The transcription factor family FOXO and FOXO3a in particular is of central importance in the long-term maintenance of haematopoietic stem cell (HSC) quiescence, cell number, reactive oxygen species (ROS) scavenging and long-term repopulation potential. ROS scavengers such as n-acetyl-L-cysteine (NAC) are able to rescue FOXO deficient HSCs from some of these abnormalities. In many cell types the PI3K/AKT pathway is regarded as the major regulatory pathway of FOXO function. PI3K/AKT activation by cytokines, growth factors or loss of suppressors such as PTEN results in the phosphorylation and cytosolic sequestration of FOXOs which generally causes transcriptional suppression of FOXO target genes. The PI3K pathway has also been implicated in maintenance of HSC number and function, however the effects were found to be independent of FOXO. Specifically, the mTORC1 arm of PI3K signaling was implicated by both PTEN and TSC1 deletion.Citation1,2 In the PTEN deletion model FOXOs remained constitutively nuclear despite hyperactivation of Akt, strongly suggesting FOXO activity is decoupled from PI3K/AKT signaling in HSCs. Recently, this phenomena of PI3K/AKT/FOXO3a decoupling was again observed in HSCs within the background of FANCD2 knockout.Citation3 FANCD2 deletion caused FOXO3a to be localized in the cytosol and this was even found to be the case for a FOXO3a construct where the three AKT phosphorylation sites were ablated; clearly demonstrating that FANCD2 was dominant to PI3K/AKT signaling in regard to FOXO3a localization.
In a recent paper published in Cell Cycle Liang et. alCitation4 describe an alternative regulatory mechanism for FOXO3a in HSCs. They show that PI3K signaling can re-establish control over FOXO3a subcellular localization in the absence of the NAD+ dependent class III histone deacetylase SIRT1. SIRT1 is a well-established regulator of FOXO function that directly deacetylates lysines 242 and 245 of FOXO which are acetylated by p300 and PCAF. HSCs with a deletion in the SIRT1 catalytic domain demonstrate enhanced cytosolic sequestration of FOXO3a which is reversed by PI3K inhibition only in lineage restricted haematopoietic progenitor cells and not in primitive haematopoietic stem and progenitor cells (LSK). Interestingly, this effect is found to be specific to FOXO3a as the localization pattern of FOXO1 was not perturbed by SIRT1 deletion; FOXO1 having a strong cytosolic localization under all conditions tested. This important finding may explain why HSCs with catalytically inactive SIRT1 exhibit elevated ROS levels, reduced quiescence and decreased repopulation potential.
This study complements previous work demonstrating that FOXOs are more efficiently phosphorylated at their AKT sites and/or more readily detach from DNA when they are acetylated following inactivation of SIRT1 or other classes of HDACs.Citation5,6 Methylation has also been found to influence FOXO phosphorylation by AKT and FOXO subcellular localization. Methylation of FOXOs at arginines 248 and 250 by the methyltransferase PRMT1 strongly impairs the ability of AKT to phosphorylate and thereby sequester FOXOs in the cytoplasm.Citation7 Due to the physical proximity of these modifications it might be of interest to determine if FOXO3a acetylation blocks methylation by PRMT1 or if methylation blocks acetylation; tuning FOXO3a phosphorylation and localization in the process (). It has also been proposed that acetylation influences FOXO3a function without necessarily altering its nuclear localization. Essentially, acetylation may switch the functions of nuclear FOXOs between driving apoptosis (acetylated state) to promoting stress resistance (deacetylated state). Alteration of nuclear function would require nuclear FOXO selecting different target genes. It would then be important to determine whether target selection is influenced by DNA motif recognition or differences in cofactor associations. Given the mechanistic possibilities and phenotypic consequences associated with FOXO acetylation status further investigation of these interactions will be warranted in HSCs and beyond.
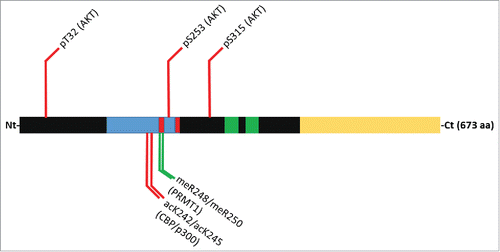
Figure 1. Domain map and post-translational modifications that influence FOXO3a subcellular localization. Within the domain structure the forkhead domain is colored blue, nuclear localization signals are red, nuclear export signals are green and the transactivation domain is yellow. Modifications that promote cytosolic localization are marked with red lines while green lines mark modifications that promote nuclear retention.
References
- Lee JY, et al. Cell Stem Cell 2010; 7(5):593-605; PMID:21040901; http://dx.doi.org/10.1016/j.stem.2010.09.015
- Chen C, et al. J Exp Med 2008; 205(10):2397-408; PMID:18809716; http://dx.doi.org/10.1084/jem.20081297
- Li X, et al. J Biol Chem 2015; 290(5):2715-27; PMID:25505262; http://dx.doi.org/10.1074/jbc.M114.619536
- Liang R, et al. Cell Cycle 2016; 15(6):861-7; PMID:26929388; http://dx.doi.org/10.1080/15384101.2015.1123355
- Matsuzaki H, et al. J Biochem 2005; 138(4):485-91; PMID:16272144; http://dx.doi.org/10.1093/jb/mvi146
- Mihaylova MM, et al. Cell 2011; 145(4):607-21; PMID:21565617; http://dx.doi.org/10.1016/j.cell.2011.03.043
- Yamagata K, et al. Mol Cell 2008; 32(2):221-31; PMID:18951090; http://dx.doi.org/10.1016/j.molcel.2008.09.013
