The accurate replication of the human genome in S-phase and the faithful segregation of sister chromatids in mitosis are fundamental events required for the maintenance of chromosome stability. Cells that are copying their DNA in preparation for division can suffer from replication stress, which refers to the various impediments that can slow or stall replication forks and which is a major source of pathological processes, including cancer, premature aging and other disorders associated with genomic instability.
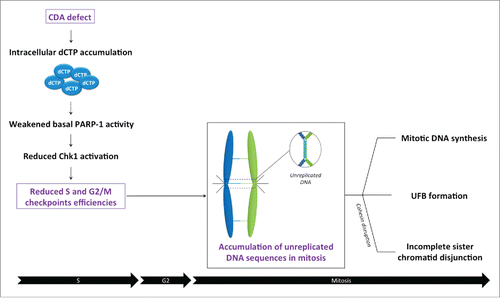
Accurate DNA replication and genetic stability is highly dependent on dNTP concentration.Citation1 Amor-Guéret's team previously reported such a situation in Bloom syndrome (BS), a genetic disease displaying one of the strongest known correlations between chromosomal instability and an increased risk of malignancy. BS results from the mutation of the BLM gene, encoding BLM, a RecQ 3’-5’ DNA helicase. BS cells are characterized by a high frequency of sister chromatid exchanges (SCEs), a high level of endogenous replication stress and mitotic abnormalities such as anaphase bridges, including chromatin bridges and the ultrafine anaphase bridges (UFBs). These recent years, Amor-Gueret and colleagues have identified a novel mechanism by which nucleotide pool disequilibrium compromises the completion of DNA replication and chromosome segregation. They reported that BLM-deficient cells present a drastic downregulation of cytidine deaminase (CDA), resulting in a pyrimidine pool imbalance, which in turn generates replication stress leading to segregation defects.Citation2,3 CDA is an enzyme of the pyrimidine salvage pathway that catalyzes the hydrolytic deamination of cytidine and deoxycytidine into uridine and deoxyuridine, respectively. CDA deficiency leads to intracellular accumulation of dC and dCTP, decreasing the basal activity of poly(ADP-ribose) polymerase 1 (PARP-1),Citation2 a multifunctional enzyme involved in many cellular processes, including the response to DNA damage. The resulting low levels of PARP-1 activity disturb the activation of the ATR-Chk1 and G2/M checkpoint pathways, leading to the transmission in mitosis of under-replicated DNA at some “difficult-to-replicate” loci in the genome, such as centromeres and fragile sites, and consequently to excess UFB formationCitation2,3 ().
Figure 1. Cytidine deaminase deficiency leads to dCTP intracellular accumulation reducing basal PARP-1 activity. In consequence, Chk1 activation and the subsequent S and G2/M checkpoint efficiencies are reduced allowing the premature entry of cells into mitosis before complete DNA replication. During mitosis, the accumulation of unreplicated DNA sequences, in particular at difficult-to-replicate sites such as centromeres and common fragile sites, leads to mitotic DNA synthesis, ultrafine anaphase bridge (UFB) formation and incomplete sister chromatid disjunction in cohesin-depleted cells.
Amor-Gueret and colleagues have also reported incomplete sister chromatid disjunction in BS cells when depleted of cohesion.Citation4 On the basis of these results, Gemble et al.Citation5 investigated whether defective sister chromatid disjunctions were resulting from the loss of BLM activity per se, or were the consequence of the CDA defect associated with BLM deficiency. Sister chromatids are held together by cohesin until mitosis: at the metaphase-anaphase transition, Rad21, the cleavable subunit of the cohesion complex is cleaved by separase, leading to sister chromatid disjunction and chromosome segregation. Rad21 depletion results in chromosomes that are fully disjoined due to cohesion depletion, but some metaphase cells present separated sister chromatids that are still physically linked, reflecting incomplete disjunction. They showed first that expressing CDA in BLM-deficient cells was sufficient to prevent the excess of Rad21-depleted cells with “incomplete disjunction” phenotype, independently of BLM. Then, they found that culturing Rad21-depleted CDA proficient cells in the presence of dC, to increase intracellular levels of dC and dCTP, and thus to mimick CDA deficiency, was reproducing the excess of cells with incomplete disjunction phenotype. Finally, they demonstrated that this “incomplete disjunction” phenotype was the direct consequence of the reduction of basal PARP-1 activity resulting from the intracellular accumulation of dC and dCTP due to CDA deficiency.
In conclusion, these unexpected findings highlight a new role for both CDA and PARP-1 in the regulation of chromosome segregation. By maintaining a balanced nucleotide pool, CDA promotes optimal PARP-1 activity, which, in turn, ensures faithful sister chromatid disjunction by preventing the persistence of under-replicated DNA until mitosis (). The nucleotide pool disequilibrium resulting from CDA deficiency is, thus, a source of replication stress leading to segregations defects, known to facilitate carcinogenesis. These results might explain why CDA expression is lost in a large fraction of cancer cells and tumor tissues.Citation6 Interestingly, upregulation of CDA has been also observed in tumors,Citation7 including pancreatic adenocarcinoma, that are generally highly resistant to DNA targeting drugs, further highlighting the importance of understanding the role of nucleotide pool imbalance in genetic instability and carcinogenesis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Mathews CK. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat Rev Cancer 2015; 15:528-39; PMID:26299592; https://doi.org/10.1038/nrc3981
- Gemble S, Ahuja A, Buhagiar-Labarchède G, Onclercq-Delic R, Dairou J, Biard DS, Lambert S, Lopes M, Amor-Guéret M. Pyrimidine Pool Disequilibrium induced by a Cytidine Deaminase deficiency inhibits PARP-1 activity, leading to the under replication of DNA. PLoS Genet 2015; 11:e1005384; PMID:26181065; https://doi.org/10.1371/journal.pgen.1005384
- Gemble S, Buhagiar-Labarchède G, Onclercq-Delic R, Biard D, Lambert S, Amor-Guéret M. A balanced pyrimidine pool is required for optimal Chk1 activation to prevent ultrafine anaphase bridge formation. J Cell Sci 2016; 129:3167-77; PMID:27383768; https://doi.org/10.1242/jcs.187781
- Rouzeau S, Cordelières FP, Buhagiar-Labarchède G, Hurbain I, Onclercq-Delic R, Gemble S, Magnaghi-Jaulin L, Jaulin C, Amor-Guéret M. Bloom's syndrome and PICH helicases cooperate with topoisomerase IIalpha in centromere disjunction before anaphase. PLoS One 2012; 7:e33905; PMID:22563370; https://doi.org/10.1371/journal.pone.0033905
- Gemble S, Buhagiar-Labarchède G, Onclercq-Delic R, Jaulin C, Amor-Guéret M. Cytidine deaminase deficiency impairs sister chromatid disjunction by decreasing PARP-1 activity. Cell Cycle 2017; 16(11):1128-1135; PMID:28463527; https://doi.org/10.1080/15384101.2017.1317413
- Mameri H, Bièche I, Meseure D, Marangoni E, Buhagiar-Labarchède G, Nicolas A, Vacher S, Onclercq-Delic R, Rajapakse V, Varma S, et al. Cytidine deaminase deficiency reveals new therapeutic opportunities against cancer. Clin Cancer Res 2017; 23:2116-26; PMID:27601591; https://doi.org/10.1158/1078-0432.CCR-16-0626
- Zauri M, Berridge G, Thézénas ML, Pugh KM, Goldin R, Kessler BM, Kriaucionis S. CDA directs metabolism of epigenetic nucleosides revealing a therapeutic window in cancer. Nature 2015; 524:114-8; PMID:26200337; https://doi.org/10.1038/nature14948
