Mismatch repair (MMR) corrects mismatches in newly synthesized DNA strands.Citation1 Key factors in the recognition of the mismatched bases are the Msh2 and Msh6 ATPases, which act during DNA replication and recombination. Recently, MMR proteins were reported to also function outside of S phase, independently of DNA replication or recombination, in a process termed non-canonical MMR.Citation2 In this volume of Cell Cycle, Tanaka et al.Citation3 identify a new function for MMR proteins during the G1 phase of the cell cycle: Msh2 and Msh6 are implicated in the degradation of the DNA licensing factor Cdt1 following Ultra-violet (UV) irradiation.
Cdt1 is a key cell cycle regulator, which licenses DNA for a new round of replication.Citation4 Cdt1 is specifically expressed during the G1 phase and it has been postulated to link replication licensing and the DNA damage response. Following DNA damage by a variety of agents, Cdt1 is degraded within minutes and this regulation is considered a novel checkpoint in the DNA damage response (DDR).Citation4 Cdt1 is targeted for proteolysis through interactions with PCNA and the CRL4Cdt2 ubiquitin ligase on damaged chromatin. It was previously shown that the main pathway responsible for targeting Cdt1 for degradation in response to UV irradiation is the nucleotide excision repair pathway (NER).Citation5,Citation6 Whether other pathways could also mediate Cdt1 degradation in UV-irradiated cells, however, remained elusive.
In the present study, Tanaka et al.Citation3 observed delayed Cdt1 proteolysis in response to UV damage in the absence of NER. In cells deficient for the NER factor XPA, Cdt1 proteolysis occurred 30 to 60 minutes later than in normal cells. They showed that proteolysis is dependent on PCNA and CRL4Cdt2, similar to XPA proficient cells. Using a micropore assay to inflict localized UV damage, they followed the recruitment kinetics of PCNA, Cdt2 and Cdt1 at the site of damage in XPA deficient cells. The authors observed a delay in the recruitment of these factors, in accordance with the delayed proteolysis of Cdt1.
Since Cdt1 proteolysis was evident in the absence of the NER pathway, the authors asked which other pathway could be responsible. To address this question, the authors examined the possible involvement of Base Excision Repair (BER) and Mismatch Repair (MMR) in cells defective for NER. To this end, XPA deficient cells synchronized in G1 were locally irradiated and the recruitment of Ape1 (BER), Msh2 and Msh6 (MMR) was assessed. They found that Msh2 and Msh6 were recruited at the site of damage with kinetics similar to the delayed degradation of Cdt1. The possible implication of Msh2 and Msh6 in Cdt1 degradation was confirmed by RNAi experiments in XPA cells: in cells depleted of Msh2 or Msh6, Cdt1 was stabilized after UV damage. Similarly, Cdt1 was stabilized in other cell lines, when MMR factors and XPA were simultaneously depleted. Importantly, in normal cells, where NER was active, knock-down of Msh2 or Msh6 led to a delay in Cdt1 degradation. This delay was similar to the one observed when XPA was depleted. This shows that the MMR pathway is involved in the degradation of Cdt1 both in concert with, and in the absence of NER (). In addition, the authors were able to show that Cdt2 directly interacts with Msh2 and Msh6 after UV irradiation.
Why is Cdt1 degradation so important in response to DNA damage? Tanaka et al., found that in the presence of non-degradable Cdt1, DNA synthesis, a vital step for DNA repair, is diminished. Consequently, Cdt1 degradation appears a prerequisite for efficient DNA repair ().
In conclusion, the recent study from Tanaka et al.Citation3 identifies MMR as a repair mechanism implicated in Cdt1 degradation following UV irradiation during G1. This result highlights the importance of regulating Cdt1 by multiple pathways in cells with damaged DNA, to ensure repair. MMR was recently linked with repair events outside of S phase. Of note, MMR was reported to be involved in the proteolysis of p21, another CRL4Cdt2 substrate, in G1 phase, following DNA alkylation.Citation7 The observations presented by Tanaka et al.Citation3 establish a firm link between non-canonical MMR and DNA damage responses in the G1 phase of the cell cycle. As non-canonical MMR may be error-prone, future work will establish the consequences of this new pathway for genome stability.
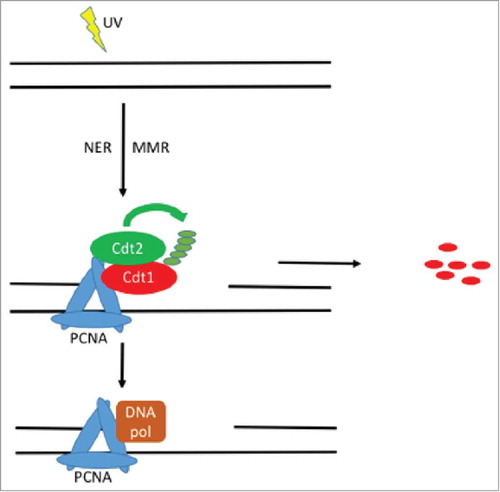
Figure 1. A model for Cdt1 proteolysis in G1 phase. UV irradiation damages DNA and induces the formation of helix distorting lesions. Both NER and MMR are activated and result in the degradation of Cdt1 through ubiquitylation by the CRL4Cdt2 complex. The degradation of Cdt1 facilitates the recruitment of DNA polymerases responsible for DNA synthesis of the excised DNA strand.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Reyes GX, Schmidt TT, Kolodner RD, Hombauer H. New insights into the mechanism of DNA mismatch repair. Chromosoma 2015; 124(4):443-62; PMID: 25862369; https://doi.org/10.1007/s00412-015-0514-0
- Crouse GF, Non-canonical actions of mismatch repair. DNA Repair (Amst) 2016; 38:102-9; PMID: 26698648; https://doi.org/10.1016/j.dnarep.2015.11.020
- Tanaka M, Takahara M, Nukina K, Hayashi A, Sakai W, Sugasawa K, Shiomi Y, Nishitani H. Mismatch repair proteins recruited to ultraviolet light-damaged sites lead to degradation of licensing factor Cdt1 in the G1 phase. Cell Cycle 2017; 16(7):673-84; PMID: 28275001; http://dx.doi.org/10.1101/gad.290379.116
- Pozo PN, Cook JG. Regulation and function of Cdt1; A key factor in cell proliferation and genome stability. Genes (Basel) 2016; 8(1):pii: E2; PMID: 28025526; http://dx.doi.org/10.3390/genes8010002
- Raman M, Havens CG, Walter JC, Harper JW. A genome-wide screen identifies p97 as an essential regulator of DNA damage-dependent CDT1 destruction. Mol Cell 2011; 44(1):72-84; PMID: 21981919; https://doi.org/10.1016/j.molcel.2011.06.036
- Shiomi Y, Hayashi A, Ishii T, Shinmyozu K, Nakayama J, Sugasawa K, Nishitani H. Two different replication factor C proteins, Ctf18 and RFC1, separately control PCNA-CRL4Cdt2-mediated Cdt1 proteolysis during S phase and following UV irradiation. Mol Cell Biol 2012; 32(12):2279-88; PMID: 22493068; https://doi.org/10.1128/MCB.06506-11
- Jascur T, Fotedar R, Greene S, Hotchkiss E, Boland CR. N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) triggers MSH2 and Cdt2 protein-dependent degradation of the cell cycle and mismatch repair (MMR) inhibitor protein p21 Waf1/Cip1. J Biol Chem 2011; 286(34):29531-9; PMID: 21725088; https://doi.org/10.1074/jbc.M111.221341
