ABSTRACT
Acetaldehyde, a primary metabolite of alcohol, forms DNA adducts and disrupts the DNA replication process, causing genomic instability, a hallmark of cancer. Indeed, chronic alcohol consumption accounts for approximately 3.6% of all cancers worldwide. However, how the adducts are prevented and repaired after acetaldehyde exposure is not well understood. In this report, we used the fission yeast Schizosaccharomyces pombe as a model organism to comprehensively understand the genetic controls of DNA damage avoidance in response to acetaldehyde. We demonstrate that Atd1 functions as a major acetaldehyde detoxification enzyme that prevents accumulation of Rad52-DNA repair foci, while Atd2 and Atd3 have minor roles in acetaldehyde detoxification. We found that acetaldehyde causes DNA damage at the replication fork and activates the cell cycle checkpoint to coordinate cell cycle arrest with DNA repair. Our investigation suggests that acetaldehyde-mediated DNA adducts include interstrand-crosslinks and DNA-protein crosslinks. We also demonstrate that acetaldehyde activates multiple DNA repair pathways. Nucleotide excision repair and homologous recombination, which are both epistatically linked to the Fanconi anemia pathway, have major roles in acetaldehyde tolerance, while base excision repair and translesion synthesis also contribute to the prevention of acetaldehyde-dependent genomic instability. We also show the involvement of Wss1-related metalloproteases, Wss1 and Wss2, in acetaldehyde tolerance. These results indicate that acetaldehyde causes cellular stresses that require cells to coordinate multiple cellular processes in order to prevent genomic instability. Considering that acetaldehyde is a human carcinogen, our genetic studies serve as a guiding investigation into the mechanisms of acetaldehyde-dependent genomic instability and carcinogenesis.
Introduction
In 2012, the World Health Organization (WHO) reported that approximately 5.9% of all deaths were associated with alcohol consumption. Chronic alcohol consumption also accounts for approximately 3.6% of all cancers worldwide; including those of the upper aerodigestive tract, liver, colorectum, and breast.Citation1-5 These correlations are most likely attributed to the carcinogenic effects of acetaldehyde, the primary metabolite of ethanol.Citation6 Acetaldehyde is highly reactive and forms adducts including N2-ethyl-2′-deoxyguanosine (N2-Et-dG) and 1,N2-propano-2′-deoxyguanosine (PdG). Although N2-Et-dG appears to have less impact on genomic integrity, PdG adducts allow generation of DNA-protein crosslinks (DPCs) and DNA interstrand crosslinks (ICLs). Both DPCs and ICLs have a propensity to disrupt the DNA replication process, causing DNA damage and genomic instability, hallmarks of cancers.Citation6
The Fanconi anemia (FA) pathway plays a critical role in the repair of ICLs,Citation7-10 although the role of this pathway in DPC repair has not yet been well understood.Citation11 Fanconi anemia is an inherited disease that is characterized by bone marrow failure and a strong predisposition to cancer.Citation12 At least 19 genes have been implicated in the FA pathway,Citation13 which is thought to coordinate at least 3 downstream repair processes; including homologous recombination (HR), nucleotide excision repair (NER), and translesion DNA synthesis (TLS). In response to crosslinking DNA damage, 8 FA proteins (FANCA, B, C, E, F, G, L, and M) form the FA core complex at the site of DNA damage, promoting ubiquitination of the FANCD2-FANCI complex. This ubiquitination initiates a signaling cascade, leading to the activation of downstream effector FA proteins FANCP/SLX4, FANCQ/XPF, FANCD1/BRCA1, and FANCO/RAD51C, altogether promoting the nucleolytic processing of crosslinking DNA damage, followed by DNA repair via homologous recombination.Citation7-10
Acetaldehyde appears to activate several DNA repair mechanisms. Studies have demonstrated that CHO cells deficient for the HR protein Rad51D show elevated sensitivity to acetaldehyde and display chromosome aberrations in response to acetaldehyde, whereas cells deficient for base excision repair (BER) and non-homologous end-joining (NHEJ) show subtle increases in chromosome aberration.Citation14 Interestingly, cells that lack FANCQ/XPF or FANCG also show significant levels of chromosomal aberrations when cells are treated with acetaldehyde.Citation14,15 These findings indicate that the FA pathway plays a critical role in repair of acetaldehyde-mediated DNA adducts. Indeed, FANCD2 is monoubiquitinated in response to ethanol or aldehyde treatment in human cells.Citation16,17 Additionally, mice defective for both aldehyde dehydrogenase and the FA pathway show developmental defects, bone marrow failure, and acute leukemia in response to alcohol.Citation18 As adults, these mice develop aplastic anemia, probably due to failures in haematopoietic stem cell function.Citation19 Such phenotypes correlate with elevated levels of acetaldehyde-dependent DNA damage in these mice, indicating the importance of the FA pathway as a cytoprotective mechanism against acetaldehyde.
Aforementioned studies have suggested roles of various DNA repair pathways in acetaldehyde tolerance. However, since acetaldehyde exposure can lead to a variety of lesions, it is challenging to understand cytoprotective mechanisms against acetaldehyde toxicity.Citation6 In addition, it is often difficult to compare multiple studies that have used various mammalian cell lines with different genetic profiles. In an effort to understand the cellular response against acetaldehyde, a genome-wide screening was performed using the budding yeast Saccharomyces cerevisiae, an important and genetically tractable model organism.Citation20 This screening revealed the critical roles of the pentose phosphate pathway and oleic acid biosynthesis; as well as aldehyde dehydrogenases ALD3 and ALD6 in acetaldehyde tolerance. This study also identified 2 DNA repair-related genes, MSH1 and RAD14, which are involved in mitochondrial DNA repair and NER, respectively. However, this study does not mention the role of aldehyde dehydrogenases and DNA repair proteins in the protection of cells against genomic instability.Citation20 Furthermore, although acetaldehyde appears to cause DNA damage and activates Pso2 nuclease-dependent DNA repair in S. cerevisiae,Citation21,22 acetaldehyde-dependent DNA repair mechanisms have not been well characterized in this organism.
While powerful genetic toolkits are available in S. cerevisiae, the genetic variations documented in this model organism have made interpretations of biological results relatively complicated.Citation23-25 In contrast, it has been regarded that nearly all laboratory strains are isogenic in another well-characterized yeast species, the fission yeast Schizosaccharomyces pombe. This is due to the fact that all laboratory S. pombe strains are derived from the same standard strains, allowing for straightforward genetic interpretations.Citation26-28 Furthermore, S. pombe appeared to have experienced less evolutionary changes since it diverged from the common ancestor. Thus, similarities between S. pombe and mammals are generally considered to be higher than similarities between S. cerevisiae and mammals.Citation27,29 Therefore, in this study, we dissect the genetic pathways that are required for acetaldehyde tolerance by using S. pombe, in which multiple isogenic strains can be used. We show that acetaldehyde causes increased levels of DNA damage when cells are deficient for aldehyde dehydrogenase. Acetaldehyde appears to cause replication fork collapse, which is prevented by the replication fork protection complex and the ATR-dependent cell cycle checkpoint. We also show genetic evidence that the FA pathway, HR, and NER have primary roles in preventing or repairing acetaldehyde-mediated DNA adducts, while base excision repair (BER) and TLS also contribute to cellular tolerance to acetaldehyde. Since acetaldehyde may produce different types of adducts and DNA damage, we will discuss potential DNA lesions that can be repaired by different DNA repair pathways. Therefore, our genetic study serves as a guiding investigation into understanding the cytoprotective mechanisms against acetaldehyde-mediated genomic instability.
Results
Genes involved in acetaldehyde detoxification in S. pombe
In order to understand how cells tolerate acetaldehyde toxicity, we first searched the S. pombe genome for genes that potentially encode aldehyde dehydrogenases. This bioinformatics analysis identified 2 genes that are closely related to human aldehyde dehydrogenase ALDH2, the major enzyme required for acetaldehyde detoxification.Citation30 The SPAC9E9.09c protein, which consists of 503 amino acids, showed 55% identity and 72% similarity to ALDH2 (query coverage, 91%). The other protein, SPAC922.07c, which consists of 469 amino acids, showed 48% identity and 67% similarity to ALDH2 (query coverage, 92%). In addition, we found 3 additional proteins that have more than 30% identity at amino acid level over 90% of the ALDH2 query amino acid sequence. These are: SPCC550.10 (39% identity, 57% similarity), SPAC139.05 (37% identity, 54% similarity), SPAC1002.12c (36% identity, 53% similarity). SPAC139.05 and SPAC1002.12c are annotated as succinate-semialdehyde dehydrogenases in PomBase (http://www.pombase.org), the fission yeast database. Based on their amino acid sequence similarities to ALDH2, the 3 most similar proteins, SPAC9E9.09c, SPAC922.07c, and SPCC550.10 were designated atd1, atd2, and atd3 (putative AceTaldehyde Dehydrogenase), respectively.
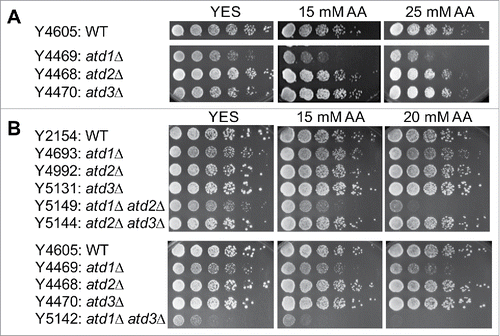
To determine whether Atd1, Atd2 and Atd3 are required for acetaldehyde detoxification in S. pombe, we examined acetaldehyde sensitivity of their corresponding deletion mutants. As described in the Materials and Methods section, acetaldehyde is highly volatile and it is difficult to control its concentrations in the growth medium. Although the same strains show varied acetaldehyde sensitivity levels in different experiments, the relative sensitivity among different mutants display similar trends. As shown in , atd1Δ cells showed hypersensitivity to acetaldehyde, indicating the major role of Atd1 in acetaldehyde detoxification. In contrast, atd2Δ and atd3Δ cells were not significantly sensitive to acetaldehyde. In order to determine whether Atd2 and Atd3 have a minor role in acetaldehyde detoxification, we constructed S. pombe strains lacking 2 ALDH2-related genes. Accordingly, atd1Δ atd2Δ, atd1Δ atd3Δ, and atd2Δ atd3Δ double mutants were generated. atd1Δ atd2Δ and atd1Δ atd3Δ cells were significantly more sensitive than atd1Δ single mutant cells, whereas atd2Δ atd3Δ showed no detectable acetaldehyde sensitivity (). In addition, atd1Δ atd3Δ cells showed significant growth defects even in the absence of acetaldehyde (). We also constructed atd1Δ atd2Δ atd3Δ triple mutants. However, growth and acetaldehyde sensitivity of these cells were not consistent, probably due to the accumulation of extra mutations that could arise during genetic cross and proliferation; they had low fitness during the early division phase, however, they improved fitness after multiple divisions. Nevertheless, our results show that Atd1 is most important for acetaldehyde detoxification, while both Atd2 and Atd3 have minor roles in acetaldehyde detoxification.
Figure 1. Atd1, Atd2 and Atd2 are involved in acetaldehyde detoxification. (A, B) Five-fold serial dilutions of cells of the indicated genotypes were grown on YES agar medium containing 0, 15, and 25 mM acetaldehyde. Cells were incubated for 3 to 5 d at 30°C. As described in the methods section, due to the difficulty in preventing acetaldehyde evaporation, the initial concentrations of acetaldehyde in the medium are shown. Representative images of repeat experiments are shown.
Acetaldehyde causes DNA damage, which is prevented by Atd1 and Atd2
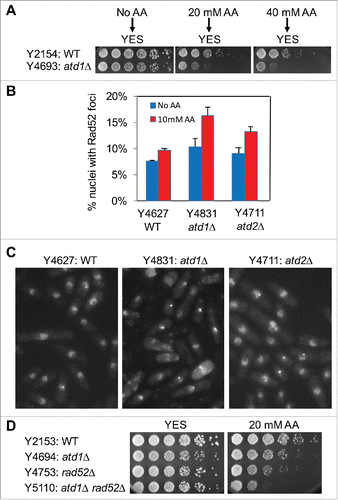
We then investigated whether acetaldehyde causes DNA damage in S. pombe cells. Wild-type, atd1Δ, and atd2Δ cells were engineered to express Rad52 recombinase fused to yellow fluorescent protein (Rad52-YFP) from the rad52 gene locus. Rad52 is required to repair DNA and recruited to sites of DNA damage, forming DNA repair foci.Citation31,32 To monitor Rad52 DNA repair foci, we needed to treat cells with acetaldehyde in liquid culture. As shown in , atd1Δ cells were sensitive to acute acetaldehyde treatment. Importantly, acetaldehyde induced a mild increase in Rad52 foci formation in wild-type nuclei, indicating that acetaldehyde causes DNA damage (). Importantly, we observed significantly elevated levels of DNA damage in atd1Δ cells in response to acetaldehyde (), which is consistent with their sensitivity to acetaldehyde (). Although atd2Δ cells failed to show sensitivity to acetaldehyde (), these cells also displayed significant accumulation of Rad52-YFP foci when cells were treated with acetaldehyde (), suggesting that Atd2 also plays a role in detoxification of acetaldehyde. Consistent with these results, rad52Δ atd1Δ cells showed higher acetaldehyde sensitivity than either single mutant cells, indicating the importance of Rad52 recombinase in the repair of acetaldehyde-mediated DNA damage ().
Figure 2. Acetaldehyde induces DNA damage in the absence of acetaldehyde dehydrogenase. (A) Cells were incubated for 3 h in YES liquid medium containing the indicated concentrations of acetaldehyde. Then, 5-fold serial dilutions of cells were prepared, plated on YES agar medium without acetaldehyde, and incubated for 3 to 5 d at 30°C. (B) Cells of the indicated genotypes were engineered to express Rad52-YFP, grown in YES medium at 25°C until mid-log phase, and treated with 10 mM acetaldehyde (AA) for 2 h. The percentages of nuclei with at least one Rad52-YFP focus are shown. At least 200 cells were counted for each strain. Error bars correspond to standard errors of the mean (SEM) obtained from 3 independent experiments. (C) Representative images of the acetaldehyde-treated cells used in B are shown. (D) Five-fold serial dilutions of cells of the indicated genotypes were grown on YES agar medium containing 0 and 20 mM acetaldehyde. Cells were incubated for 3 to 5 d at 30°C.
Involvement of replication fork protection and cell cycle checkpoints in response to acetaldehyde
Acetaldehyde forms DNA adducts and may interfere with the replication process. Therefore, we determined acetaldehyde sensitivity of cells deficient for Swi1, a component of the replication fork protection complex (FPC). Swi1 plays a critical role in the stabilization of stalled replication forks created in response to DNA damage and adducts, which can interfere with the DNA replication process.Citation33,34 swi1Δ cells were slightly sensitive to acetaldehyde at the concentration we used, although they were highly sensitive to replication-stressing agents including camptothecin (CPT) and hydroxyurea (HU) as reported previouslyCitation31,35 (). Interestingly, swi1Δ cells also showed sensitivity to cisplatin, which is an ICL-inducing agent that induces replication fork breakage (), suggesting a role of Swi1 in preventing or repairing crosslinking DNA damage during DNA replication. We reasoned that acetaldehyde-mediated adducts are efficiently repaired in swi1Δ cells because cells activate cell cycle checkpoints to allow time for DNA repair. Therefore, to inactivate the checkpoints, we deleted the rad26 gene, which encodes S. pombe ATRIP that works together with Rad3ATR and is essential for activation of cell cycle checkpoints that arrest the cell cycle in response to DNA damage.Citation36 Although rad26Δ cells were not sensitive to acetaldehyde at the concentrations we used, swi1Δ rad26Δ cells were much more sensitive than either single mutant (). Similar effects were observed when swi1Δ rad26Δ cells were exposed to CPT, HU and cisplatin, all of which compromise replication fork integrity (). These results suggest that acetaldehyde induces replication abnormalities that activate the ATR-dependent cell cycle checkpoint.
Figure 3. Acetaldehyde causes replication stress that activates the ATR-dependent checkpoint. (A, B) Five-fold serial dilutions of the indicated mutants were incubated on YES agar medium supplemented with the indicated drugs for 3 to 5 d at 30°C. Representative images of repeat experiments are shown. (C) PFGE analysis of chromosome samples prepared from wild-type and swi1Δ cells. Cells were grown in the presence of 20 mM acetaldehyde for 3 h and returned to fresh medium. Cells were collected at 0 (AA), 2, and 4 h after acetaldehyde treatment and processed for PFGE. Chromosomes prepared from exponentially growing cells are also analyzed (Log). Representative results of repeat experiments are shown. swi1Δ cells are known to display shorter chromosome III due to the elevated recombination rate at rDNA repeatsCitation50,60.
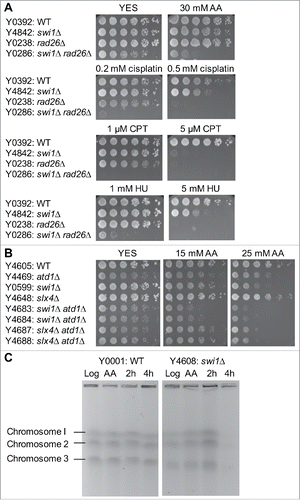
Swi1's involvement in acetaldehyde response suggests that DNA damage occurs at the replication fork. Consequently, swi1Δ atd1Δ cells were much more sensitive to acetaldehyde than either single mutant (), suggesting a role of Swi1 in replication recovery in response to acetaldehyde accumulation. To test this possibility, we analyzed chromosomal DNA isolated from wild-type and swi1Δ mutant cells and investigated whether acetaldehyde causes DNA replication problems. For this purpose, we treated cells with 20 mM acetaldehyde for 3 h (AA) and returned to fresh medium without acetaldehyde for 2 h and 4 h, and then prepared chromosome samples. We also prepared chromosomes from exponentially growing cells without acetaldehyde treatment (Log). These chromosomes were analyzed by PFGE, which allows only fully replicated chromosomes to migrate into the gel.Citation37 Intact chromosomes from exponentially growing wild-type and swi1Δ cells were clearly visible on the gel (). Wild-type chromosomes were still readily detected after acetaldehyde treatment. In contrast, the levels of chromosomes from acetaldehyde-treated swi1Δ cells were much lower than those from wild-type cells at 4 h after acetaldehyde treatment (). This result suggests that acetaldehyde induces DNA damage at the replication fork and affects fork recovery when Swi1 is not present.
NER and HR processes are critical for cellular tolerance to acetaldehyde
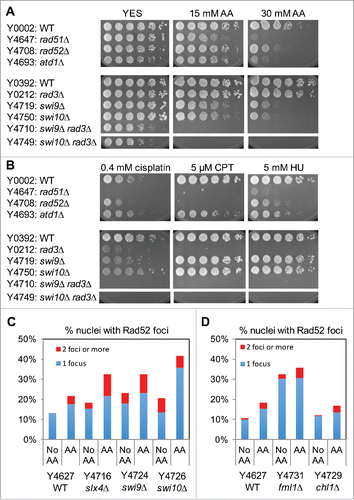
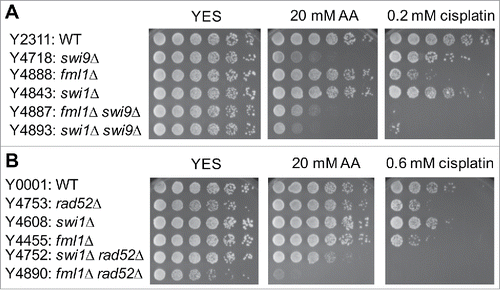
Acetaldehyde is thought to induce various DNA adducts including ICLs.Citation6 ICLs activate the Fanconi Anemia (FA) DNA repair pathway, which is thought to coordinate downstream DNA repair processes, including NER, TLS, and HR.Citation7-10 S. pombe has several proteins that are potential orthologs of FA proteins, including Fml1FANCM, Chl1FANCJ, Slx4FANCP, Rad51FANCO/Rad51, and Swi9FANCQ/XPF, although roles of these proteins in acetaldehyde response are not known. It is important to note that Rad51 is involved in HR and that Swi9 is involved in nucleolytic process during NER.Citation7 Among the deletion mutants of FA-related factors, only swi9Δ and rad51Δ cells showed strong sensitivity to acetaldehyde (), suggesting that HR and NER process are essential for the repair of acetaldehyde-mediated DNA damage in S. pombe. Consistently, deletion of another HR factor, Rad52, also rendered S. pombe cells highly sensitive to acetaldehyde (). In addition, loss of Swi10ERCC,Citation1 which is known to form a nuclease complex with Swi9FANCQ/XPF, also led to hypersensitivity to acetaldehyde (). Furthermore, swi9Δ and swi10Δ cells showed significant accumulation of Rad52-YFP DNA repair foci in response to acetaldehyde (). These results suggest that the Swi9-Swi10-dependent nucleolytic process is required for preventing accumulation of acetaldehyde-mediated DNA damage. Interestingly, swi9Δ and swi10Δ cells had increased levels of DNA damage even in the absence of acetaldehyde (), suggesting a role of Swi9-Swi10 in preventing spontaneous DNA damage generated during cell metabolism.
Figure 4. NER and HR play critical roles in repair of acetaldehyde-induced DNA damage. (A, B) Five-fold serial dilutions of the indicated mutants were incubated on YES agar medium supplemented with the indicated drugs for 3 to 5 d at 30°C. NER and HR mutants show strong sensitivity to acetaldehyde. Representative images of repeat experiments are shown. (C, D) Rad52-YFP foci analysis of the indicated cells was performed as described in . The percentages of nuclei with at least one Rad52-YFP focus and 2 or more foci are shown. Representative images of repeat experiments are shown.
Acetaldehyde forms DNA adducts that are removed by NER
In order to understand the nature of acetaldehyde-mediated DNA adducts, we also treated NER and HR mutants with other DNA damaging agents, including CPT, HU and cisplatin. CPT traps topoisomerase I on DNA by forming a single-strand nick, leading to replication fork breakage during DNA synthesis. HU depletes the dNTP pool by inhibiting ribonucleotide reductase and causes arrest of the replication fork. Cisplatin forms DNA adducts including ICLs that lead to replication fork breakage. As expected from previous studies,Citation38,39 HR (rad51Δ and rad52Δ) mutants were highly sensitive to CPT, HU, and cisplatin (), indicating the requirement of HR in repair of various types of DNA damage. However, NER mutants, swi9Δ and swi10Δ cells were not sensitive to CPT or HU but were highly sensitive to cisplatin (). Considering that swi9Δ and swi10Δ cells show hypersensitivity to acetaldehyde and cisplatin, our results suggest that acetaldehyde produces DNA adducts that are similar to those generated by cisplatin and that NER plays a critical role in repair of acetaldehyde-mediated DNA adducts.
Furthermore, swi9Δ and swi10Δ cells had synergistic genetic interaction with rad3Δ cells; swi9Δ rad3Δ and swi10Δ rad3Δ cells were much more sensitive to acetaldehyde than corresponding single mutants (). Taken together, our results suggest that acetaldehyde-mediated DNA adducts activate the Rad3ATR-dependent cell cycle checkpoint pathway to allow time for DNA repair by NER.
Acetaldehyde may form DPC in fission yeast
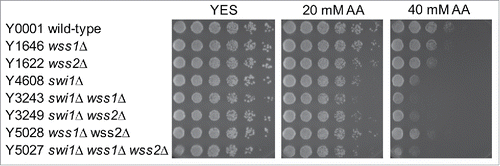
Next, we tested whether acetaldehyde exposure results in DNA-protein crosslinking. Although the mechanism of DPC repair remains largely elusive, recent studies reported that, in S. cerevisiae, the metalloprotease Wss1 removes proteins crosslinked on DNA during DNA replication to facilitate DPC repair.Citation40 This proteolytic process is suggested to occur at the replication fork.Citation11,40 Accordingly, we found that the S. pombe genome contains 2 Wss1-related protein-coding genes; we named them wss1 (SPAC521.02) and wss2 (SPCC1442.07c). Although single mutant wss1Δ and wss2Δ cells only had mild sensitivity to acetaldehyde, wss1Δ wss2Δ, swi1Δ wss1Δ, swi1Δ wss2Δ, and swi1Δ wws1Δ wss2Δ cells were more sensitive than corresponding single mutants (). These results suggest that acetaldehyde may also form DPCs, whose repair is facilitated by Wss1-related proteases, although we cannot exclude the possibility that Wss1-related proteins have non-proteolytic roles in replication fork maintenance when cells are treated with acetaldehyde.
Figure 5. Wss1-like proteases are involved in cellular tolerance to acetaldehyde. Five-fold serial dilutions of the indicated mutants were incubated on YES agar medium supplemented with the indicated amounts of acetaldehyde (AA) for 3 to 5 d at 30°C. wss1Δ and swi1Δ have additive genetic interaction in acetaldehyde sensitivity. Representative images of repeat experiments are shown.
Fml1FANCM, Chl1FANCJ, and Slx4FANCP augment DNA repair after acetaldehyde exposure
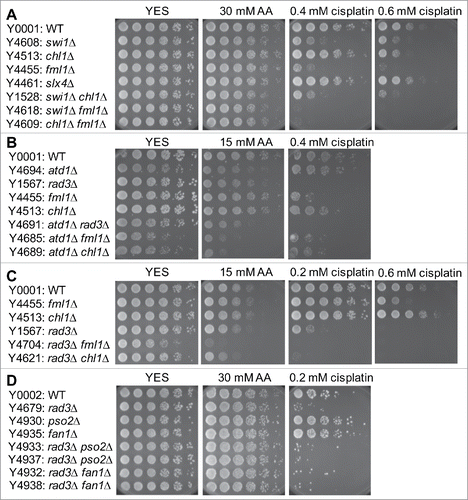
In contrast to the strong acetaldehyde sensitivity of cells deficient for Rad51, Rad52, and Swi9-Swi10, other FA mutant cells, including fml1Δ, chl1Δ, and slx4Δ cells, were not significantly sensitive to acetaldehyde, although fml1Δ and chl1Δ cells were sensitive to an ICL-inducing agent, cisplatin (). To further investigate the role of Fml1, Chl1, and Slx4 in acetaldehyde tolerance, we crossed atd1Δ cells with fml1Δ, chl1Δ, and slx4Δ cells to construct double deletion mutants. atd1Δ fml1Δ, atd1Δ chl1Δ, and atd1Δ slx4Δ cells were more sensitive to acetaldehyde than corresponding single mutants (), suggesting that Fml1, Chl1, and Slx4 play a role in DNA damage prevention in response to acetaldehyde. Consistently, slx4Δ cells showed elevated levels of Rad52-YFP foci in response to acetaldehyde (), indicating a role of Slx4 in preventing DNA damage in response to acetaldehyde. Interestingly, fml1Δ cells accumulated DNA damage even in the absence of acetaldehyde, although acetaldehyde treatment did not significantly enhance DNA damage in fml1Δ cells (). The level of DNA damage in chl1Δ cells was similar to that in wild-type cells (). However, rad3Δ fml1Δ and rad3Δ chl1Δ cells were more sensitive to acetaldehyde than corresponding single mutant (), indicating that fml1Δ and chl1Δ cells require the ATR-dependent DNA damage response pathway to survive in the presence of acetaldehyde. Nevertheless, our results indicate that FA-related proteins, Fml1, Chl1, and Slx4 have ancillary roles in the repair of acetaldehyde-mediated DNA damage.
Figure 6. Involvement of the FA pathway in acetaldehyde-mediated DNA damage response. Five-fold serial dilutions of the indicated mutants were incubated on YES agar medium supplemented with the indicated drugs for 3 to 5 d at 30°C. (A, B, C) FA mutants show significant acetaldehyde sensitivity when combined with rad3Δ. (D) pso2Δ and fan1Δ nuclease mutants failed to show significant acetaldehyde sensitivity. Representative images of repeat experiments are shown.
Swi9XPF/FANCQ-Swi10ERCC1 may function as the major endonuclease to prevent acetaldehyde-dependent DNA damage
Several endonucleases are implicated in processing cross-linked DNA. These nucleases include Swi9XPF/FANCQ-Swi10ERCC1, Slx1-Slx4FANCP, Fan1FAN1, and Pso2SNM1 in S. pombe. Although swi9Δ and swi10Δ cells were strongly sensitive to acetaldehyde (), slx4Δ, fan1Δ and pso2Δ cells were not sensitive (). Furthermore, rad3Δ fan1Δ and rad3Δ pso2Δ double mutants displayed no significant sensitivity (). However, other FA related mutations showed additive acetaldehyde sensitivity when combined with rad3Δ; rad3Δ swi9Δ, rad3Δ swi10Δ, rad3Δ fml1Δ, rad3Δ chl1Δ were more sensitive to acetaldehyde than corresponding single mutants as described above (). Therefore, our results suggest that Swi9XPF/FANCQ-Swi10ERCC1 is the major endonuclease that prevents acetaldehyde-dependent DNA damage in S. pombe.
NER acts downstream of Fml1FANCM, while HR and Swi1 function independently of Fml1FANCM during acetaldehyde DNA damage response in S. pombe
Studies in human cells suggest that FANCM functions during early steps of crosslink repair to facilitate downstream DNA repair processes, including HR and NER,Citation7 although FANCM appears to have a role in genome maintenance independent of the FA pathway.Citation41,42 In S. pombe, Fml1 is shown to play a role in promoting HR at stalled replication forks.Citation43 Importantly, our present studies identified NER and HR as the major DNA repair mechanisms involved in acetaldehyde response. To understand the relationship between the FA proteins and downstream DNA repair processes such as NER and HR, we performed epistasis analyses. fml1Δ swi9Δ cells were as sensitive to acetaldehyde as swi9Δ single mutant cells (). Therefore, swi9Δ is epistatic to fml1Δ for acetaldehyde sensitivity, suggesting that Fml1FANCM (FA protein) and Swi9FANCQ (NER) operate in the same pathway for acetaldehyde response or Swi9 is more critical for acetaldehyde tolerance than Fml1. Strikingly, fml1Δ rad52Δ cells were much more sensitive to acetaldehyde than either single mutant (). We also noticed that swi1Δ swi9Δ double mutant cells were more sensitive to acetaldehyde than either single mutant (). Furthermore, swi1Δ fml1Δ cells have higher acetaldehyde sensitivity than either single mutant (). Therefore, our results suggest that Fml1FANCM functions independently of HR and Swi1-dependent replication fork protection in acetaldehyde-mediated stress response.
Figure 7. Epistasis analysis of FA, NER, HR and swi1Δ mutants. Five-fold serial dilutions of the indicated mutants were incubated on YES agar medium supplemented with the indicated drugs for 3 to 5 d at 30°C. (A) fml1Δ shows epistatic interaction with swi9Δ but not with swi1Δ. (B) fml1Δ has additive or synergistic genetic interaction with rad52Δ. Representative images of repeat experiments are shown.
Involvement of base excision repair and translesion DNA synthesis in acetaldehyde response
Aforementioned results indicate that HR and NER have critical roles in the repair of acetaldehyde-dependent DNA damage. Because the FA pathway is thought to facilitate TLS, besides HR and NER,Citation7 we examined sensitivities of mutants compromised for TLS. Deletion of individual TLS polymerases, including Eso1 (pol η), Kpa1/DinB (pol κ), and Rev3 (Pol ζ) failed to confer sensitivity to acetaldehyde (), although rev3Δ kpa1Δ eso1Δ triple mutant cells showed mild acetaldehyde sensitivity (). Interestingly, rad3Δ eso1Δ double mutants showed significant acetaldehyde sensitivity, while rad3Δ rev3Δ kpa1Δ eso1Δ quadruple mutant cells showed a dramatic increase in acetaldehyde sensitivity (). These results indicate that these TLS polymerases, especially Eso1 (pol η), have critical roles in repair of acetaldehyde-mediated DNA damage.
Figure 8. Involvement of TLS and BER in acetaldehyde-mediated DNA damage response. Five-fold serial dilutions of the indicated mutants were incubated on YES agar medium supplemented with the indicated drugs for 3 to 5 d at 30°C. (A) Single TLS mutants failed to show acetaldehyde and cisplatin sensitivity. (B) TLS polymerases become more important for the cellular tolerance to acetaldehyde when Rad3 is absent. (C) BER is involved in acetaldehyde-mediated DNA damage response. Representative images of repeat experiments are shown.

We also investigated the effect of BER deficiency on acetaldehyde tolerance. Cells deficient for Endonuclease III Nth1 (nth1Δ) or AP-endonuclease Apn2 (apn2Δ) were not significantly sensitive to acetaldehyde (). However, rad3Δ nth1Δ and rad3Δ apn2Δ double mutant cells were more sensitive than corresponding single mutants (). Interestingly, such additive genetic interactions were not observed in response to cisplatin (), which causes ICLs; suggesting that in addition to ICLs, acetaldehyde causes DNA adducts that are repaired by the BER pathway.
Discussion
In this report, for the first time, we characterized aldehyde dehydrogenase related genes in S. pombe. We found that Atd1 is a major aldehyde dehydrogenase, while Atd2 and Atd3 also play minor roles in acetaldehyde detoxification. We also found that Atd1 and Atd2 prevent DNA damage in response to acetaldehyde. Importantly, acetaldehyde is one of the major environmental carcinogensCitation6; it is of high significance to elucidate how acetaldehyde-mediated DNA damage is repaired or prevented. Therefore, in this study, using powerful genetic approaches available in S. pombe, we dissected roles of DNA repair-related genes in the preservation of genomic integrity in response to acetaldehyde. Our genetic investigation revealed that acetaldehyde causes a variety of DNA damage, which induces multiple genome maintenance proteins involved in fork protection, cell cycle checkpoint, proteolysis, and multiple DNA repair mechanisms including NER, HR, TLS, BER, and the FA pathway.
Our studies indicate that acetaldehyde forms DNA adducts that interfere with the DNA replication process. In the absence of Swi1, an FPC subunit required for replication fork protection, cells experience difficulties in completing DNA replication and activate the Rad3ATR-dependent cell cycle checkpoint for survival (). Therefore, stalled replication forks at acetaldehyde-dependent DNA adducts need to be stabilized by the FPC to prevent fork breakage.
Once the fork is stalled, DNA adducts need to be removed in order to resume the DNA replication process. Then, how might the DNA adducts be removed? It may depend on the type of DNA adducts. Studies suggest that acetaldehyde can cause ICLs, DPCs, and monoadducts.Citation6 Consistent with a role of the FA pathway in ICL repair,Citation7 mutations in FA proteins rendered S. pombe cells highly sensitive to acetaldehyde when combined with rad3 deletion. Furthermore, our studies also suggest the involvement of Wss1-like proteases in cellular tolerance to acetaldehyde, supporting the notion that these proteases digest proteins crosslinked on DNA.Citation40 It is important to note that these proteases appear to leave a small peptide still crosslinked on DNA.Citation11,40 As in the case of ICLs and monoadducts, these peptides may still need to be removed via nucleolytic processes in order to complete DNA replication (). Among possible structure-specific endonucleases that may process cross-linking adducts, loss of Swi9 or Swi10 alone led to significant sensitivity to acetaldehyde (). The Swi9-Swi10 complex is homologous to the XPF-ERCC endonuclease that is proposed to process stalled forks at DNA adducts.Citation7 Failure in this process may lead to non-specific DNA breakage at DNA adducts, leading to Rad52 DNA repair foci. Consistently, swi9 and swi10 cells accumulated Rad52 DNA repair foci in response to acetaldehyde ().
Figure 9. Model of cellular responses to prevent acetaldehyde-dependent DNA damage. For details, see text.
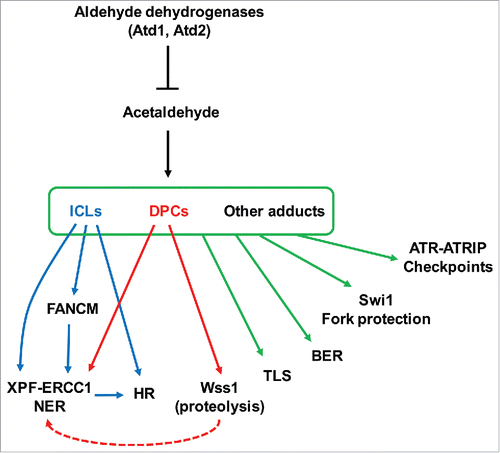
In mammalian cells, FANCM is proposed to recognize stalled replication forks, leading to the recruitment of the FA core complex that functions as an E3-ubiquitin ligase for the FANCD2-FANCI complex. This ubiquitination seems to trigger efficient recruitment of nucleases and other enzymes required for processing stalled replication forks.Citation7 However, the fission yeast genome does not possess genes that encode the components of the FA core complex or FANCD2-FANCI.Citation28 Therefore, it seems reasonable to suggest that Fml1, the S. pombe FANCM ortholog, is critical for efficient recruitment of nucleolytic enzymes such as Swi9XPF-Swi10ERCC1 (). Consistently, our genetic studies revealed that fml1Δ is epistatic to swi9Δ, suggesting that Fml1FANCM and Swi9XPF-Swi10ERCC1 function in the same pathway ().
Because XPF-ERCC1-dependent DNA cleavage may generate a double-strand break activating HR, it is proposed that HR functions downstream of FANCM.Citation7 Studies in S. pombe also described a role of Fml1 in HR.Citation43,44 However, fml1Δ rad52Δ showed much higher acetaldehyde sensitivity than either single mutant. This result suggests that, although HR may still function downstream of Fml1FANCM as previously suggested in mammalian cells and S. pombe,Citation7,43,44 HR is also initiated in a manner independent of the FA pathway (). Considering that fml1Δ cells failed to show significant acetaldehyde sensitivity, it is highly possible that Swi9XPF-Swi10ERCC1 is recruited to DNA adducts in an Fml1FANCM-independent manner. This would result in double-strand breaks and promote HR at DNA adducts. Therefore, it appears that Fml1FANCM has only a minor role in acetaldehyde-dependent DNA repair. In contrast, fml1Δ cells were highly sensitive to cisplatin, an ICL-causing agent. It is possible that ICL may only constitute a small portion of DNA adducts induced by acetaldehyde. Interestingly, swi9Δ and swi10Δ cells showed significant sensitivity to both cisplatin and acetaldehyde (). These results are consistent with the notion that acetaldehyde-mediated DNA adducts are processed by the Swi9XPF-Swi10ERCC1 endonuclease. Considering the involvement of Wss1-like proteases in acetaldehyde response, our results also suggest that acetaldehyde induces DPCs, which are repaired by Swi9XPF-Swi10ERCC1 in a manner independent of Fml1FANCM (). This notion is supported by an experiment using a site-specific DPC, where it was demonstrated that FANCD2-FANCI depletion had no effect on DPC repair in Xenopus egg extracts.Citation11
The FA pathway is also proposed to promote TLS.Citation7 Although single TLS polymerase mutants failed to show acetaldehyde sensitivity, triple TLS mutant cells (eso1Δ kpa1Δ rev3Δ) were significantly sensitive to acetaldehyde. Because rad3Δ rev3Δ double mutants failed to show additive acetaldehyde sensitivity compared to either single mutant (), Rev3 (DNA polymerase ζ) may not play a major role in acetaldehyde response. However, rad3Δ eso1Δ and rad3Δ kpa1Δ cells were significantly more sensitive than respective single mutants (), suggesting that Eso1 (DNA polymerase η) and Kpa1 (DNA polymerase κ) are required for repair of acetaldehyde-mediated DNA damage. Particularly, Eso1 seems to have a major role in this process because rad3Δ eso1Δ cells were more sensitive to acetaldehyde than rad3Δ kpa1Δ cells (). Consistently, DNA polymerases η and ζ are recruited to DNA damage sites by FANCD2 in human cells and Xenopus egg extracts, respectively.Citation45,46 Further investigations are warranted to further address roles of TLS polymerases at acetaldehyde-mediated DNA adducts.
In summary, our work has provided a comprehensive analysis of DNA repair pathways required for cellular tolerance in response to acetaldehyde in fission yeast. Acetaldehyde induces a variety of cytoprotective mechanisms to reduce the burden of acetaldehyde-dependent cytotoxicity. Such a multifactorial nature of acetaldehyde response warrants future investigation using systems-level approaches to understand the network of cellular response mechanisms to prevent mutagenic effects of acetaldehyde. Furthermore, considering that fundamental cellular processes are highly conserved between yeast and humans, our studies serve as a guiding investigation to shape future studies in humans.
Materials and methods
General techniques
The methods used for genetic and molecular biology analyses of fission yeast have been described previously.Citation47,48 PCR amplification of DNA was performed using Ex Taq DNA polymerase (TaKaRa, Ohtsu, Japan), and the accuracy of PCR products were confirmed by DNA sequencing analysis.
S. pombe strains
The S. pombe strains used in this study were constructed using standard techniques,Citation47,48 and their genotypes and sources are listed in Supplemental Table S1. To detect Rad52-YFP DNA repair foci (originally termed Rad22-YFP in S. pombe), pJK210-Rad52-YFPCitation49,50 was digested by AflII and inserted at the rad52 locus of various S. pombe strains. atd2Δ (atd2::hphMX6), rad52Δ (rad52::hphMX6), wss1Δ (wss1::hphMX6), and wss2Δ (wss2::kanMX6) were constructed by a PCR-based method,Citation51 to replace the open reading frame with the hphMX6 or kanMX6 gene. atd3Δ (atd3::natMX6) was generated by a one-step marker switch methodCitation52 using atd3::kanMX4.
Mutations and epitope-tagged genes have been previously described for swi1Δ (swi1::kanMX6),Citation31 rad26Δ (rad26::ura4+),Citation53 slx4Δ (slx4::kanMX6),Citation54 rad51Δ (rad51::kanr),Citation55 swi9Δ (swi9::ura4+),Citation56 swi10Δ (swi10::ura4+),Citation57 rad3Δ (rad3::kanMX6),Citation35 chl1Δ (chl1::hphMX6),Citation35 fml1Δ (fml1::natMX4)Citation44, eso1Δ (eso1::kanMX6), kap1Δ (kap1::bleMX6), rev3Δ (rev3::hphMX6),Citation58 nth1Δ (nth1::ura4+), apn2Δ (apn2::ura4+),Citation59 and rad52-YFP (rad52-YFP:ura4+).Citation50 SPAC22A12.01cΔ (pso2::kanMX4) and SPBC146.06cΔ (fan1::kanMX4), SPAC9E9.09cΔ (atd1::kanMX4), SPAC922.07cΔ (atd2::kanMX4), and SPCC550.10Δ (atd3::kanMX4) cells were obtained from Bioneer Inc.
Drug sensitivity assays
Drug sensitivity assays, except for acetaldehyde sensitivity assay, were performed as described in our previous studies.Citation37 Acetaldehyde is highly volatile, with a boiling point of 20.2°C. To prevent acetaldehyde evaporation, acetaldehyde was kept at −20°C before use and diluted in ice-cold water. Pipet tips and tubes used for preparation of acetaldehyde solutions were also kept at 4°C. The appropriate amount of acetaldehyde solution was spread onto cold agar plates in a room maintained at 4°C. Agar plates were then sealed by Parafilm and stored at 4°C for 16 to 20 h to allow acetaldehyde to be absorbed into agar medium.
Fivefold serial dilutions of exponentially growing S. pombe cells were plated on acetaldehyde-containing plates, and the plates were sealed and incubated at 32°C for 3 to 5 d to allow cell growth. With this method, wild-type S. pombe cells were able to grow in the presence of up to 40 mM of acetaldehyde. At 40 mM acetaldehyde, wild-type cells also started to cease growth. Therefore, 15 – 40 mM of acetaldehyde was used to test sensitivity of S. pombe cells. Since it is difficult to completely prevent acetaldehyde evaporation and to control accurate acetaldehyde concentration, the initial concentrations of acetaldehyde prepared in each experiment are indicated throughout this study.
Detection of Rad52-YFP DNA repair foci by fluorescent microscopy
Cells expressing Rad52-YFP from its own promoter were grown at 25°C in YES liquid medium until mid-log phase. This was done because cells grown at 25°C yield more stable YFP fluorescence with lower background signal. Cells were then treated with acetaldehyde in a sealed test tube for 2 h, and live-cell imaging analysis of Rad52-YFP was performed using an Olympus PROVIS AX70 microscope equipped with a Retiga EXi camera (QImaging, Surrey, BC, Canada). Images were acquired with iVision software (BioVision Technologies, Exton, PA) and analyzed with ImageJ software (National Institutes of Health, Bethesda, MD). At least 100 cells were counted for each experiment as previously described.Citation37
Pulsed-field gel electrophoresis
Exponentially growing cells were treated with 20 mM of acetaldehyde in a sealed test tube for 3 h, then returned to fresh medium without acetaldehyde for 4 h. Cells (2 × 108 cells) were then collected, processed for DNA preparation, and analyzed by pulsed-field gel electrophoresis as previously described in our earlier studies.Citation37
Data availability
All S. pombe strains (Supplemental Table S1) and oligonucleotide sequences used in this study are available upon request.
Abbreviations
| AA | = | acetaldehyde |
| BER | = | base excision repair |
| CPT | = | camptothecin |
| DPC | = | DNA-protein crosslink |
| FA | = | Fanconi anemia |
| FPC | = | fork protection complex |
| HR | = | homologous recombination |
| HU | = | hydroxyurea |
| ICL | = | interstrand crosslink |
| NER | = | nucleotide excision repair |
| TLS | = | translesion synthesis |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary files
Download PDF (56 KB)Acknowledgments
We thank Drs. Shogo Ikeda, Matthew O'Connell, Paul Russell, Matthew Whitby, and National BioResource Project Japan for S. pombe strains. We also thank Dr. Toru Nakamura for his comments, and Jordan Asam, Prajakti Awade, Maxwell Boamah, and Kody Schneider for technical assistance. Members of the Noguchi laboratory are thanked for their support and encouragement.
Funding
This work was supported by Drexel University College of Medicine, the Aging Initiative at Drexel University College of Medicine (to E.N.), Drexel STAR Undergraduate Scholars Program (to V.A. and G.G.), Raptor Pharmaceuticals (to H.N.), and NIH (K26RR032714 to H.N. and P30-ES013508 University of Pennsylvania Center of Excellence in Environmental Toxicology).
References
- WHO. Global status report on alcohol and health 2014. World Health Organization: http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf
- Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J. The burden of cancer attributable to alcohol drinking. Int J Cancer 2006; 119:884-7; PMID:16557583; http://dx.doi.org/10.1002/ijc.21903
- Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C. Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012. Alcohol Alcohol 2012; 47:204-12; PMID:22459019; http://dx.doi.org/10.1093/alcalc/ags011
- Nelson DE, Jarman DW, Rehm J, Greenfield TK, Rey G, Kerr WC, Miller P, Shield KD, Ye Y, Naimi TS. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health 2013; 103:641-8; PMID:23409916; http://dx.doi.org/10.2105/AJPH.2012.301199
- Rehm J, Shield KD. Global alcohol-attributable deaths from cancer, liver cirrhosis, and injury in 2010. Alcohol Res 2013; 35:174-83; PMID:24881325
- Brooks PJ, Zakhari S. Acetaldehyde and the genome: beyond nuclear DNA adducts and carcinogenesis. Environ Mol Mutagen 2014; 55:77-91; PMID:24282063; http://dx.doi.org/10.1002/em.21824
- Clauson C, Scharer OD, Niedernhofer L. Advances in understanding the complex mechanisms of DNA interstrand cross-link repair. Cold Spring Harb Perspect Biol 2013; 5:a012732; PMID:24086043; http://dx.doi.org/10.1101/cshperspect.a012732
- Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature 2013; 493:356-63; PMID:23325218; http://dx.doi.org/10.1038/nature11863
- Moldovan GL, D'Andrea AD. How the fanconi anemia pathway guards the genome. Annu Rev Genet 2009; 43:223-49; PMID:19686080; http://dx.doi.org/10.1146/annurev-genet-102108-134222
- Walden H, Deans AJ. The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder. Annu Rev Biophys 2014; 43:257-78; PMID:24773018; http://dx.doi.org/10.1146/annurev-biophys-051013-022737
- Duxin JP, Dewar JM, Yardimci H, Walter JC. Repair of a DNA-protein crosslink by replication-coupled proteolysis. Cell 2014; 159:346-57; PMID:25303529; http://dx.doi.org/10.1016/j.cell.2014.09.024
- Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res 2009; 668:4-10; PMID:19622403; http://dx.doi.org/10.1016/j.mrfmmm.2009.01.013
- Dong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, Vasiliou V. Update of the human and mouse Fanconi anemia genes. Hum Genomics 2015; 9:32; PMID:26596371; http://dx.doi.org/10.1186/s40246-015-0054-y
- Mechilli M, Schinoppi A, Kobos K, Natarajan AT, Palitti F. DNA repair deficiency and acetaldehyde-induced chromosomal alterations in CHO cells. Mutagenesis 2008; 23:51-6; PMID:17989147; http://dx.doi.org/10.1093/mutage/gem042
- Lorenti Garcia C, Mechilli M, Proietti De Santis L, Schinoppi A, Kobos K, Palitti F. Relationship between DNA lesions, DNA repair and chromosomal damage induced by acetaldehyde. Mutat Res 2009; 662:3-9; PMID:19084543; http://dx.doi.org/10.1016/j.mrfmmm.2008.11.008
- Abraham J, Balbo S, Crabb D, Brooks PJ. Alcohol metabolism in human cells causes DNA damage and activates the Fanconi anemia-breast cancer susceptibility (FA-BRCA) DNA damage response network. Alcohol Clin Exp Res 2011; 35:2113-20; PMID:21919919; http://dx.doi.org/10.1111/j.1530-0277.2011.01563.x
- Marietta C, Thompson LH, Lamerdin JE, Brooks PJ. Acetaldehyde stimulates FANCD2 monoubiquitination, H2AX phosphorylation, and BRCA1 phosphorylation in human cells in vitro: implications for alcohol-related carcinogenesis. Mutat Res 2009; 664:77-83; PMID:19428384; http://dx.doi.org/10.1016/j.mrfmmm.2009.03.011
- Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature 2011; 475:53-8; PMID:21734703; http://dx.doi.org/10.1038/nature10192
- Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature 2012; 489:571-5; PMID:22922648; http://dx.doi.org/10.1038/nature11368
- Matsufuji Y, Fujimura S, Ito T, Nishizawa M, Miyaji T, Nakagawa J, Ohyama T, Tomizuka N, Nakagawa T. Acetaldehyde tolerance in Saccharomyces cerevisiae involves the pentose phosphate pathway and oleic acid biosynthesis. Yeast 2008; 25:825-33; PMID:19061187; http://dx.doi.org/10.1002/yea.1637
- Brendel M, Marisco G, Ganda I, Wolter R, Pungartnik C. DNA repair mutant pso2 of Saccharomyces cerevisiae is sensitive to intracellular acetaldehyde accumulated by disulfiram-mediated inhibition of acetaldehyde dehydrogenase. Genet Mol Res 2010; 9:48-57; PMID:20082270; http://dx.doi.org/10.4238/vol9-1gmr695
- Ristow H, Seyfarth A, Lochmann ER. Chromosomal damages by ethanol and acetaldehyde in Saccharomyces cerevisiae as studied by pulsed field gel electrophoresis. Mutat Res 1995; 326:165-70; PMID:7529880; http://dx.doi.org/10.1016/0027-5107(94)00165-2
- Fritsch ES, Schacherer J, Bleykasten-Grosshans C, Souciet JL, Potier S, de Montigny J. Influence of genetic background on the occurrence of chromosomal rearrangements in Saccharomyces cerevisiae. BMC Genomics 2009; 10:99; PMID:19267901; http://dx.doi.org/10.1186/1471-2164-10-99
- Ralser M, Kuhl H, Ralser M, Werber M, Lehrach H, Breitenbach M, Timmermann B. The Saccharomyces cerevisiae W303-K6001 cross-platform genome sequence: insights into ancestry and physiology of a laboratory mutt. Open Biol 2012; 2:120093; PMID:22977733; http://dx.doi.org/10.1098/rsob.120093
- Schacherer J, Ruderfer DM, Gresham D, Dolinski K, Botstein D, Kruglyak L. Genome-wide analysis of nucleotide-level variation in commonly used Saccharomyces cerevisiae strains. PLoS One 2007; 2:e322; PMID:17389913; http://dx.doi.org/10.1371/journal.pone.0000322
- Forsburg SL, Rhind N. Basic methods for fission yeast. Yeast 2006; 23:173-83; PMID:16498704; http://dx.doi.org/10.1002/yea.1347
- Hoffman CS, Wood V, Fantes PA. An Ancient Yeast for Young Geneticists: A Primer on the Schizosaccharomyces pombe model system. Genetics 2015; 201:403-23; PMID:26447128; http://dx.doi.org/10.1534/genetics.115.181503
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, et al. The genome sequence of Schizosaccharomyces pombe. Nature 2002; 415:871-80; PMID:11859360; http://dx.doi.org/10.1038/nature724
- Sipiczki M. Where does fission yeast sit on the tree of life? Genome Biol 2000; 1: reviews1011; http://dx.doi.org/10.1186/gb-2000-1-2-reviews1011
- Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev 2014; 94:1-34; PMID:24382882; http://dx.doi.org/10.1152/physrev.00017.2013
- Noguchi E, Noguchi C, Du LL, Russell P. Swi1 prevents replication fork collapse and controls checkpoint kinase Cds1. Mol Cell Biol 2003; 23:7861-74; PMID:14560029; http://dx.doi.org/10.1128/MCB.23.21.7861-7874.2003
- Lisby M, Rothstein R, Mortensen UH. Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci U S A 2001; 98:8276-82; PMID:11459964; http://dx.doi.org/10.1073/pnas.121006298
- Leman AR, Noguchi E. Local and global functions of Timeless and Tipin in replication fork protection. Cell Cycle 2012; 11:3945-55; PMID:22987152; http://dx.doi.org/10.4161/cc.21989
- Noguchi E, Noguchi C, McDonald WH, Yates JR, 3rd, Russell P. Swi1 and Swi3 are components of a replication fork protection complex in fission yeast. Mol Cell Biol 2004; 24:8342-55; PMID:15367656; http://dx.doi.org/10.1128/MCB.24.19.8342-8355.2004
- Ansbach AB, Noguchi C, Klansek IW, Heidlebaugh M, Nakamura TM, Noguchi E. RFCCtf18 and the Swi1-Swi3 complex function in separate and redundant pathways required for the stabilization of replication forks to facilitate sister chromatid cohesion in Schizosaccharomyces pombe. Mol Biol Cell 2008; 19:595-607; PMID:18045993; http://dx.doi.org/10.1091/mbc.E07-06-0618
- Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA Damage and Replication Checkpoints. Annu Rev Genet 2002; 36:617-56; PMID:12429704; http://dx.doi.org/10.1146/annurev.genet.36.060402.113540
- Noguchi E, Ansbach AB, Noguchi C, Russell P. Assays used to study the DNA replication checkpoint in fission yeast. Methods Mol Biol 2009; 521:493-507; PMID:19563125; http://dx.doi.org/10.1007/978-1-60327-815-7_28
- Doe CL, Osman F, Dixon J, Whitby MC. DNA repair by a Rad22-Mus81-dependent pathway that is independent of Rhp51. Nucleic Acids Res 2004; 32:5570-81; PMID:15486206; http://dx.doi.org/10.1093/nar/gkh853
- Khasanov FK, Salakhova AF, Chepurnaja OV, Korolev VG, Bashkirov VI. Identification and characterization of the rlp1+, the novel Rad51 paralog in the fission yeast Schizosaccharomyces pombe. DNA Repair (Amst) 2004; 3:1363-74; PMID:15336631; http://dx.doi.org/10.1016/j.dnarep.2004.05.010
- Stingele J, Schwarz MS, Bloemeke N, Wolf PG, Jentsch S. A DNA-dependent protease involved in DNA-protein crosslink repair. Cell 2014; 158:327-38; PMID:24998930; http://dx.doi.org/10.1016/j.cell.2014.04.053
- Collis SJ, Ciccia A, Deans AJ, Horejsi Z, Martin JS, Maslen SL, Skehel JM, Elledge SJ, West SC, Boulton SJ. FANCM and FAAP24 function in ATR-mediated checkpoint signaling independently of the Fanconi anemia core complex. Mol Cell 2008; 32:313-24; PMID:18995830; http://dx.doi.org/10.1016/j.molcel.2008.10.014
- Kuo HK, McMahan S, Rota CM, Kohl KP, Sekelsky J. Drosophila FANCM helicase prevents spontaneous mitotic crossovers generated by the MUS81 and SLX1 nucleases. Genetics 2014; 198:935-45; PMID:25205745; http://dx.doi.org/10.1534/genetics.114.168096
- Sun W, Nandi S, Osman F, Ahn JS, Jakovleska J, Lorenz A, Whitby MC. The FANCM ortholog Fml1 promotes recombination at stalled replication forks and limits crossing over during DNA double-strand break repair. Mol Cell 2008; 32:118-28; PMID:18851838; http://dx.doi.org/10.1016/j.molcel.2008.08.024
- Nandi S, Whitby MC. The ATPase activity of Fml1 is essential for its roles in homologous recombination and DNA repair. Nucleic Acids Res 2012; 40:9584-95; PMID:22844101; http://dx.doi.org/10.1093/nar/gks715
- Budzowska M, Graham TG, Sobeck A, Waga S, Walter JC. Regulation of the Rev1-pol zeta complex during bypass of a DNA interstrand cross-link. EMBO J 2015; 34:1971-85; PMID:26071591; http://dx.doi.org/10.15252/embj.201490878
- Fu D, Dudimah FD, Zhang J, Pickering A, Paneerselvam J, Palrasu M, Wang H, Fei P. Recruitment of DNA polymerase eta by FANCD2 in the early response to DNA damage. Cell Cycle 2013; 12:803-9; PMID:23388460; http://dx.doi.org/10.4161/cc.23755
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 1991; 194:795-823; PMID:2005825; http://dx.doi.org/10.1016/0076-6879(91)94059-L
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast: A laboratory course manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1993.
- Noguchi C, Rapp JB, Skorobogatko YV, Bailey LD, Noguchi E. Swi1 associates with chromatin through the DDT domain and recruits Swi3 to preserve genomic integrity. PLoS One 2012; 7:e43988; PMID:22952839; http://dx.doi.org/10.1371/journal.pone.0043988
- Rapp JB, Noguchi C, Das MM, Wong LK, Ansbach AB, Holmes AM, Arcangioli B, Noguchi E. Checkpoint-dependent and -independent roles of Swi3 in replication fork recovery and sister chromatid cohesion in fission yeast. PLoS One 2010; 5:e13379; PMID:20967229; http://dx.doi.org/10.1371/journal.pone.0013379
- Krawchuk MD, Wahls WP. High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast 1999; 15:1419-27; PMID:10509024; http://dx.doi.org/10.1002/(SICI)1097-0061(19990930)15:13%3c1419::AID-YEA466%3e3.0.CO;2-Q
- Sato M, Dhut S, Toda T. New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 2005; 22:583-91; PMID:15942936; http://dx.doi.org/10.1002/yea.1233
- al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJ, Lehmann AR, Carr AM. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell 1994; 5:147-60; PMID:8019001; http://dx.doi.org/10.1091/mbc.5.2.147
- Coulon S, Noguchi E, Noguchi C, Du LL, Nakamura TM, Russell P. Rad22Rad52-dependent repair of ribosomal DNA repeats cleaved by Slx1-Slx4 endonuclease. Mol Biol Cell 2006; 17:2081-90; PMID:16467377; http://dx.doi.org/10.1091/mbc.E05-11-1006
- Lambert S, Watson A, Sheedy DM, Martin B, Carr AM. Gross chromosomal rearrangements and elevated recombination at an inducible site-specific replication fork barrier. Cell 2005; 121:689-702; PMID:15935756; http://dx.doi.org/10.1016/j.cell.2005.03.022
- Carr AM, Schmidt H, Kirchhoff S, Muriel WJ, Sheldrick KS, Griffiths DJ, Basmacioglu CN, Subramani S, Clegg M, Nasim A, et al. The rad16 gene of Schizosaccharomyces pombe: a homolog of the RAD1 gene of Saccharomyces cerevisiae. Mol Cell Biol 1994; 14:2029-40; PMID:8114734; http://dx.doi.org/10.1128/MCB.14.3.2029
- Rodel C, Kirchhoff S, Schmidt H. The protein sequence and some intron positions are conserved between the switching gene swi10 of Schizosaccharomyces pombe and the human excision repair gene ERCC1. Nucleic Acids Res 1992; 20:6347-53; PMID:1475195; http://dx.doi.org/10.1093/nar/20.23.6347
- Sheedy DM, Dimitrova D, Rankin JK, Bass KL, Lee KM, Tapia-Alveal C, Harvey SH, Murray JM, O'Connell MJ. Brc1-mediated DNA repair and damage tolerance. Genetics 2005; 171:457-68; PMID:15972456; http://dx.doi.org/10.1534/genetics.105.044966
- Sugimoto T, Igawa E, Tanihigashi H, Matsubara M, Ide H, Ikeda S. Roles of base excision repair enzymes Nth1p and Apn2p from Schizosaccharomyces pombe in processing alkylation and oxidative DNA damage. DNA Repair (Amst) 2005; 4:1270-80; PMID:16076563; http://dx.doi.org/10.1016/j.dnarep.2005.06.009
- Sommariva E, Pellny TK, Karahan N, Kumar S, Huberman JA, Dalgaard JZ. Schizosaccharomyces pombe Swi1, Swi3, and Hsk1 are components of a novel S-phase response pathway to alkylation damage. Mol Cell Biol 2005; 25:2770-84; PMID:15767681; http://dx.doi.org/10.1128/MCB.25.7.2770-2784.2005
