected cells were washed twice with 1× PBS (pH 7.4) and re-fed with DMEM containing 2% FBS. At various time points post-infection, cell-free culture supernatants and cell lysates were harvested and stored at −80 °C until use.
To examine the effect of FMDV VP3 on the expression of the JAK1, HEK293T cells were co-transfected with JAK1 and VP3 or an empty vector for 18 h and subsequently treated with DMSO, MG132 (20 μM), 3-MA (0.5 mg/ml), NH4Cl (20 mM) or Leupeptin (400 μg/ml) for 6 h. The samples were harvested and analyzed using protein gel blotting.
Construction of a stable cell line overexpressing 3A and VP3
HEK293T cells were co-transfected with 10 μg of pMSCV-3A, pMSCV-VP3 or pMSCV, 10 μg of gag-pol, and 3 μg of VSVg. At 6 hpt, the medium was replaced with fresh DMEM containing 5% PBS. After 48 h of incubation, the supernatant of the transfected cells was harvested and filtered through a membrane with a 0.22-μm pore size. The filtrates were ultra-centrifuged to concentrate the recombinant retrovirus expressing an empty vector, Flag-3A, or Flag-VP3. Subsequently, BHK21 or HEK293T cells were transduced with the retroviruses at 10 transduction units per cell. The expression of empty vector, Flag-3A, or Flag-VP3 in the transduced cells was examined by western blotting.
Real-time PCR assays
The expression of IRF1, GBP1, STAT1, and CXCL9 was examined using relative quantitative real-time RT-PCR. HEK293T cells were transfected with 0.5 μg of pCAGGS or pCAGGS-Flag-VP3 using calcium phosphate for 24 h. The cells were treated with IFN-γ (100 ng/ml) or left untreated for 3 h. The total RNA was extracted from the cells with TRIzol and treated with DNase I to remove potential genomic DNA contaminants. The isolated RNA was then reverse transcribed into cDNA with Moloney murine leukemia virus reverse transcriptase (TaKaRa) according to the manufacturer's instructions. The level of mRNA transcription was quantified by real time RT-PCR with SYBR Premix Ex Taq II (TaKaRa) in a LightCycler 480 II real-time PCR system (Roche).Citation37,48 The target gene expression was normalized to the expression of the glyceraldehyde-3-phosphate dehydrogenase gene. The primers used for the investigated genes are listed in . Relative fold changes in gene expression were determined using the threshold cycle (2–ΔΔCt) method.
The viral genome copies in FMDV-infected BHK21 cells were quantified using real-time quantitative PCR. Total RNA was extracted from the FMDV-infected cells using TRIzol, as described above. The quantification of the genome copies of FMDV was performed as described previously.Citation49
Statistical analysis
Statistical analysis was performed using the SPSS17.0 software. The student's t test was performed for the experiments. The level of significance is shown in figures (*P < 0.05; **P < 0.01). Densitometry analysis of the original protein gel blots was performed using the Image J software.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank other members of Shu's laboratory for stimulating discussion and technical help.
Funding
This work was supported by the following funding sources: the National Natural Science Foundation of China (Nos. 31402179, 31302118, 31402179, U1501213), the National Science & Technology Support Plan Program (No.2015BAD12B04), the Gansu Science Foundation for Distinguished Young Scholars (No. 145RJDA328), the Key Technologies R&D Program of Gansu Province (No. 1302NKDA027) and the Ministry of agriculture 948 project (2015-Z6). The funding bodies had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Woolhouse M, Chase-Topping M, Haydon D, Friar J, Matthews L, Hughes G, Shaw D, Wilesmith J, Donaldson A, Cornell S, et al. Epidemiology. Foot-and-mouth disease under control in the UK. Nature 2001; 411:258-9; PMID:11357118; http://dx.doi.org/10.1038/35077149
- Belsham GJ. Distinctive features of foot-and-mouth disease virus, a member of the picornavirus family; aspects of virus protein synthesis, protein processing and structure. Prog Biophys Mol Biol 1993; 60:241-60; PMID:8396787; http://dx.doi.org/10.1016/0079-6107(93)90016-D
- Toka FN, Golde WT. Cell mediated innate responses of cattle and swine are diverse during foot-and-mouth disease virus (FMDV) infection: a unique landscape of innate immunity. Immunol Lett 2013; 152:135-43; PMID:23727070; http://dx.doi.org/10.1016/j.imlet.2013.05.007
- Golde WT, Nfon CK, Toka FN. Immune evasion during foot-and-mouth disease virus infection of swine. Immunol Rev 2008; 225:85-95; PMID:18837777; http://dx.doi.org/10.1111/j.1600-065X.2008.00672.x
- Grubman MJ, Moraes MP, Segundo FDS, Pena L, de los Santos T. Evading the host immune response: how foot-and-mouth disease virus has become an effective pathogen. Fems Immunol Med Mic 2008; 53:8-17; http://dx.doi.org/10.1111/j.1574-695X.2008.00409.x
- Summerfield A, Guzylack-Piriou L, Harwood L, McCullough KC. Innate immune responses against foot-and-mouth disease virus: current understanding and future directions. Vet Immunol Immunop 2009; 128:205-10; http://dx.doi.org/10.1016/j.vetimm.2008.10.296
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev 2001; 14:778-809; PMID:11585785; http://dx.doi.org/10.1128/CMR.14.4.778-809.2001
- Yao QX, Huang QF, Cao Y, Qian P, Chen HC. Porcine interferon-gamma protects swine from foot-and-mouth disease virus (FMDV). Vet Immunol Immunop 2008; 122:309-11; http://dx.doi.org/10.1016/j.vetimm.2007.09.004
- Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat Rev Immunol 2008; 8:559-68; PMID:18575461; http://dx.doi.org/10.1038/nri2314
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005; 5:375-86; PMID:15864272; http://dx.doi.org/10.1038/nri1604
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: From interferons to cytokines. J Biol Chem 2007; 282:20059-63; PMID:17502367; http://dx.doi.org/10.1074/jbc.R700016200
- Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem 1998; 67:227-64; PMID:9759489; http://dx.doi.org/10.1146/annurev.biochem.67.1.227
- Raghavan B, Cook CH, Trgovcich J. The Carboxy Terminal Region of the Human Cytomegalovirus Immediate Early 1 (IE1) Protein Disrupts Type II Inteferon Signaling. Viruses-Basel 2014; 6:1502-24; http://dx.doi.org/10.3390/v6041502
- Goodbourn S, Didcock L, Randall RE. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol 2000; 81:2341-64; PMID:10993923; http://dx.doi.org/10.1099/0022-1317-81-10-2341
- Guzylack-Piriou L, Bergamin F, Gerber M, McCullough KC, Summerfield A. Plasmacytoid dendritic cell activation by foot-and-mouth disease virus requires immune complexes. Eur J Immunol 2006; 36:1674-83; PMID:16783856; http://dx.doi.org/10.1002/eji.200635866
- de Los Santos T, de Avila Botton S, Weiblen R, Grubman MJ. The leader proteinase of foot-and-mouth disease virus inhibits the induction of β interferon mRNA and blocks the host innate immune response. J Virol 2006; 80:1906-14; PMID:16439546; http://dx.doi.org/10.1128/JVI.80.4.1906-1914.2006
- Wen X, Kudo T, Payne L, Wang X, Rodgers L, Suzuki Y. Predominant interferon-gamma-mediated expression of CXCL9, CXCL10, and CCL5 proteins in the brain during chronic infection with Toxoplasma gondii in BALB/c mice resistant to development of toxoplasmic encephalitis. J Interf Cytok Res 2010; 30:653-60; http://dx.doi.org/10.1089/jir.2009.0119
- Ostrowski M, Vermeulen M, Zabal O, Geffner JR, Sadir AM, Lopez OJ. Impairment of thymus-dependent responses by murine dendritic cells infected with foot-and-mouth disease virus. J Immunol 2005; 175:3971-9; PMID:16148145; http://dx.doi.org/10.4049/jimmunol.175.6.3971
- de Los Santos T, Diaz-San Segundo F, Grubman MJ. Degradation of nuclear factor kappa B during foot-and-mouth disease virus infection. J Virol 2007; 81:12803-15; PMID:17881445; http://dx.doi.org/10.1128/JVI.01467-07
- Medina M, Domingo E, Brangwyn JK, Belsham GJ. The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology 1993; 194:355-9; PMID:8386879; http://dx.doi.org/10.1006/viro.1993.1267
- Du Y, Bi J, Liu J, Liu X, Wu X, Jiang P, Yoo D, Zhang Y, Wu J, Wan R, et al. 3Cpro of foot-and-mouth disease virus antagonizes the interferon signaling pathway by blocking STAT1/STAT2 nuclear translocation. J Virol 2014; 88:4908-20; PMID:24554650; http://dx.doi.org/10.1128/JVI.03668-13
- Li X, Wang J, Liu J, Li Z, Wang Y, Xue Y, Li X, Cao H, Zheng SJ. Engagement of soluble resistance-related calcium binding protein (sorcin) with foot-and-mouth disease virus (FMDV) VP1 inhibits type I interferon response in cells. Vet Microbiol 2013; 166:35-46; PMID:23764275; http://dx.doi.org/10.1016/j.vetmic.2013.04.028
- Ostler N, Britzen-Laurent N, Liebl A, Naschberger E, Lochnit G, Ostler M, Forster F, Kunzelmann P, Ince S, Supper V, et al. Gamma Interferon-Induced Guanylate Binding Protein 1 Is a Novel Actin Cytoskeleton Remodeling Factor. Mol Cell Biol 2014; 34:196-209; PMID:24190970; http://dx.doi.org/10.1128/MCB.00664-13
- Cheng YS, Colonno RJ, Yin FH. Interferon induction of fibroblast proteins with guanylate binding activity. J Biol Chem 1983; 258:7746-50; PMID:6305951
- Lubeseder-Martellato C, Guenzi E, Jorg A, Topolt K, Naschberger E, Kremmer E, Zietz C, Tschachler E, Hutzler P, Schwemmle M, et al. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am J Pathol 2002; 161:1749-59; PMID:12414522; http://dx.doi.org/10.1016/S0002-9440(10)64452-5
- Rosowski EE, Nguyen QP, Camejo A, Spooner E, Saeij JP. Toxoplasma gondii Inhibits gamma interferon (IFN-gamma)- and IFN-β-induced host cell STAT1 transcriptional activity by increasing the association of STAT1 with DNA. Infect Immun 2014; 82:706-19; PMID:24478085; http://dx.doi.org/10.1128/IAI.01291-13
- Schindler C, Strehlow I. Cytokines and STAT signaling. Adv Pharmacol 2000; 47:113-74; PMID:10582086; http://dx.doi.org/10.1016/S1054-3589(08)60111-8
- Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth F R 2008; 19:383-94; http://dx.doi.org/10.1016/j.cytogfr.2008.08.004
- Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nat Genet 2003; 33:388-91; PMID:12590259; http://dx.doi.org/10.1038/ng1097
- Sung YY, Kim HK. Illicium verum extract suppresses IFN-gamma-induced ICAM-1 expression via blockade of JAK/STAT pathway in HaCaT human keratinocytes. J Ethnopharmacol 2013; 149:626-32; PMID:23872327; http://dx.doi.org/10.1016/j.jep.2013.07.013
- Andl CD, Mizushima T, Oyama K, Bowser M, Nakagawa H, Rustgi AK. EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am J Physiol-Gastr L 2004; 287:G1227-G37
- Baum A, Garcia-Sastre A. Differential recognition of viral RNA by RIG-I. Virulence 2011; 2:166-9; PMID:21422808; http://dx.doi.org/10.4161/viru.2.2.15481
- Rodriguez JJ, Parisien JP, Horvath CM. Nipah virus V protein evades α and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J Virol 2002; 76:11476-83; PMID:12388709; http://dx.doi.org/10.1128/JVI.76.22.11476-11483.2002
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J GenVirol 2008; 89:1-47
- Wang D, Fang L, Luo R, Ye R, Fang Y, Xie L, Chen H, Xiao S. Foot-and-mouth disease virus leader proteinase inhibits dsRNA-induced type I interferon transcription by decreasing interferon regulatory factor 3/7 in protein levels. Biochem Bioph Res Co 2010; 399:72-8; http://dx.doi.org/10.1016/j.bbrc.2010.07.044
- Wang D, Fang L, Liu L, Zhong H, Chen Q, Luo R, Liu X, Zhang Z, Chen H, Xiao S. Foot-and-mouth disease virus (FMDV) leader proteinase negatively regulates the porcine interferon-lambda1 pathway. Mol Immunol 2011; 49:407-12; PMID:21975014; http://dx.doi.org/10.1016/j.molimm.2011.09.009
- Wang D, Fang L, Li K, Zhong H, Fan J, Ouyang C, Zhang H, Duan E, Luo R, Zhang Z, et al. Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling. J Virol 2012; 86:9311-22; PMID:22718831; http://dx.doi.org/10.1128/JVI.00722-12
- Borca MV, Pacheco JM, Holinka LG, Carrillo C, Hartwig E, Garriga D, Kramer E, Rodriguez L, Piccone ME. Role of arginine-56 within the structural protein VP3 of foot-and-mouth disease virus (FMDV) O1 Campos in virus virulence. Virology 2012; 422:37-45; PMID:22036313; http://dx.doi.org/10.1016/j.virol.2011.09.031
- Parisien JP, Lau JF, Rodriguez JJ, Sullivan BM, Moscona A, Parks GD, Lamb RA, Horvath CM. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology 2001; 283:230-9; PMID:11336548; http://dx.doi.org/10.1006/viro.2001.0856
- Ulane CM, Horvath CM. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology 2002; 304:160-6; PMID:12504558; http://dx.doi.org/10.1006/viro.2002.1773
- Garcin D, Marq JB, Strahle L, le Mercier P, Kolakofsky D. All four Sendai virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology 2002; 295:256-65; PMID:12033784; http://dx.doi.org/10.1006/viro.2001.1342
- Komatsu T, Takeuchi K, Yokoo J, Gotoh B. Sendai virus C protein impairs both phosphorylation and dephosphorylation processes of Stat1. Febs Lett 2002; 511:139-44; PMID:11821064; http://dx.doi.org/10.1016/S0014-5793(01)03301-4
- Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J Virol 2003; 77:7635-44; PMID:12805463; http://dx.doi.org/10.1128/JVI.77.13.7635-7644.2003
- Takeuchi K, Kadota SI, Takeda M, Miyajima N, Nagata K. Measles virus V protein blocks interferon (IFN)-α/β but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. Febs Lett 2003; 545:177-82; PMID:12804771; http://dx.doi.org/10.1016/S0014-5793(03)00528-3
- Rodriguez JJ, Wang LF, Horvath CM. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J Virol 2003; 77:11842-5; PMID:14557668; http://dx.doi.org/10.1128/JVI.77.21.11842-11845.2003
- Rodriguez JJ, Cruz CD, Horvath CM. Identification of the nuclear export signal and STAT-binding domains of the Nipah virus V protein reveals mechanisms underlying interferon evasion. J Virol 2004; 78:5358-67; PMID:15113915; http://dx.doi.org/10.1128/JVI.78.10.5358-5367.2004
- Leffers H, Dejgaard K, Celis JE. Characterisation of two major cellular poly(rC)-binding human proteins, each containing three K-homologous (KH) domains. Eur J Biochem 1995; 230:447-53; PMID:7607214; http://dx.doi.org/10.1111/j.1432-1033.1995.tb20581.x
- Paulus C, Krauss S, Nevels M. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. PNAS 2006; 103:3840-5; PMID:16497831; http://dx.doi.org/10.1073/pnas.0600007103
- Moniwa M, Clavijo A, Li MY, Collignon B, Kitching RP. Performance of a foot-and-mouth disease virus reverse transcription-polymerase chain reaction with amplification controls between three real-time instruments. J Vet Diagn Invest 2007; 19:9-20; PMID:17459827; http://dx.doi.org/10.1177/104063870701900103
