In embryonic stem cells (ESC), epigenetic mechanisms contribute to maintain the expression of pluripotency genes and the repression of lineage-specific genes, which must remain in a silenced state in order to avoid exit from pluripotency. Upon differentiating stimuli, epigenetic mechanisms orchestrate the expression of developmental programs spatially and temporally to ensure heritability of existing or newly acquired phenotypic states.Citation1 One of the best known epigenetic machineries required for cell fate determination involves complexes of the Polycomb group (PcG) proteins. In mouse and human pluripotent ESCs, Polycomb complexes are recruited to lineage-specific gene promoters, where they are required to maintain gene repression. In a critical step during ESC differentiation, Polycomb complexes are specifically relocated in the genome to allow the expression of a specific group of proteins.Citation2 The molecular mechanisms and factors involved in establishing such exquisite gene regulation networks remain largely elusive.
The Mediator complex controls gene transcription of most RNA Polymerase II (Pol II)-regulated transcripts, which include protein-coding and noncoding RNA genes.Citation3 Mediator subunits Med1 and Med12 regulate ESC self-renewal, and are recruited to active enhancers and promoters to regulate enhancer-promoter interactions.Citation4 Interestingly, Mediator composition is dynamic, and specific Mediator variants differentially regulate gene expression. Also, Mediator directly associates with transcription factors (TFs) and the cyclin-dependent kinase 8 (Cdk8)-module. The Cdk8-module is well-known as a negative regulator of transcription, and induces a functional switch in the Mediator-dependent gene activation function.Citation3 Thus, gene regulation is greatly influenced by the architecture of the Mediator complexes.
In a recent paper published in Cell Cycle, Papadopoulou et al. investigated the Mediator-mediated mechanisms that govern gene regulation in mouse ESCs.Citation5 Genome-wide analysis of the core Mediator subunit Med12, Cdk8 and the Polycomb Repressive Complex 1 (PRC1) subunit Ring1b revealed that PRC1 and Mediator complexes co-occupy, and negatively regulate, a large set of developmental genes. Interestingly, opposite to what might be expected, Cdk8 and Med12 were found to co-target highly active genes. These results, not only suggested that Mediator and PRC1 are functionally linked, but also that Mediator and the Cdk8-module positively regulate gene transcription in ESCs (). The authors then focused on delineating the molecular mechanism behind these 2 important observations. The authors found that Med12 associated to chromatin was greatly reduced upon Ring1b depletion, most probably due to the destabilization of the Mediator complex. Interestingly, gene ontology (GO) analysis of the 598 co-deregulated genes in Ring1b and Med12 depleted cells suggested that PRC1 and Mediator co-regulate mesodermal genes (blood vessel and skeletal system development). This observation suggests that PRC1 and Mediator might functionally co-operate in mesoderm cell fate determination of ESCs.
Figure 1. Proposed model. In pluripotent ESCs, while developmental genes are co-regulated by PRC1 and Mediator complexes, the Mediator-Cdk8 module regulates metabolic genes. During ESCs differentiation, Zrf1 is associated with Cdk8, and displaces Ring1b from the ncRNA Dlx1as, permitting Hoxd11 expression by Mediator-Cdk8.
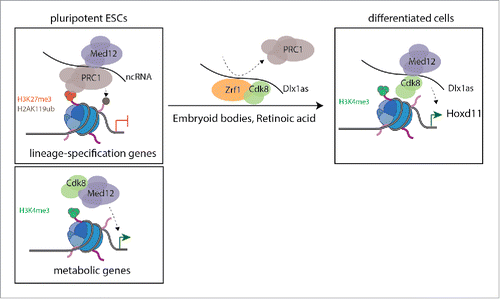
The authors then examined the interplay between Mediator, PRC1 and Cdk8 during early ESCs differentiation. Interestingly, expression of developmental genes was differentially affected in Med12, Ring1b and Cdk8-depleted embryoid bodies (EBs). While developmental genes were aberrantly upregulated in Med12-depleted EBs, the same set of genes was downregulated upon Ring1b depletion, suggesting that Mediator and Ring1b play opposite roles during early differentiation. Using the Hoxd11 gene as a read-out for their mechanistic studies the authors found that the ncRNA Dlx1as regulates Hoxd11 expression during differentiation. Interestingly, Med12 and Ring1b interacted with Dlx1as, in a Ring1b dependent manner. Also, this interaction was abrogated upon differentiation. These results not only suggested that ncRNAs stabilize Med12 and Ring1b interaction, but also that Ring1b inhibits the activation function of Dlx1as. Further mechanistic studies indicated that Zrf1 (Zuotin-related factor 1), a factor known to displace PRC1 from chromatin upon cell differentiation,Citation6 interacted with Dlx1as during differentiation, facilitating the dissociation of Ring1b with Dlx1as to enhance Hoxd11 activation. To further investigate the function of Zrf1 in the context of Mediator-mediated gene activation the authors found that Zrf1 interacts with Cdk8 during differentiation. Importantly, Zrf1 was required for proper loading of Cdk8 into chromatin and therefore recruitment to the Hoxd11 promoter.
Collectively, the results of Papadopoulou et al. shed new light on the epigenetic mechanisms that regulate pluripotency and differentiation. Specifically, these results suggest for the first time a functional interplay between Mediator, PRC1 and ncRNAs. Despite these remarkable observations, several questions remain. The key questions appear to center on how the Mediator-PRC1-ncRNAs axis is recruited and dissociated from target genes in pluripotent and differentiating stem cells, and which ncRNAs molecules interact with Mediator and Polycomb complexes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Morey L et al. Mol Cell Biol 2015 Aug; 35(16):2716-28; http://dx.doi.org/10.1128/MCB.00266-15
- Di Croce L, Helin K. Nat Struct Mol Biol 2013 Oct; 20(10):1147-55; http://dx.doi.org/10.1038/nsmb.2669
- Allen BL, Taatjes DJ. Nat Rev Mol Cell Biol 2015 Mar; 16(3):155-66; http://dx.doi.org/10.1038/nrm3951
- Kagey MH et al. Nature 2010 Sep 23; 467(7314):430-5; http://dx.doi.org/10.1038/nature09380
- Papadopoulou et al. Cell Cycle 2016; PMID:27096886
- Richly H et al. Nature 2010 Dec 23; 468(7327):1124-8; http://dx.doi.org/10.1038/nature09574
