Sphingolipids are important constituents of all eukaryotic cell membranes. In addition to a mere structural role, sphingolipids are being identified as second messengers in a growing number of pathways with impact on diverse biological processes. Similar as for other lipids, the disturbance of sphingolipid homeostasis is associated to the pathobiology of several diseases.Citation1,2
Sphingolipid metabolism is tightly controlled and evolutionary conserved. Biosynthesis starts in the endoplasmic reticulum (ER) to form ceramide and continues in the Golgi compartment with synthesis of complex sphingolipids. Most reactions are reversible, allowing for a rapid interconversion of metabolic intermediates. Ceramide can also be catabolized by ceramidases to regenerate sphingosine, while complex sphingolipids are hydrolyzed by glycohydrolases and sphingomyelinases to recycle ceramide. Both ceramide and sphingosine can be phosphorylated to produce ceramide-1-phosphate and sphingosine-1-phosphate. Besides biosynthesis and breakdown, sphingolipids are also subject to uptake and secretion. In general, a relative increase in the level of ceramide or sphingosine is associated to anti-proliferation, senescence and apoptosis, while an augmentation of ceramide-1-phosphate or sphingosine-1-phosphate levels usually promotes cell growth and survival.Citation1,2
Sphingolipid biology is complex and the current picture is far from clear. In fact, sphingolipid metabolism and signaling coordinate a network that is strongly dependent on other metabolic inputs. The latter is evidenced by the targets and effector pathways that act in conjunction with sphingolipid signaling. These include key players known to operate at the intersect of different signaling routes, like 14-3-3 proteins, the protein phosphatase PP2A, the protein kinases AKT-PKB/Sch9 and AMPK/Snf1 (mammalian/yeast), or the multiprotein complexes, TORC1 and TORC2.Citation1, 2 Recent work by the group of Vitor Costa extended the coordinative role of sphingolipid signaling toward sugar metabolism and glucose signaling.Citation3 By performing a comparative phosphoproteomic analysis of wild-type yeast cells or cells lacking the ceramide-activated type 2A-like protein phosphatase Sit4, they identified several proteins with significantly altered expression levels, most of which are involved in carbohydrate metabolism and energy production. They also identified some proteins with altered phosphorylation and this included the hexokinase Hxk2, which displays increased serine-15 (S15) phosphorylation in glucose grown sit4Δ cells. Hxk2 is a dual-function kinase that shuttles between the cytosol and nucleus. Besides its role in sugar uptake and the initial step of glycolysis, it has a regulatory role in glucose signaling. During fermentative growth, when glucose levels are high, Hxk2 interacts with the transcription factor Mig1 and the AMPK-ortholog Snf1 to form a nuclear complex that represses genes involved in the utilization of alternative carbon sources, like SUC2, gluconeogenesis and respiratory growth. Upon glucose limitation, Hxk2-S15 phosphorylation and the subsequent phosphorylation of Mig1 by Snf1 trigger disintegration of the repressor complex and exit of both Hxk2 and Mig1 from the nucleus.Citation4 The enhanced phosphorylation of Hxk2 in sit4Δ cells is consistent with previous reports showing that already during growth on glucose respiration is derepressed in sit4Δ cells and that mitochondrial respiration is indeed essential because sit4Δ cells fail to grow anaerobically.Citation5 Moreover, Costa's group also linked the Hxk2 phosphorylation status to other phenotypes, such as the enhanced oxidative stress resistance and extended lifespan of the sit4Δ mutant and, conversely, the premature aging of mutant cells lacking the sphingomyelinase Isc1.Citation3 Notably, Isc1 translocates in a Sch9-dependent manner from the ER to the mitochondria during the diauxic shift, thereby gaining activity to allow for the adaptation from fermentation to respiration.Citation6
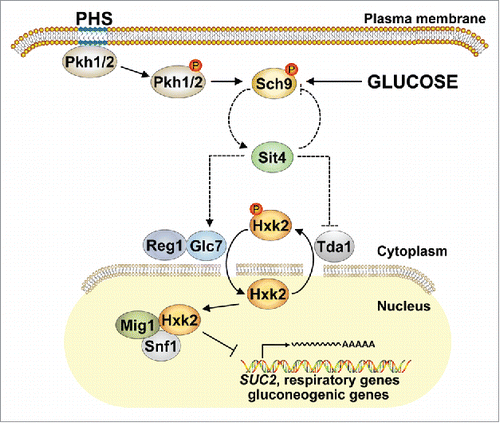
How Sit4 controls Hxk2 phosphorylation still needs to be clarified. The Costa group found no evidence for the possibilities that Hxk2 would be a direct target of Sit4 or that Sit4 would influence Hxk2 phosphorylation indirectly through inhibition of Snf1.Citation3 Whether Sit4 acts via the protein phosphatase complex Reg1-Glc7, known to dephosphorylate Hxk2,Citation7 or via the protein kinase Tda1, known to be essential for Hxk2-S15 phosphorylation,Citation4 still needs to be confirmed (). Also Sch9 could be involved since this kinase appears to modulate Hxk2 phosphorylation in response to glucose availability.Citation4 Moreover, Sch9 is an established effector of sphingolipid signaling that is activated by phytosphingosine through phosphorylation by the yeast PDK1 orthologues, Pkh1 and Pkh2, and it acts as gatekeeper of ceramide production through transcriptional repression of the ceramidase-encoding genes, YDC1 and YPC1, as well as through the above mentioned control of the sphingomyelinase Isc1.Citation6
Figure 1. Hypothetical model for Sit4-mediated control of Hxk2 phosphorylation. The phosphorylation (red dot) status of Hxk2 controls its subcellular localization and is regulated indirectly via the ceramide-activated phosphatase Sit4, possibly through Reg1-Glc7 or Tda1. As an important regulator and effector of sphingolipid metabolism, Sch9 is likely involved through modulation of Sit4 activity. Dashed lines indicate putative connections. PHS, phytosphingosine.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
References
- Hla T, Dannenberg AJ. Sphingolipid signaling in metabolic disorders. Cell Metab 2012; 16:420-34; PMID: 22982021; http://dx.doi.org/10.1016/j.cmet.2012.06.017
- Teixeira V, Costa V. Unraveling the role of the Target of Rapamycin signaling in sphingolipid metabolism. Prog Lipid Res 2016; 61:109-33; PMID: 26703187; http://dx.doi.org/10.1016/j.plipres.2015.11.001
- Barbosa AD, Pereira C, Osório H, Moradas-Ferreira, Costa V. The ceramide-activated protein phosphatase Sit4p controls lifespan, mitochondrial function and cell cycle progression by regulating hexokinase 2 phosphorylation. Cell Cycle 2016; 15:1620-30; PMID: 27163342; http://dx.doi.org/10.1080/15384101.2016.1183846
- Kaps S, Kettner K, Migotti R, Kanashova T, Krause U, Rödel G, Dittmar G, Kriegel TM. Protein kinase Ymr291w/Tda1 is essential for glucose signaling in Saccharomyces cerevisiae on the level of hexokinase isoenzyme ScHxk2 phosphorylation. J Biol Chem 2015; 290:6243-55; PMID: 25593311; http://dx.doi.org/10.1074/jbc.M114.595074
- Jablonka W, Guzmán S, Ramírez J, Montero-Lomeli M. Deviation of carbohydrate metabolism by the SIT4 phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta 2006; 1760:1281-91; PMID: 16764994; http://dx.doi.org/10.1016/j.bbagen.2006.02.014
- Swinnen E, Wilms T, Idkowiak-Baldys J, Smets B, De Snijder P, Accardo S,Ghillebert R, Thevissen K, Cammue B, De Vos D, et al. The protein kinase Sch9 is a key regulator of sphingolipid metabolism in Saccharomyces cerevisiae. Mol Biol Cell 2014; 25:196-211; PMID: 24196832; http://dx.doi.org/10.1091/mbc.E13-06-0340
- Randez-Gil F, Sanz P, Entian KD, Prieto JA. Carbon source-dependent phosphorylation of hexokinase PII and its role in the glucose-signaling response in yeast. Mol Cell Biol 1998; 18:2940-8; PMID: 9566913
