ABSTRACT
DNA replication is a key biological process that involves different protein complexes whose assembly is rigorously regulated in a successive order. One of these complexes is a replicative hexameric helicase, the MCM complex, which is essential for the initiation and elongation phases of replication. After the assembly of a double heterohexameric MCM2-7 complex at replication origins in G1, the 2 heterohexamers separate from each other and associate with Cdc45 and GINS proteins in a CMG complex that is capable of unwinding dsDNA during S phase. Here, we have reconstituted and characterized the purified human MCM2-7 (hMCM2-7) hexameric complex by co-expression of its 6 different subunits in insect cells. The conformational variability of the complex has been analyzed by single particle electron microscopy in the presence of different nucleotide analogs and DNA. The interaction with nucleotide stabilizes the complex while DNA introduces conformational changes in the hexamer inducing a cylindrical shape. Our studies suggest that the assembly of GINS and Cdc45 to the hMCM2-7 hexamer would favor conformational changes on the hexamer bound to ssDNA shifting the cylindrical shape of the complex into a right-handed spiral conformation as observed in the CMG complex bound to DNA.
Introduction
All living organisms require DNA duplication for survival. Replisomes are the molecular machines responsible for replicating genetic material. Their dynamic architecture, and a large adaptable network of protein-protein and protein-DNA interactions characterize these multi-protein assemblies.Citation1 It is imperative for the cell to ensure correct spatial and temporal recruitment of the components of the replisome and their subsequent activation in order to duplicate DNA in a timely and accurate manner, securing the exact copy of the entire amount of genetic functional information.Citation2 Errors in this process lead to DNA damage, genetic instabilities and cell death. The MCM2-7 complex plays an essential role during the initiation of DNA replication and the progression of the replisome.Citation3 Dysregulation of these processes is linked to genomic instability and cancer progression.Citation4 However, despite impressive advances in our global understanding of DNA replication, the mechanism by which helicases unwind dsDNA during initiation and elongation remains unclear.Citation5,6
The core of the eukaryotic replicative DNA helicase MCM consists of 6 homologous proteins, the mini-chromosome maintenance proteins 2–7 (MCM2-7), which form a heterohexameric complex.Citation7 Each subunit contains an N-terminal DNA interacting and assembly domain and an ATP-binding C-terminal ATP binding AAA+ domain.Citation8 The MCM2-7 complexes are loaded as double hexamers at the origins of replication around dsDNA during the G1-phase of the cell cycle.Citation9,10 A recent cryo-EM structure of the double hexamer suggests a possible mechanism for the melting of double stranded into single stranded DNA.Citation11 MCM loading onto DNA is assisted by 3 additional elements: origin recognition complex (ORC), Cdc6 and Cdt1. As cells enter S-phase, MCM2-7 is activated through phosphorylation of different MCM2-7 subunits by the Dbf4-dependent Cdc7 kinase (DDK),Citation12,13 while almost simultaneously the cyclin-dependent kinase (CDK) promotes the assembly of additional protein components Cdc45 and the GINS heterotetramer to the complex generating the active helicase, the CMG complex.Citation14,Citation15 Therefore MCM2-7 activation requires extensive remodeling of the double hexamer complex that is initially engaged with DNA. First, the N-terminal interface that forms the double hexamer must be disrupted to allow independent translocation of 2 MCM2-7 helicases. In addition dsDNA at the origin must be melted as during its translocation in 3′–5′ direction MCM2-7 retains only the leading strand DNA in its central channel while excluding the lagging strand DNA template.
In contrast with the archaeal MCM complexes, the eukaryotic MCM2-7 shows an open hexameric ring, displaying a “gate” between subunits MCM2 and MCM5, which has been identified as a possible site of closing and opening of the MCM2-7 ring to encircle DNA.Citation16 Structural data on eukaryotic hexameric MCM2-7 include EM analysis of Drosophila melanogaster MCM2-7 (DmeMCM2-7),Citation15 on microsporidian parasite Encephalitozoon cuniculi MCM2-7 (EcuMCM2-7),Citation17 human MCM2-7Citation18 and yeast (Saccharomyces cerevisiae).Citation10,11,19 These structures show different conformations and distinct response to nucleotide binding to the single hexamer complex. While DmeMCM2-7 displays 2 different conformations in the presence or absence of nucleotide, one a planar notched and the other a spiral, lock washer state,Citation15 the EcuMCM2-7 displays high conformational heterogeneity in absence of nucleotide, and the interaction with ATPγS stabilizes the complex in the lock washer shape. Similar conclusions arise from biochemical studies of yeast MCM proteins, in which ATP binding is required for stability of the yeast MCM2-7 complexCitation20 that makes a closed circular complex in presence of ATPγS.Citation19 It is currently unknown whether the MCM2-7 structural particularities correspond to species-specific effects or represent distinct intermediates within a general helicase-loading mechanism.
Here, we describe for the first time the reconstitution of the hMCM2-7 complex (hMCM2-7) in mg amounts for structure-function studies using insect cells. The recombinant complex is biochemically active. Furthermore, we used single-particle EM reconstructions to analyze the stabilization of hMCM2-7 complex upon ATP binding, revealing large conformational changes on the hexamer as a consequence of its binding to ssDNA. Our findings, and the method to generate this recombinant complex, open the door to a detailed mechanistic structure-function studies, by site-directed mutagenesis and in vitro assembly of different factors, to provide sequential pieces of information related to the structural basis for the activation of the human replicative helicase.
Results
Isolation and characterization of the human MCM2-7
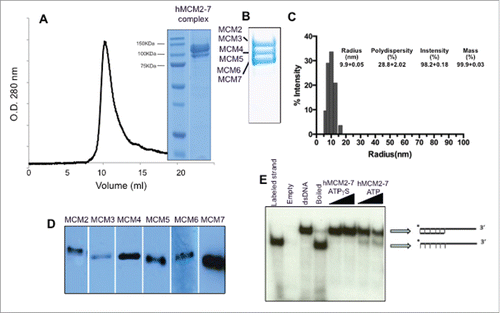
The hMCM2-7 complex was expressed and purified from insect cells infected with a single recombinant baculovirus, using the MultiBac System.Citation21 The six human MCM genes were synthesized (GeneScript) and subcloned in tandem into MultiBac donor and acceptor vectors (see Materials & Methods). To facilitate hMCM2-7 purification, the MCM4 subunit was engineered to include a His-tag, while MCM2 was tagged with a Strep-tag, both tags were located on the N-terminus and included a TEV protease cleavage site to remove them. The donor and acceptor vectors were used to generate the bacmid with all 6 hMCM cDNAs genes. This recombinant bacmid was used for insect cell transfection and later for the production of the recombinant hMCM2-7 complex (). After hMCM2-7 over-expression the assembly was purified from the soluble fraction using the 2 affinity chromatography steps, thus avoiding the presence of MCM4-6-7 hexamers, and a final gel filtration column (). The presence of the 6 different subunits in the final complex was confirmed by immunoblots with specific antibodies (). The molecular weight of the assembled complex was assessed by size exclusion chromatography and dynamic light scattering revealed a good complex homogeneity. The tagged complex eluted as a single peak at a volume consistent with a predicted molecular weight of a heterohexamer (). The final product was > 95% pure as shown by Coomassie blue-stained SDS-PAGE. The recombinant hMCM2-7 displays weak, but detectable ATP-dependent helicase activity as confirmed by strand displacement assays in vitro () as previously observed.Citation16,22
Figure 1. Protein expression and isolation of the recombinant hMCM2-7 complex. (A) The complex was isolated by tandem affinity purification using a His-Tag and a Strep-Tag was loaded onto a Supedex200 HR10/30 column. The complex eluted as a single peak at 550 kDa. (B) 15 % SDS-PAGE of the complex showing the different subunits grouped in pairs. (C) Dynamic Light scattering of the hMCM2-7 complex shows the homogeneity of the heterohexamer. Polydispersity of hMCM2-7 shows a sample having a hydrodynamic radius of 100 Å in agreement with the heterohexamer size. The inserted table shows the statistics of the sample measured. (D) Western blot of the isolated hMCM2-7 complex using specific antibodies against the human MCM subunits. (E) Helicase assay demonstrates that the recombinant hMCM2-7 displays low activity.
hMCM2-7 complex stabilization correlate with nucleotide binding
To examine the molecular architecture of the hMCM2-7 hexamer and its conformational changes upon nucleotide binding we carried out single-particle negative-staining electron microscopy studies (). We first analyzed the structure of hMCM2-7 complexes in the absence of nucleotide. Freshly purified hMCM2-7 was applied directly onto glow-discharged carbon-coated copper and negatively stained for EM analysis. A detailed examination of the EM field revealed a relatively homogeneous distribution of particles (). A set of 21434 single images was used for reference-free 2-dimensional (2D) classification. The 2D averages yield images that resemble to round top/bottom views of other eukaryotic MCM2-7 complexes with a central channel, as well as plausible side views features with 2 roughly parallel layers of protein density (). However a close look at the top/bottom 2D images showed that 6 individual MCM subunits present a flexible arrangement around the central channel. This high conformational heterogeneity in the sample disallowed the 3D reconstruction of the apo-hMCM2-7 complex in absence of a nucleotide.
Figure 2. Electron microscopy fields of the hMCM2-7 in the absence of nucleotide or after incubation with ADP, ATPγS and ATPγS–DNA. The bar in each display represents 50 nm.
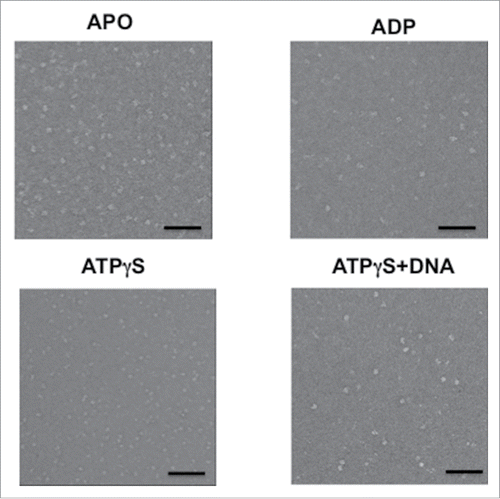
Figure 3. Detailed view of the negative stained preparations of the hMCM2-7 complex in the presence of different nucleotides. Gallery of raw particle images corresponding to top/bottom view (top row) of the apo (A), ADP (B), ATPγS (C) and ATPγS–DNA (D) preparations; reference free class-averages (middle raw); representative 2D class averages for each condition (lower raw). (E) Schematic representation of MCM2-7 complex stabilization as a consequence of binding of nucleotides and DNA.
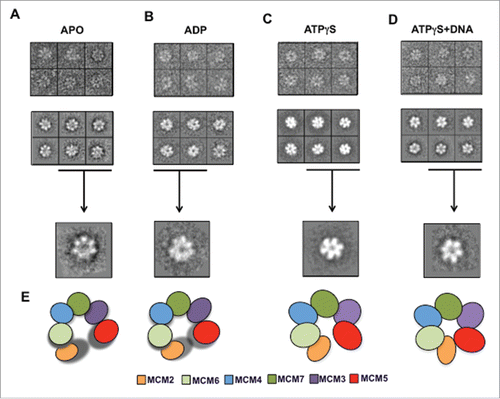
In order to stabilize the hMCM2-7 hexamer, the complex was isolated in presence of 5 mM ADP or 2 mM of the non-hydrolysable ATP homolog ATPγS, followed by negative staining electron microscopy and image analysis (). Reference free 2D averages generated using 10370 particles of hMCM2-7 complex purified in presence of ADP resembled the complex isolated in absence of nucleotide, while top/bottom views present slightly more ordered organization around the central channel (). The hMCM2-7 complex bound to ADP still exhibited high conformational heterogeneity hampering a proper 3D reconstruction.
In contrast the addition of 2 mM ATPγS yielded structurally more homogeneous negative-stained particles. The reference-free 2D averages of hMCM2-7-ATPγS complex presented either images of quasi-symmetric ring with approximate 6-fold symmetry or particles of 2 parallel layers of protein density separated by a sparse density section ().
3D reconstruction of the hMCM2-7-ATPγS complex
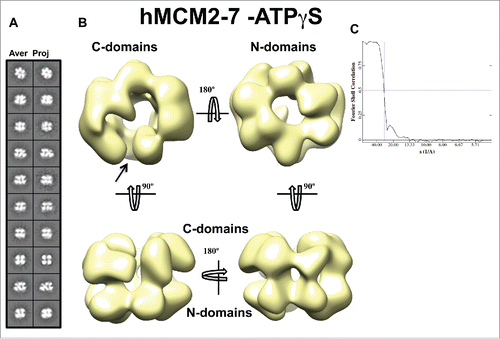
More than 20,000 hMCM2-7-ATPγS single images were selected for 3D reconstruction. As starting models we used in parallel 3 selected templates obtained by e2initialmodel.py implemented in EMAN2,Citation23 as well as low-pass filtered notched (EMD-1834) and the lock-washer (EMD-1835) DmeMCM2-7 structures.Citation15,24 All resultant hMCM2-7 3D reconstructions were highly similar indicating that in our experimental conditions the complex mainly adopts a single conformation. The chirality of eukaryotic MCM2-7 hexamer was previously calculated as a left-handed structure by docking the archaeal MCM monomer into 6-subunits of EcuMCM2-7,Citation17 in addition to tilt-pair analysis of DmeMCM2-7.Citation15 We used a visual comparison of EcuMCM2-7 to our hMCM2-7–ATPγS structure to define the handedness of the complex. Furthermore we fitted the hMCM2-7–ATPγS complex into the “notched” DmeMCM2-7 structure where the cross-correlation values favored the left-handed conformation of the complex. The hMCM2-7-ATPγS volume, despite its apparent 6-fold symmetry, presents a clear asymmetry between the N-terminal and C-terminal sides of the molecule (). The N-terminal view displays a more regular hexameric arrangement of the subunits forming a closed ring while the AAA+ domains of the complex form an open ring. Therefore, the recombinant hMCM2-7-ATPγS complex resembles the structure of the notched form of DmeMCM2-7.Citation15,24 However, our reference-free 2D averages did not show top/bottom views that correspond to an open ring with 6 lobes, characteristic of a lock-washer DmeMCM2-7 form, as the subunits are organized in a nearly closed circle. Thus, although the lock-washer form was a dominant conformation in the DmeMCM2-7-apo, in the DmeMCM2-7-ADP-BeF3 samples and it was the only form observed for ATPγS bound EcuMCM2-7 complex,Citation15,17 the gapped spiral conformation was not present in the hMCM2-7-ATPγS sample.
Figure 4. 3D reconstruction of hMCM2-7–ATPγS. (A) hMCM2-7–ATPγS reference-free class averages (left panel) and corresponding reprojections from the final structure (right panel). (B) Several views of different surface representations of a 3D reconstruction of hMCM2-7–ATPγS hexamer filtered to 28 Å. (C) Fourier Shell Correlation (FSC) curve of the 3D reconstructed map. The arrow indicates the MCM2-MCM5 “gate.”
Conformational changes of hMCM2-7 hexamer upon DNA binding
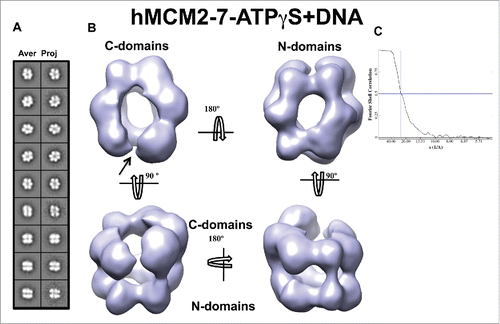
To investigate putative conformational changes of hMCM2-7 upon its binding to DNA we prepared and examined the low resolution 3D structure of hMCM2-7–ATPγS–DNA complex and compare it with our hMCM2-7–ATPγS reconstruction. We generated a 60 bp dsDNA substrate coupled to biotin molecule that included an EcoRI restriction site and a single-stranded 3′-dT(49) tail. This DNA was incubated with streptavidin-coated magnetic beads (see Materials and Methods). After washing, the beads were incubated with purified hMCM2-7 in the presence of ATPγS. The nucleotide analog was washed and the hMCM2-7–ATPγS–DNA complexes were released from the beads by EcoRI DNA cleavage. The freshly eluted fractions containing the protein-DNA complex were directly applied on carbon-coated glow discharged EM grids and negatively stained for further structural analysis. The hMCM2-7–ATPγS–DNA complexes visualized on a negative stained EM grid are single hexamers (). Reference free 2D class averages of DNA bound complex showed similar general features to hMCM2-7–ATPγS. However, the averages presented some significant differences (compare ). The top/bottom views displayed the characteristic 6-petal flower shape, but upon DNA binding each monomer becomes less twisted when compared to the hMCM2-7–ATPγS complex. Side views also showed conformational differences when compared to the complex without DNA. In the ATPγS bound form, the putative side views of the complex show that the monomers are tilted, whereas in the ATPγS-DNA bound form the hMCM2-7 monomers are straight. The complex looks like an elongated cylinder instead of a flatted one in the absence of nucleotide (). The 3D reconstruction suggests that conformational changes occurred upon DNA binding. Once the DNA is bound to hMCM2-7, the N-terminal and C-terminal domains of each monomer, which are pointing out from the central channel in absence of DNA, are bent toward the cavity resulting in a more elongated cylindrical shape of the hMCM2-7–ATPγS–DNA complex. Although DNA binding promotes a global tightening of the molecule, the MCM2-5 “gate” remains slightly open on the side where the putative C-terminal parts of MCM2 and MCM5 subunits are placed ().
Figure 5. 3D reconstruction of the hMCM2-7–ATPγS–DNA complex. (A) hMCM2-7–ATPγS-DNA reference-free class averages (left panel) and corresponding reprojections from the final structure (right panel). (B) Several views of different surface representations of a 3D reconstruction of hMCM2-7–ATPγS-DNA hexamer filtered to 24 Å. (C) Fourier correlation shell graph of the 3D reconstructed map. The arrow indicates the MCM2-MCM5 “gate.”
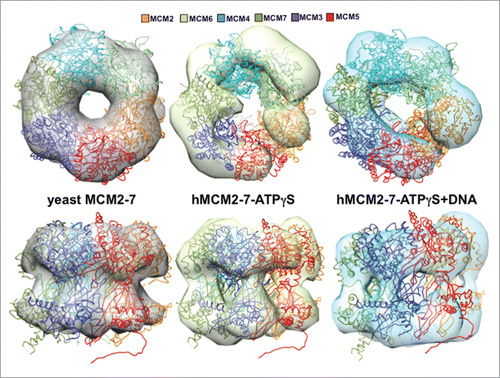
The conformational changes in the hMCM2-7 complex are evident after comparison with a single hexamer 30 Å filtered map derived from the high resolution double hexamer structure ().Citation11 The ribbon structure of a single hexamer from the yeast high-resolution structure was fitted to the different envelopes for comparison purposes, showing that large conformational changes must occur, mainly in the AAA+ and its linker with the N-domain, during the different stages leading from the dodecamer to the CMG assembly. The symmetric form of the double hexamer is broken when the ATPγS and the DNA are present, inducing specific conformational changes in the different subunits.
Figure 6. Comparison of the hMCM2-7 hexamer conformations. Top and side views of the volumes of hMCM2-7–ATPγS and hMCM2-7–ATPγS-DNA are compared to a 30 Å filtered map generated from the 3JA8 PDB file. The structure of the yeast MCM2-7 hexamer has been docked with Chimera to facilitate the comparison between the different maps and as a reference to assess the conformational changes of the different stages.
Discussion
To understand and dissect the architecture of the core of the human replicative helicase we have developed a protocol to heterologously express and purify the hMCM2-7 complex from insect cells. A previous work has co-expressed the hMCM2-7 complex in E. coli.Citation18 We have assessed the architecture of our complex by electron microscopy, showing differences with the one isolated from E. coli. The analysis of the hMCM2-7 in the absence of nucleotide reveals that the complex is highly flexible (). Previous analysis in the E. cuniculi and S. cerevisiae complexes support this observation.Citation15,17 In both cases nucleotide binding plays a role in stabilizing the MCM2-7 complex.Citation17,20 In the recent cryoEM structure of the double hexamer, the authors have suggested the presence of ADP and modeled in the nucleotide binding pockets,Citation11 although they cannot resolve unambiguously the nucleotide. Noteworthy, the double hexamer maintains the 6-fold pseudo-symmetry, indicating that the high flexibility exhibited by the single hexamer in the presence of this nucleotide can be a mechanism to facilitate the assembly of the dodecamer onto the DNA during origin licensing.
Our results show that only binding of the non-hydrolysable ATP analog (ATPγS) to the hMCM2-7 generates more homogeneous complexes. The available structural information, concerning DmeMCM2-7 and EcuMCM2-7, shows that while the drosophila complex assumes 2 different conformations, notched planar and lock-washer form, which are independent of the presence of the nucleotide; the parasite EcuMCM2-7-ATPγS complex adopts a unique left-handed, lock-washer configuration. Here, using negative staining EM we show that hMCM2-7 bound to ATPγS spontaneously and predominantly forms a planar irregular left-handed open ring. This conformation is stable over time suggesting no need of additional cofactors for hMCM2-7 complex stabilization.
The hexamerization of the MCM2-7 complex is not only favored by nucleotide binding, but also is enhanced by ssDNA binding. ssDNA binding is not an intrinsic property of all individual subunits,Citation22 however, the addition of ATP or ATPγS stimulates oligomerization, thus improving MCM2-7 binding to ssDNA. We have demonstrated that ATPγS binding not only favors oligomerization but also reduces the conformational flexibility promoting the stability of the complex (). This effect has not been observed in the case of ADP (), in agreement with the fact that ADP does not enhance binding to DNA.Citation22 A further enhancement of the hMCM2-7 conformational homogeneity is obtained by the presence of ATPγS during the assembly with a 3′-tailed DNA duplex to form the hMCM2-7-ATPγS-DNA complex. On the other hand the assembly with DNA introduces significant conformational changes in the complex when compared to the nucleotide bound complex. Once the DNA is bound the left-handed ring conformation of hMCM2-7-ATPγS is switched to a more oval non-twisted structure, while the gate between MCM2 and 5 becomes almost closed. This conformational change could be attributed to the pre-sensor-1-β (ps1β) and helix-2-insert (h2i) modules, which seem to be involved in DNA and nucleotide binding ().Citation11,25 A similar conformational change of the MCM hexamer was also observed after ssDNA binding in the hexameric N-terminal domain of the archaeal Pyrococcus furiosus MCM.Citation26
The MCM2-7 undergoes different conformational changes before is assembled with GINS and Cdc45 to form the CMG complex. The MCM2-7 is loaded on dsDNA at the replication origins as an interlocked double hexamer along the DNA axisCitation10 (). The fact that the Mcm2/5 gates are offset from each other in the eukaryotic double hexamer should result in uncoupling of the MCM2-7 ATP hydrolysis motifs restricting the DNA unwinding activity. Consequently, for MCM2-7 activation, the dissociation of the double hexamer is mandatory. Therefore, the dodecamer separation should be followed by opening of the MCM2-7 ring and simultaneous DNA meltingCitation11 (). The MCM2-7 ring would be closed around the leading strand while the lagging strand is excluded. As it is pointed out by the helicase assays, the MCM2-7 hexamer still has reduced DNA unwinding activity at this stage, before its assembly with the GINS complex and Cdc45 generates the fully active CMG helicaseCitation27,28 (). The binding of GINS and Cdc45 to MCM2-7 hexamer would promote the closure of the Mcm2/5 gate suggesting that MCM2-7 hexamer could encircle ssDNA previous to GINS and Cdc45 binding.Citation24
Figure 7. Schematic view of the hMCM2-7 conformations. (A) Eukaryotic MCM2-7 are loaded to dsDNA as double hexamer. Upon double hexamer disassembly and opening of dsDNA, MCM2-7 engages ssDNA. Subsequently GINS and Cdc45 would bind to MCM2-7 forming the active helicase complex, CMG. (B) Eukaryotic MCM2-7 in presence of nucleotide preferentially assumes a left-handed ring conformation. Upon ssDNA binding the MCM2-7 ring adopts more planar configuration. In the presence of GINS, Cdc45 and ssDNA, the AAA+ domain shifts again, but now in a right-handed spiral configuration. Hence, MCM2-7 experiences a chiral-flip in their conformation as it maturates into a functional helicase.
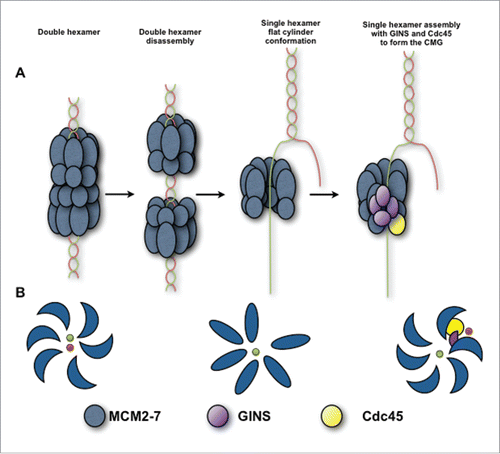
In the presence of ATPγS, the MCM2-7 complex, preferentially assumes a left-handed spiral shapeCitation17 while addition of GINS, Cdc45 and ssDNA shift the left handed spiral into a right-handed conformation of MCM2-7Citation24 (). Our work provides structural evidence for a conformational change in hMCM2-7 in the presence of DNA prior to the binding to Cdc45 and GINS. Our data indicate the possibility of an intermediate step in origin licensing including the existence of a “pre-translocase state” of hMCM2-7 encircling ssDNA (). In this conformation, MCM2-7 shows a relaxed interaction with the ssDNA, lacking full helicase activity. Following this state, the assembly with GINS and Cdc45 would introduce additional conformational changes in the core of the helicase complex, from non-twisted oval MCM2-7/DNA conformation to the spiral right-handed arrangement, stabilizing its interaction with the DNA and thus enhancing its helicase activity.Citation24
Material and methods
Expression and purification of the recombinant hMCM2-7 protein complex
cDNAs coding for each subunit of the human MCM2-7 were PCR amplified and cloned into pCR-Blunt II TOPO vector and subcloned in the corresponding MCS1 and MCS2 of each binary intermediate vectors of MultiBac expression system.Citation21 The MCM genes were synthesized and subcloned into the pFL, pSpL and pUCDM vectors. After Cre-lox specific recombination a multi expression cassette vector with all 6 MCM2-7 subunits was obtained. DH10Bac E.coli cells containing the bacmid were transformed with the recombinant vector and the positive clones were selected. The isolated recombinant bacmid DNA was used for Sf9 cells transfection. The protein expression was performed using the previously published protocol.Citation29 Infected Sf21 cells were incubated during 60-72h at 27°C. Cells were harvested by centrifugation at 800 × g for 5 min at 4°C, washed once with ice-cold PBS, and then frozen in liquid nitrogen and stored at −80°C until used.
The frozen pellet was thawed on ice and resuspended in buffer A (50 mM NaHPO4 pH8.0, 100 mM K-Acetate, 5 mM MgCl2, 10 mM imidazole, 10% glycerol, 0.1% NP-40, and protease inhibitors EDTA-free (Roche) plus 0.4 mM PMSF. The cells were lysed by sonication and centrifuged at 40,000 × g for 50 min at 4°C. Purification was performed by tandem affinity purification using a His-tag and a Strep-Tag inserted in MCM2 and MCM4 respectively. The cleared lysate was filtered and loaded onto a Hi-Trap column (GE Life Science) attached to an Akta system. The column was developed with an imidazole step gradient following the manufacturer instructions. Samples containing the MCM complex were pooled and loaded into a Strep Trap HP column (GE Life Science). The sample was eluted with dexthobiotin following the instructions of the manufacturer. The fraction containing hMCM2-7 complex was concentrated by using a 100 KDa cutoff Amicon Ultra centrifugal filters (MerckMilipore) and loaded into Superdex 200 16/60 (GE) column pre-equilibrated with buffer B (20 mM Tris-HCl, pH 7.0, 150 mM K-Glutamate, 5 mM MgCl2, 2 mM DTT, 10% glycerol). The hMCM2-7 heterohexamer eluted at ∼600 kDa molecular weight. The protein complex was concentrated and stored at −80°C.
Dynamic light scattering (DLS)
DLS measurements were carried out using a Wyatt Nano Star at a protein concentration of 6.7 µM at 298 K in a disposable microcuvette. The protein sample was filtered using 0.1uM Microcon centifugal filters and the measurements were performed in triplicate.
Western blot analysis
The hMCM2-7 was analyzed by Western Blot using specific primary antibodies for each subunit kindly provided by the DNA replication Group at CNIO (Madrid, Spain). Detection was done using ECL prime western blotting detection reagent (GE Healthcare) after the membrane was treated with Goat anti-rabbit HRP (Dako) conjugate for anti MCMs.
Helicase assay
We used a 3′ overhang DNA structure to determine the helicase activity of the hMCM2-7 complex. Oligo A (5′-ACTGGCCGTCGTTTTACAACGTCGTGACTGGGAAAACCCTGGCGTTACCCAACTTAATCG-3′) was radiolabeled with [γ32P] ATP (Perkin Elmer) at the 5′ end using T4 polynucleotide kinase (Roche), and then hybridized with Oligo B (5′-GTTGTAAAACGACGGCCAGT-3′) overnight in 150 mM sodium chloride. The helicase reaction was performed at 37°C for 60 min in presence of 0.75 μM or 1.5 μM of hMCM2-7 complex and 0.5 nM DNA in a buffer containing 20 mM BisTris pH 7.5, 150 mM K-Glutamate, 10 mM Mg-Acetate, 10% glycerol, 0.1 mg/ml BSA, 2 mM DTT, supplemented with 5 mM ATP or ATPγS, in a final volume of 20 ul. The reaction was stopped by addition of 5x stop solution (10 mM Tris-HCl pH 7.6, 100 mM EDTA, 0.2% SDS, 60 % glycerol, 0,03 % bromophenol and 0,03 % xylen cyanol). The products were separated by electrophoresis through 12% (w/v) non-denaturing polyacrylamide gel, and visualized using a Typhoon TRIO phosphorimager (GE Healthcare) or regular autoradiography.
Assembling the hMCM2-7/DNA complex
Oligonucleotides used for DNA-protein complex reconstitution were synthesized by SIGMA and shipped as lyophilized pellets. We have use oligo GM3 (5′ATCTATACATACACGTGCCTTCGATAATGGGAATTCAAACAGTAGCATGCATACACCGTA-3′), and the oligo GM5, which contained 60 nucleotides of complementary sequence including EcoRI site (in bold), overhang poly(dT)50 at 3′-end and dT-biotinilated at 5′-end: (5′-Biotin-TACGGTGTATGCATGCTACTGTTTGAATTCCCATTATCGAAGGCACGTG
TATGTATAGAT(dT)50-3′). For annealing oligonucleotides were dissolved in the water and mixed in equimolar amounts (100 mM) in the presence of 10 mM Tris-HCl, pH 8,0 and 15 mM NaCl, briefly heated to 95°C and slow-cooled to 25°C and stored at 4°C. The resulting DNA fork has 5′ (dT) biotinylated 60 bp double strand region that includes EcoRI restrictios site (bold) and 49 nucleotides long poly T arm. Formed duplex DNA was confirmed by 4% Metaphor agarose gel analysis. 20 µL of streptavidin-coated magnetic beads (DynaBeads M280 Streptavidin, Invitrogen) were washed twice with water and with MCM2-7 gel filtration buffer B and resuspended in 100 µL of the same buffer. 3 µL of hybridized DNA was added to magnetic beads and incubated with gentle agitation at room temperature during 1 h. DNA binding beads were washed 3 times with gel filtration buffer and 100 µL of purified hMCM2-7 (0.2 mg/mL) in the presence of 2 mM ATPγS was added to the beads. The mixture was incubated at 4°C with gentle agitation during 3 h. Unbound hMCM2-7 was removed and beads was washed 3 times as above and resuspended in 30 µL of gel filtration buffer. 1 µL (10 U) of EcoRI (Roche) was added to Biotin-DNA-hMCM2-7 complex and incubated at 4°C overnight as above. After EcoRI digestion the beads were collected with the magnet and the supernatant fraction containing the hMCM2-7 complex bound to DNA was pooled and used for electron microscopy (EM) analysis.
Negative stain sample preparation
For negative staining 4 μl (∼0.1 mg/ml) of the each sample were applied onto a freshly glow-discharged carbon-coated 400 mesh copper EM grids (Electron Microscopy Sciences) and incubated for 1.5 min at the room temperature. The grids were sequentially laid on top of 2 distinct 50 μl drops of MilliQ water, and striped gently for 2 s than laid on the top of 2 distinct 50 μl drops of a freshly prepared 2% uranyl formate solution for 1.5 min, striped gently for 10 sec and air dried.
Electron microscopy data collection
Data were collected on a Tecnai 12 transmission EM (FEI, Netherlands) with Lanthanum hexaboride cathode operated at 120 keV. Images were recorded under low-dose conditions with an electron dose of 8–10 e−/Å2 at 61,320x nominal magnification on a 4kx4k TVIPS TemCam-F416 CMOS camera resulting in a final pixel size at the specimen level of 2.5 Å.
Particle picking and dataset generation
The contrast transfer function (CTF) was determined on micrographs with ctffind3Citation30 and its effects were corrected with bctf from bsoft program (http://lsbr.niams.nih.gov/bsoft/programs/bctf.html). Particle picking was performed manually using boxer (EMAN1.9) or semi-automatically with e2boxer.py in EMAN2.Citation23 A total of 21434 particles were selected for apo hMCM2-7, 10370 for ADP-bound hMCM2-7, 20394 for ATPγS-bound hMCM2-7 and 18268 particles for hMCM2-7 –ATPγS –ssDNA complex. A 128×128 pixel size window was used for all data sets.
Two-dimensional images analysis
For 2D classification images were down sampled by a factor of 2. Reference-free 2D classification and averaging of the raw datasets were carried out in refine2d.py implemented in EMAN and CL2D from the Xmipp package.Citation31 A large number of class averages (up to 1000) were produced with around 20 particles in each class.
3D reconstruction
For both 3D structures, ATPγS-bound hMCM2-7 and hMCM2-7–ATPγS–ssDNA, we used 2D averages as input into the program e2initialmodel.py implemented in EMAN2, to produce 8 different starting models. These models were carefully inspected for consistency between original reference-free 2D averages and the models re-projections. For each 3D refinement 3 different models were selected, low-pass filtered to minimize model bias and used as starting models. We have also used a low-pass filtered “notched” (EMD-1834) and lock-washer (EMD-1835) D. melanogaster MCM2-7 structures. 3D volumes were calculated using an iterative projection-matching approach using libraries from EMAN1.9 and XMIPP. The resolution of both structures was estimated as 28 Å for hMCM2–7 –ATPγS and 24 Å for hMCM2–7 –ATPγS –ssDNA using Fourier Shell Correlation, with 0.5 cut-off criterion. The resulting volumes were filtered in Spider to calculated resolution using Butter low-pass filtering. The docking of atomic coordinates was performed with UCSF Chimera.Citation32
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Alison Catherine Lilley for excellent technical assistance. We thank Oscar Llorca for useful comments.
Funding
This work was supported by the Ministerio de Economía y Competitividad [BFU2011-23815/BMC to G.M., BFU2013-49153-P to J.M., the Fundación Ramón Areces to G.M., the Comunidad Autónoma de Madrid [CAM-S2010/BMD-2305 to G.M]. The Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (Grant agreement NNF14CC0001).
References
- Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem 2002; 71:333-74; PMID:12045100; http://dx.doi.org/10.1146/annurev.biochem.71.110601.135425
- Mechali M. Eukaryotic DNA replication origins: many choices for appropriate answers. Nat Rev Mol Cell Biol 2010; 11:728-38; PMID:20861881; http://dx.doi.org/10.1038/nrm2976
- Hills SA, Diffley JF. DNA replication and oncogene-induced replicative stress. Curr Biol 2014; 24:R435-44; PMID:24845676; http://dx.doi.org/10.1016/j.cub.2014.04.012
- Blow JJ, Gillespie PJ. Replication licensing and cancer–a fatal entanglement? Nat Rev Cancer 2008; 8:799-806; PMID:18756287; http://dx.doi.org/10.1038/nrc2500
- Frigola J, Remus D, Mehanna A, Diffley JF. ATPase-dependent quality control of DNA replication origin licensing. Nature 2013; 495:339-43; PMID:23474987; http://dx.doi.org/10.1038/nature11920
- Yeeles JT, Deegan TD, Janska A, Early A, Diffley JF. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature 2015; 519:431-5; PMID:25739503; http://dx.doi.org/10.1038/nature14285
- Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev 2004; 68:109-31; PMID:15007098; http://dx.doi.org/10.1128/MMBR.68.1.109-131.2004
- Bochman ML, Schwacha A. The Mcm complex: unwinding the mechanism of a replicative helicase. Microbiol Mol Biol Rev 2009; 73:652-83; PMID:19946136; http://dx.doi.org/10.1128/MMBR.00019-09
- Evrin C, Clarke P, Zech J, Lurz R, Sun J, Uhle S, Li H, Stillman B, Speck C. A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proc Natl Acad Sci U S A 2009; 106:20240-5; PMID:19910535; http://dx.doi.org/10.1073/pnas.0911500106
- Remus D, Beuron F, Tolun G, Griffith JD, Morris EP, Diffley JF. Concerted loading of MCM2-7 double hexamers around DNA during DNA replication origin licensing. Cell 2009; 139:719-30; PMID:19896182; http://dx.doi.org/10.1016/j.cell.2009.10.015
- Li N, Zhai Y, Zhang Y, Li W, Yang M, Lei J, Tye BK, Gao N. Structure of the eukaryotic MCM complex at 3.8 A. Nature 2015; 524:186-91; PMID:26222030
- Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev 2010; 24:1208-19; PMID:20551170; http://dx.doi.org/10.1101/gad.1933010
- Tanaka S, Araki H. Helicase activation and establishment of replication forks at chromosomal origins of replication. Cold Spring Harb Perspect Biol 2013; 5:a010371; PMID:23881938; http://dx.doi.org/10.1101/cshperspect.a010371
- Heller RC, Kang S, Lam WM, Chen S, Chan CS, Bell SP. Eukaryotic origin-dependent DNA replication in vitro reveals sequential action of DDK and S-CDK kinases. Cell 2011; 146:80-91; PMID:21729781; http://dx.doi.org/10.1016/j.cell.2011.06.012
- Costa A, Ilves I, Tamberg N, Petojevic T, Nogales E, Botchan MR, Berger JM. The structural basis for MCM2-7 helicase activation by GINS and Cdc45. Nat Struct Mol Biol 2011; 18:471-7; PMID:21378962; http://dx.doi.org/10.1038/nsmb.2004
- Bochman ML, Schwacha A. The MCM2-7 complex has in vitro helicase activity. Mol Cell 2008; 31:287-93; PMID:18657510; http://dx.doi.org/10.1016/j.molcel.2008.05.020
- Lyubimov AY, Costa A, Bleichert F, Botchan MR, Berger JM. ATP-dependent conformational dynamics underlie the functional asymmetry of the replicative helicase from a minimalist eukaryote. Proc Natl Acad Sci U S A 2012; 109:11999-2004; PMID:22778422; http://dx.doi.org/10.1073/pnas.1209406109
- Hesketh EL, Parker-Manuel RP, Chaban Y, Satti R, Coverley D, Orlova EV, Chong JP. DNA induces conformational changes in a recombinant human minichromosome maintenance complex. J Biol Chem 2015; 290:7973-9; PMID:25648893; http://dx.doi.org/10.1074/jbc.M114.622738
- Samel SA, Fernandez-Cid A, Sun J, Riera A, Tognetti S, Herrera MC, Li H, Speck C. A unique DNA entry gate serves for regulated loading of the eukaryotic replicative helicase MCM2-7 onto DNA. Genes Dev 28:1653-66; PMID:25085418; http://dx.doi.org/10.1101/gad.242404.114
- Coster G, Frigola J, Beuron F, Morris EP, Diffley JF. Origin licensing requires ATP binding and hydrolysis by the MCM replicative helicase. Mol Cell 2014; 55:666-77; PMID:25087873; http://dx.doi.org/10.1016/j.molcel.2014.06.034
- Fitzgerald DJ, Berger P, Schaffitzel C, Yamada K, Richmond TJ, Berger I. Protein complex expression by using multigene baculoviral vectors. Nat Methods 2006; 3:1021-32; PMID:17117155; http://dx.doi.org/10.1038/nmeth983
- Bochman ML, Schwacha A. Differences in the single-stranded DNA binding activities of MCM2-7 and MCM467: MCM2 and MCM5 define a slow ATP-dependent step. J Biol Chem 2007; 282:33795-804; PMID:17895243; http://dx.doi.org/10.1074/jbc.M703824200
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 2007; 157:38-46; PMID:16859925; http://dx.doi.org/10.1016/j.jsb.2006.05.009
- Costa A, Renault L, Swuec P, Petojevic T, Pesavento JJ, Ilves I, MacLellan-Gibson K, Fleck RA, Botchan MR, Berger JM. DNA binding polarity, dimerization, and ATPase ring remodeling in the CMG helicase of the eukaryotic replisome. Elife 2014; 3:e03273; PMID:25117490
- Sanchez-Berrondo J, Mesa P, Ibarra A, Martinez-Jimenez MI, Blanco L, Mendez J, Boskovic J, Montoya G. Molecular architecture of a multifunctional MCM complex. Nucleic Acids Res 2012; 40:1366-80; PMID:21984415; http://dx.doi.org/10.1093/nar/gkr831
- Froelich CA, Kang S, Epling LB, Bell SP, Enemark EJ. A conserved MCM single-stranded DNA binding element is essential for replication initiation. Elife 2014; 3:e01993; PMID:24692448; http://dx.doi.org/10.7554/eLife.01993
- Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/MCM2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci U S A 2006; 103:10236-41; PMID:16798881; http://dx.doi.org/10.1073/pnas.0602400103
- Ilves I, Petojevic T, Pesavento JJ, Botchan MR. Activation of the MCM2-7 helicase by association with Cdc45 and GINS proteins. Mol Cell 2010; 37:247-58; PMID:20122406; http://dx.doi.org/10.1016/j.molcel.2009.12.030
- Bieniossek C, Richmond TJ, Berger I. MultiBac: multigene baculovirus-based eukaryotic protein complex production. Curr Protoc Protein Sci 2008; 51:5.20.1-5.20.26; PMID:18429060
- Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol 2003; 142:334-47; PMID:12781660; http://dx.doi.org/10.1016/S1047-8477(03)00069-8
- Scheres SH, Nunez-Ramirez R, Sorzano CO, Carazo JM, Marabini R. Image processing for electron microscopy single-particle analysis using XMIPP. Nat Protoc 2008; 3:977-90; PMID:18536645; http://dx.doi.org/10.1038/nprot.2008.62
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 2004; 25:1605-12; PMID:15264254; http://dx.doi.org/10.1002/jcc.20084
