ABSTRACT
Interference with the expression and/or functions of the multifunctional tumor suppressor BRCA1 leads to a high risk of breast and ovarian cancers. BRCA1 expression is usually activated by the estrogen (E2) liganded ERα receptor. Activated ERα is considered as a potent transcription factor which activates various genes expression by 2 pathways. A classical pathway, ERα binds directly to E2-responsive elements (EREs) in the promoters of the responsive genes and a non-classical pathway where ERα indirectly binds with the appropriate gene promoter. In our previous study, HTLV-1Tax was found to strongly inhibit ERα induced BRCA1 expression while stimulating ERα induced ERE dependent genes. TPA is a strong PKC activator which found to induce the expression of HTLV-1. Here we examined the effect of TPA on the expression of BRCA1 and genes controlled by ERE region in MCF-7 cells and on Tax activity on these genes. Our results showed strong stimulatory effect of TPA on both BRCA1 and ERE expression without treatment with E2. Tax did not show any significant effect on these TPA activities. It seems that TPA activation of BRCA1 and ERE expression is dependent on PKC activity but not through the NFκB pathway. However, 53BP1 may be involved in this TPA activity because its overexpression significantly reduced the TPA stimulatory effect on BRCA1 and ERE expression. Additionally, our Chip assay results probably exclude possible involvement of ERα pathway in this TPA activity because TPA did not interfere with the binding of ERα to both BRCA1 promoter and ERE region.
Introduction
The majority of breast cancer (BC) cases are sporadic and are affected by various factors related to lifestyle and exposure to environmental pollutionsCitation1-8 However, about 10% of BC cases arise in a hereditary manner.Citation9 Although it was found that dysfunction of different genes leads to BC development,Citation10 however the first identified breast cancer susceptibility gene BRCA1 is the most important one in BC cases. BRCA1 is a multifunctional tumor suppressor,Citation11 involved in many cellular processes such as gene expression,Citation12 ubiquitination,Citation13 genome stabilizationCitation14 and enhancing DNA damage repairCitation15-17 chromatin remodeling,Citation18 induction of cell cycle arrestCitation19 and apoptosis.Citation20 Lack of expression or functionality of BRCA1 is related mainly to breast and ovarian cancers,Citation21 most likely due to the response of these organs to estrogen (E2) and estrogen receptor α (ERα) on each other.Citation12 In addition, it was found that a significant portion (40–45%) of the hereditary BCs are linked with germline mutations in BRCA1 gene.Citation22 On the other hand, although BRCA1 mutations are rare (2–3%) in sporadic BCs,Citation22 there was no expression of BRCA1 in 30–40% of them, indicating that BRCA1 malfunction can lead to sporadic BC also.Citation23-27
It is known that E2 activates ERα by direct ligation with it in the cell nucleus. The activated ERα is acting as a transcription factor which is responsible for activating various genes by 2 different pathways: (a) Classical pathway, by direct binding of the E2 activated ERα to the promoters of the target genes at E2-responsive elements (EREs) located in these promoters. As a result of this binding and after recruitment of appropriate co-activators and co-factors there will be a stimulation of the transcription of the appropriate gene.Citation28 (b) Non-classical pathways, by indirect binding of the E2 activated ERα with promoters lacking ERE elements through interacting with other specific transcription factors which are bound directly to these promoters and recruiting various co-activators and co-factors, which promote the activity of these transcription factors on their specific target gene.Citation29 For instance, in the case of BRCA1 promoter, although it has not ERE elements still it is activated by the E2 activated ERα.Citation30 This activation is induced by the non-classical pathway, in which the E2- ERα complex binds to the CBP/p300 co-activators and then interacts with Jun/Fos transcription factor which is bound with its AP-1 site located in the BRCA1 promoter.Citation29 In addition, it has been shown previously that this BRCA1 expression by E2- ERα requires the recruitment of different other cofactors like Sp1, CREB,Citation31 AhR,Citation32 E2F transcription factor family and other co-factors.Citation31,33,34
Human T-cell leukemia virus type-1 (HTLV-1) is the cause of the aggressive malignancy adult T-cell leukemia (ATL)Citation1 and different other severe clinical disorders such as tropical spastic paraparesis/HTLV-1 associated myelopathy.Citation35 It is generally believed that the HTLV-1 Tax protein is responsible for HTLV-1 pathogenicity.Citation36
In contrast to the tumor suppressor nature of BRCA1, Tax is a potent oncoprotein which most of its activities are strictly opposing those of BRCA1. In our previous study it was found that introducing Tax into breast epithelial cells strongly blocked the E2-ERα-mediated activation of BRCA1 expression.Citation37 Therefore, expression of HTLV-1Tax in breast epithelial cells probably will increase the risk for developing breast cancer in these women. It is important to note that breastfeeding is a major route of mother-to-infant HTLV-1 transmission, especially in communities with traditional prolonged breastfeeding.Citation38-41 Normal breast milk contains large numbers of T-lymphocytes, monocytes and epithelial cells during the entire long lactation periods of over 5 y.Citation42,43 Breast milk of HTLV-1 infected women is loaded also with HTLV-1-producing T-cells.Citation38,40,44 It has been shown that the milk-born epithelial cells can be infected with HTLV-1 by co-cultivation with HTLV-1-producing T-cells and that these infected cells can transmit the virus to other breast cells and to primary T-cells.Citation42,43 Southern et alCitation42 have suggested that such infection of breast epithelial cells can likely occur also in vivo and that these in vivo infected cells are presumably the virus reservoir for its milk-born transmission.
Phorbol and diterpen esters are tumor promoting plant products,Citation45-50 potentially accessible to human contact or consumption.Citation45 The most intensively investigated member of this group, 12-O-tetradecanoylphorbol-13-acetate (TPA), is a potent activator of protein kinase C (PKC) and the majority of its biologic effects, including tumor promotion, are related to PKC.Citation51,52 In addition, it has been previously shown that TPA strongly activate HTLV-1 expression,Citation46,48,49,53 and therefore, it might be considered as possible environmental co-factors related to HTLV-1 pathogenicity.Citation46
On this ground, it was important to elucidate the effect of TPA on the activity of HTLV-1Tax on BRCA1 and ERE expression.
Materials and methods
Cells and culture conditions
MCF-7 epithelial-like breast cancer cells (weakly invasive ER-α positive)Citation54 were obtained from Etta Livne (from our department) and used in this study. These cells were grown and maintained in Dulbeco's Modified Eagle's Medium (DMEM) with 2mM L-glutamine, 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
When indicated, the cells were treated with 50 nM TPA (Sigma Chemicals Israel Ltd., Holon, Isarel) in the absence or presence of 1 mM of PKC inhibitor BI (Calbiochem, La Jolla, CA).
Plasmids and transfection
The reporter firefly luciferase (Luc) driven by the BRCA1 promoter (BRCA1-Luc) and Luc reporter driven by estrogen response elements (EREs-Luc) were provided by Haim Werner (Clinical Biochemistry, Tel-Aviv University, Israel). CBP and p300 plasmids were provided by addgene company. The ERα-expressing pCDNA3 vectorCitation55 was from Michael Danilenko (Clinical Biochemistry, Ben-Gurion University, Beer-Sheva, Israel). The plasmid expressing the Renilla luciferase, was purchased from Promega (Madison WI, USA).Citation56
Transfection of plasmids was done by jetPRIMTM kit (Polyplus-transfection company, France, www.polyplus-transfection.com) according to the manufacturer's instructions. Breifly, 1 µg of each plasmid DNA was transfected into 5 × 105 cells/well of 6 well plate and the total DNA was completed in each transfection mixture to 3 µg with an empty plasmid (dLTR). The efficiency of the transfections, determined with GFP-expressing plasmid, was found by FACS analysis in our cells between 70 to 80% (not shown). Each transfection mixture included the pRL-renilla plasmid (0.2 μg) as control for efficiency. At 24 h post transfection the cells were extracted and the enzymatic activity of luciferase was measured by 20/20 Luminometer (Promega).
The cell cultures were treated with 20 nM estrogen (E2, Sigma-Aldrich Chemical Co.) at 5 hr before cell harvest. The Luc activity was normalized to that of renilla and presented as fold of the relevant control.
Antibodies, cell fractionation, Western blot analyses and co-immunoprecipitation
Monoclonal antibodies against BRCA1, Tax, ER-α, CBP, CREB and Ps2 were all purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Extracts of the whole cell and sub-cellular fractions were prepared by NucBuster Kit (Calbiochem, Catalog No. 71183–3) according to the Manufacturer's protocol.
Aliquots of the tested extracts (80 µg protein) were analyzed with the respective antibodies for Western analyses as described previously.Citation57
Aliquots of the nuclear extracts (200 µg protein) were immunoprecipitated with the specified mouse antibodies and analyzed by Western blot for co-precipitated proteins with the respective rabbit antibodies as described previously.Citation58
Chromatin immunoprecipitation (ChIP)
MCF-7 (2 × 107) cells were transfected with Tax expressing plasmid by jetPRIMTM kit and at 24 hr post-transfection the cells were treated with E2 for 5 hr. The chromatin mmunoprecipitation was performed by EZ Chip kit (Millipore) according to the manufacturer's instruction. The BRCA1 promoter region flanking the Sp/AP-1/CRE binding sites (171 base pairs) in the obtained DNA was amplified by real time PCR, using the following primers: forward: 5′-GACAGATGGGTATTCTTTGACG-3′ and reverse: 5′-GCATATTCCAGTTCC TATCACGAG-3′.Citation31
The ERE region of the pS2 promoter was amplified using the following primers:
forward: PS2 5′-TATGAATCACTTCTGCAGTGAG-3′ and reverse PS2 5′- GAGCGTTAGATAACATTTGCC-3′.
Results
Effect of TPA on the expression of BRCA1 and genes controlled by estrogen responsive elements (ERE)
In our previous study it was found that HTLV-1 Tax strongly prevented the E2-ERα induced expression of BRCA1, while it stimulated the E2-ERα induced expression of genes that contain estrogen responsive elements (EREs) in their promotors.Citation59 Based on these results we hypothesized that the expression of HTLV-1 Tax in breast epithelial cells may increase the risk for breast cancer development.
On the other hand, it was found previously that TPA highly induced the expression of HTLV-1,Citation46,48,49,53 and that both TPA and Tax synergistically stimulate HTLV-1 expression (unpublished).
In this study we investigated the effect of TPA on BRCA1 expression and on the inhibitory effect of Tax on BRCA1 expression. This was done by transfecting the reporter (Luc) driven by the BRCA1 promoter (BRCA1-Luc) into MCF7 cells and examining the effect of TPA on the expression of this promotor. The results of these experiments show that TPA strongly activated BRCA1 expression by 17–18 folds of its basal level (, column 4) while treatment with E2 induced this expression by 9–10 folds (, columns 2). Treatment of the cells with both E2 and TPA caused additive stimulation of BRCA1 expression of about 25–26 folds. Although the expression of HTLV-1 Tax in these cells drastically inhibited the E2 induced activation of BRCA1, it has only moderate inhibitory effect (about 10%) on the TPA induced activation of this gene. Accordingly, it can be seen that when these cells were treated with both E2 and TPA, Tax was able to reduce mainly the E2 activation part (, column 8).
Figure 1. Effect of TPA on BRCA1 and ERE expression. MCF-7 were co-transfected with either a plasmid expressing BRCA1-Luc (1 μg) (A) or ERE-Luc (1 μg) (B) alone or together with Tax expressing plasmid (1 μg) with or without TPA (50 nM) or E2 (20 nM) treatment. The TPA and E2 were added to the cultures 5 and 24hr respectively before harvesting the cells for analyzing the reporter expression. The presented results are an average of 3 repeated experiments ± SE.
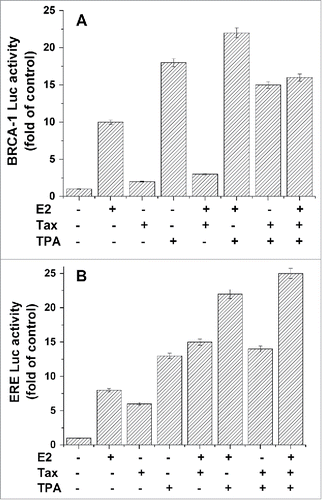
Furthermore, the effect of TPA on the expression of ERE containing genes was examined by transfecting the reporter driven by estrogen response elements (EREs-Luc) into MCF7 cells and testing the effect of TPA on the expression of this promotor. The results presented in show that TPA strongly activated ERE-Luc expression by 12–13 folds of its basal level while treatment with E2 induced this expression by 8 folds (, columns 2 and 4). Treatment with both E2 and TPA resulted in an additive stimulatory effect (about 20 folds, , column 6). In contrast to its effect on BRCA1 expression, Tax stimulated both the basal and the E2 induced expression of ERE-Luc. Treatment with TPA caused further enhancement of this Tax-E2 induced expression of ERE-Luc (, columns 8).
TPA effect on BRCA1 and ERE expression is mediated by PKC activity
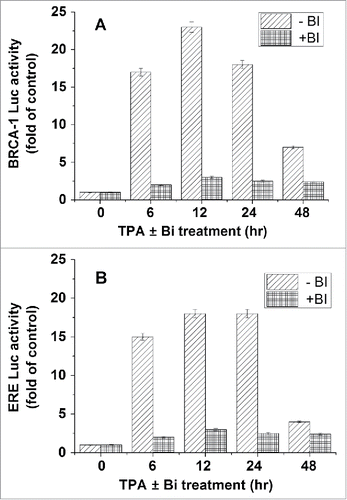
As mentioned in the introduction section, TPA is a potent activator of PKCCitation51,52 and that a long exposure to TPA cause down regulation of PKC expression.Citation60 Therefore, MCF-7 transfected with plasmids expressing either BRCA1-Luc or ERE-Luc were treated with TPA alone or with BI (a potent inhibitor of PKC activity) for different periods of time. Our results presented in showed that long exposure to TPA strongly reduced its stimulatory effect both on BRCA1 () and ERE () expression. The highest stimulatory effect was obtained at 12h of treatment. Furthermore, it can be seen that BI completely abolished the TPA induced activation of both promotors.
Figure 2. Involvement of PKC in TPA effect on BRCA1 and ERE expression. MCF-7 cells were transfected with plasmids expressing either BRCA1-Luc (1 μg) (A) or ERE-Luc (1 μg) (B) and treated with TPA for different periods of time alone or with 2nM BI (a potent inhibitor of PKC activity). Bi was added 2h before the treatment with TPA. The cells were harvested at 24h post transfection for analyzing the reporter expression. The presented results are an average of 3 repeated experiments ± SE.
Differential effects of PKC isoforms on BRCA1 and ERE expression
Based on the above results showed that the TPA induced activation of BRCA1 and ERE is mediated by PKC activity, it was important to test which PKC isoforms are responsible for this activity. For achieving that, MCF-7 (containing BRCA1-Luc or ERE-Luc) were treated with TPA for 24hs to deplete all cellular PKCs and then transfected with plasmids expressing different PKC isoforms. The results presented in shows that indeed treatment with TPA for 24h or more caused complete depletion in the protein levels of the various tested PKC isoforms. However, at 24h post transfection of the cells with the different PKC isoforms there were high levels of expression of the various PKC isoforms (). shows that PKCα, β1, δ and η strongly induce the expression of BRCA1 (columns 4, 6, 10 and 14 respectively), while PKCβ2 and ϵ completely reduce this expression (, columns 8 and 12 respectively).
Figure 3. Effect of PKC isoforms on BRCA1 and ERE expression. (A) MCF-7 cells were treated with 50 nM TPA and at different periods of time post treatment the cells were extracted and examined for the different PKC isoforms expression by western blot analysis with the appropriate monoclonal antibodies. (B) Cells were treated with TPA for 24hs to deplete all cellular PKCs and then transfected with plasmids expressing the PKC isoforms (1 μg) and at different periods of time post treatment PKC isoforms expression was determined by western blot analysis. In (C) and (D) cells were transfected with plasmids expressing either BRCA1-Luc (1 μg) or ERE-Luc (1 μg) respectively, treated with TPA for 24hs and then transfected with plasmids expressing different PKC isoforms (1 μg). The cells were harvested at 24h post transfection for analyzing the reporter expression. The presented results are an average of 3 repeated experiments ± SE.
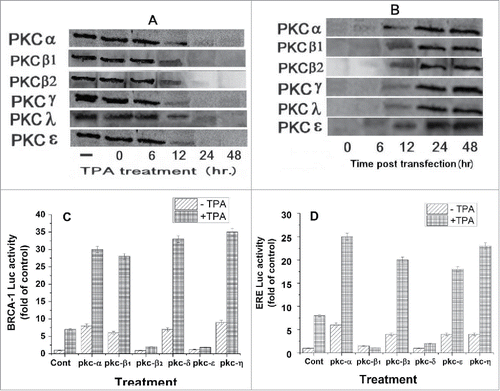
Interestingly, it can be seen in that PKCα, PKCβ2, PKCϵ and PKCη strongly induced ERE-Luc expression (columns 4, 8, 12 and 14respectively), while PKCβ1 and PKCδ blocked this expression (, columns 6 and 10 respectively).
Effect of TPA and Tax on the expression of BRCA1 and pS2 proteins
Protein levels of BRCA1 and pS2, an endogenous ERE responsive gene,Citation61 in MCF-7 cells which were treated with TPA (for different periods of time) or E2 in the presence or absence of Tax were examined by western blot analysis. The results presented in show that a short treatment (12–24 hrs in the case of BRCA1 and 6–12h in the case of pS2) with TPA induced both BRCA1 (lanes 3 and 4) and pS2 (lanes 2 and 3) protein expression. However, long treatment (24 or 48 hrs in the case of pS2 or BRCA1 respectively) with TPA has no effect on the basal expression of both BRCA1 (lane 5) and pS2 (lanes 4 and 5).
Figure 4. Effect of TPA and Tax on the expression of BRCA1 and pS2 proteins. MCF-7 cells were transfected with aTax expressing plasmid (1 μg) and treated with TPA (for different periods of time) or E2 at 5 hr before extracting the cells for western blot analysis. The whole cell extracts of the cells were examined for BRACA1 and pS2 expression by western blot analysis with anti BRCA1 and pS2 monoclonal antibodies.
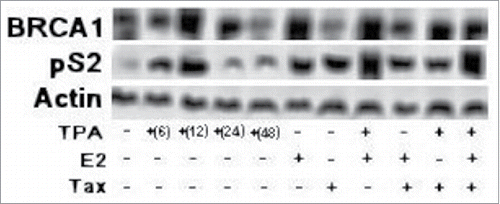
Treatment with E2 also strongly induced both BRCA1 and pS2 proteins expression levels (, lane 6), while Tax alone induces only the expression of pS2 protein without any effect on the expression of BRCA1 protein (, lane 7). It can be seen also that treatment of the cells with both TPA and E2 caused strong expression of both BRCA1 and pS2 proteins (lane 8). However, when Tax expressing cells were treated with E2, Tax almost completely blocked the E2 induced BRCA1 protein expression (lane 9), while when these cells were treated with TPA there was no or minor effect of Tax on the induced BRCA1 protein expression (, lane 10).
Are NFκB and/or 53Bp1 involved in the TPA induced activation of BRCA1 and ERE controlled genes?
Possible involvement of NFκB
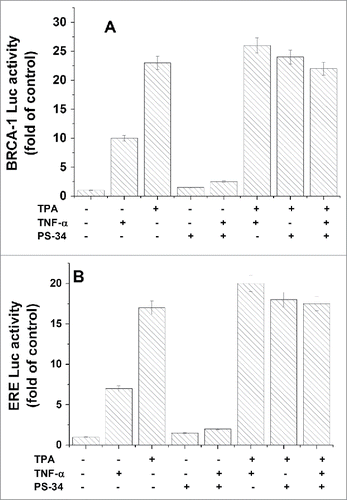
As mentioned above TPA is a potent activator of PKCs which are involved in the activation of various transcriptional factors such as NFκB.Citation62 The activation of NF-κB involves usually degradation of the IκBα subunit bound to the NF-κ B dimer, which allows its translocation to the nucleus, where it binds and activates NF-κ B-dependent genes. Due to the fact that NFκB has a binding site in BRCA1 promotor,Citation62 we decided to examine possible involvement of NFκB in TPA induced activation of BRCA1 and ERE. MCF-7 cells were transfected with BRCA1-Luc or ERE-Luc and treated with TPA in the presence or absence of 10 nM PS-34 (an inhibitor of the 26S proteasome which prevents the activation of NF-κ B by inhibiting the degradation of the IκBα). The treatment with PS-34 was at 2h post transfection up to the end of the experiment. As a control, cells were treated with 5 ng/ml tumor necrosis factor (TNF)-α, a pro-inflammatory cytokine which induces the activation of NF-κ B by initiating an intracellular signaling cascade, resulting in the phosphorylation and subsequent degradation of IκBα by the 26S-proteasome.Citation63 The treatment with (TNF)-α was at 24 h post-transfection and 6 h later the luciferase activity was measured in the cell lysates. The results show that both TPA and (TNF)-α significantly induced the expression of both BRCA1 () and ERE (). However, while treatment with PS-34 blocked completely the effect of (TNF)-α on both BRCA1 and ERE, it had no effect on the TPA induced expression of both BRCA1 () and ERE (). These results indicate that NF-κ B seems not to be involved in the TPA stimulatory effect on BRCA1 and ERE expression.
Figure 5. Examining of NFκB involvement in the TPA induced activation of BRCA1 and ERE. MCF-7 cells were transfected with BRCA1-Luc(A) or ERE-Luc (B) and treated with TPA in the presence or absence of 10 nM PS-34 (an inhibitor of NF-κ B activation). The treatment with PS-34 was at 2h post transfection up to the end of the experiment. Where indicated, cells were treated with 5ng/ml (TNF)-α, an inducer of NF-κ activation, at 6 h before harvesting the cells for analyzing the reporter expression. The presented results are an average of 3 repeated experiments ± SE.
Possible involvement of 53Bp1
53BP1 is a tumor suppressor which was identified previously as p53 binding protein and involves in enhancing p53 transactivation.Citation7,64 It was also found that 53BP1 is able to activate BRCA1 in absence of E2.Citation64 Our previous published results showed that when cells were transfected with 53BP1 and treated with E2, they exerted an additive stimulation on BRCA1 expression, indicating that their stimulatory effects on BRCA1 expression were unaffected by each other.Citation37 In the present study we examined possible involvement of 53BP1in TPA induced activation of BRCA1 and ERE expression. shows that both 53BP1 and TPA separately increased BRCA1 expression by 9 and 23 folds higher than its basal expression respectively, while when they were applied together they induced BRCA1 activation only by about 17 folds. Furthermore, when the expression of 53BP1was silenced by shRNA of 53BP1the effect of TPA on BRCA1 expression was recovered (, lane 6), indicating that indeed expression of 53BP1 in these cells is responsible for the reduction in the stimulatory effect of TPA on BRCA1 expression.
Figure 6. Examining of 53Bp1 involvement in the TPA induced activation of BRCA1 and ERE. MCF-7 cells were co-transfected with either a plasmid expressing BRCA1-Luc (1 μg) (A) or ERE-Luc (1 μg) (B) alone or together with 1 μg of the of 53BP1, shRNA 53BP1 or Tax expressing plasmids. The appropriate cell cultures were treated with TPA for 24h and E2 at 6 h before harvesting the cells for analyzing the reporter expression. The presented results are an average of 3 repeated experiments ± SE.
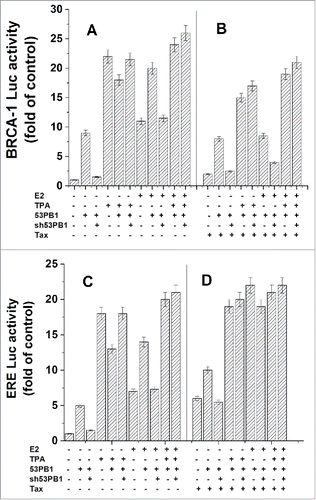
shows, as expected, that Tax had no significant effect on the induced activation of BRCA1 expression by both TPA and 53BP1, while it strongly inhibited this induced expression by E2.
When the effect of 53BP1on TPA induced activation of ERE expression was examined, our results presented in show that both 53BP1and TPA increased ERE expression by 5 and 17 folds higher than its basal expression respectively, while when they were applied together they induced ERE activation only by about 13 folds (lane 5). It seems also in the case of ERE that 53BP1 interferes in TPA induced ERE expression because when the expression of 53BP1was silenced the effect of TPA on ERE expression was recovered (, lane 6).
shows that Tax induces ERE expression and that when it was applied together with TPA or 53BP1 to these cells they additively induced ERE expression.
Effect of TPA on ERα binding to ERE and BRCA1 promoter
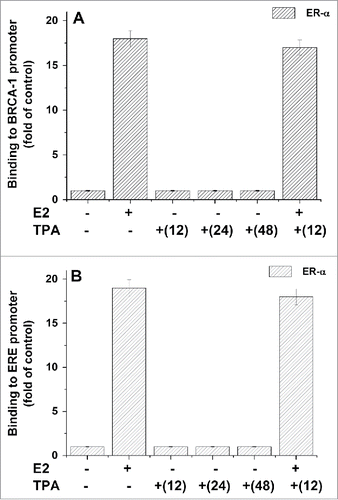
Trying to find out whether ERα is involved in TPA induced activation of BRCA1or ERE expression, we examined the effect of TPA on the binding of ERα to AP-1 DNA site at the BRCA1 promoter and to ERE region by ChIP analysis. Our results showed that while E2 strongly induced the binding of ERα to either BRCA1 promoter () or to ERE region (), TPA has no effect on the binding of ERα to these promoters.
Figure 7. Effect of TPA on ERα binding to ERE and BRCA1 promoter. MCF-7 cells which were treated with TPA (for different periods of time) and E2 at 5h before their extraction for examining the binding of ERα protein to BRCA1 promoter (A) or ERE region (B) by CHIP assay as described in Materials and Methods section. The presented results are an average of 3 repeated experiments ± SE.
Discussion
HTLV-1 Tax onc-protein was found in our previous study to stimulate the transcriptional activity of ERα through the classical pathway (inducing the expression of genes controlled by ERE sequences) while it strongly inhibited BRCA1 expression through the non-classical pathway of ERα.Citation37,59 TPA which is known as a potent PKC activator and involved in the promotion of various kinds of tumorsCitation51,52 was previously found to induce the expression of HTLV-1.Citation46,48,49,53
In the present study we examined the effect of TPA treatment on the expression of BRCA1 and genes controlled by ERE sequences in MCF-7 cells with or without Tax expression. Our results showed very strong stimulatory effect of TPA on the expression of both BRCA1 and genes controlled by ERE sequences (). This stimulatory effect was significantly higher compared with E2 induced activation of these genes. These results are interesting because TPA is known to be involved in the promotion of various kinds of tumorsCitation50-52 while BRCA1 is known as an important tumor suppressor particularly in the case of ovarian or mammary malignancies.Citation22,26 So, it is worthwhile to study possible benefits of TPA treatment as preventive against ovarian or mammary tumors development. However, on the other hand TPA strongly stimulated the expression of genes controlled by ERE sequences which part of them are responsible for enhanced replication of the cellsCitation65 and as consequences of that these cells may acquire high excess of mutations which might lead to tumors development. These results are in agreement with previous studies which showed that treatment with TPA increased pS2 mRNA expression more dramatically than the stimulation by E2.Citation66 Others also found that TPA had a synergistic effect with E2 on the stimulation of pS2 and pNR100 (E2 responsive gene) expression levels in MCF-7 cells.Citation66-68 In contrast, TPA prevented WISP-2 (other E2 responsive gene) mRNA expression.Citation67 These TPA effects on either of these gene expressions seem to be related to protein intermediates synthesis. Also, it was found that TPA induction of pS2 is mediated by ERα, while the inhibition of WISP-2 expression was independent of ERα.Citation67 From different previous studies it was concluded that ERα-mediated modulation of gene expression by protein kinase activators was not a result of changes either in the expression levels of ERα nor in its binding affinity to E2 or EREs.Citation67,69,70 Alternatively, it could be a result of stabilization or enhancement of ERα interaction with various components involved in ERα transcription activity.
Despite of that it was found that TPA, in contrast to E2, strongly reduced MCF-7 cell growth and replication.Citation66 It was concluded that the inhibitory effects of TPA on the growth stimulation of MCF-7 cells by E2 was not due to a general inhibition of the expression of E2 responsive genes but probably due to a stimulation of TGF-β 1, acting as an autocrine inhibitory growth factor.Citation66
Based on our previous studies which showed that Tax strongly inhibited the E2-ERα induced activation of BRCA1 expression while stimulating E2-ERα induced activation of ERE dependent genes,Citation37,59 it was important to find out whether Tax has any effect on TPA activities on BRCA1 and ERE responsive genes. The results of this study showed that Tax has only minor inhibitory effect on TPA stimulation of BRCA1 (), while it slightly increased the TPA stimulatory effect on ERE (). These results may indicate that the TPA effect on both BRCA1 and ERE expression is not through the ERα. However, it was found previously that in addition to the conventional way of ERα activation by E2, there are possible transduction pathways that might activate ERα without E2.Citation66,71,72 Based on the findings that signals which induce receptors activation cause phosphorylation or altered phosphorylation of the receptors or proteins associated with the receptors, it is most likely that these phosphorylations are important for ligand independent activation.Citation66
Our results showed that TPA effect on both BRCA1 and ERE is completely dependent on PKC activity () and that some of the PKC isoforms induced these activities while others blocked it (). It is interesting that the various examined PKC isoforms showed different effects on BRCA1 and ERE; for instance PKCβ2 and ϵ completely reduced BRCA1 expression while inducing ERE expression. However, PKCβ1 and PKCδ blocked ERE expression while inducing BRCA1 expression. This may indicate for different mechanism of action of TPA effect on BRCA1 and ERE expression.
Trying to explore the mechanism of TPA effect on BRCA1 and ERE expression, we examined the involvement of different appropriate transcriptional factors in this TPA activity. Our CHIP results proved that TPA has no effect on the binding of the ERα transcriptional complex to both AP1 site at the BRCA1 promoter and ERE region (). These results strongly eliminate the possibility of ERα involvement in TPA induced expression of both BRCA1 and ERE. In addition, although NFκB has a binding site in BRCA1 promotor and strongly activated by TPA,Citation62 our results () showed that NF-κ B seems also not to be involved in the TPA stimulatory effect on BRCA1 and ERE expression. However, when 53BP1 level was elevated by its ectopic expression, it caused a significant reduction in TPA induced activation of either BRCA1 or ERE expression (). It is known that the BRCA1 minimal promoter contains a binding site for 53BP164 and it was found that both SRC3 (a member of the SRC family of proteins) and 53BP1co-occupy the same region of the BRCA1 promoter.Citation34 It might be speculated that the binding of 53BP1 with BRCA1 promoter interferes with the binding of the transcriptional factor responsible for the BRCA1 activation by TPA.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Yoshida M. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 2005; 24:5931-7; PMID:16155600; https://doi.org/10.1038/sj.onc.1208981
- Nagata C, Mizoue T, Tanaka K, Tsuji I, Wakai K, Inoue M, Tsugane S. Tobacco smoking and breast cancer risk: An evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 2006; 36:387-94; PMID:16766567; https://doi.org/10.1093/jjco/hyl031
- Shrubsole MJ, Gao YT, Dai Q, Shu XO, Ruan ZX, Jin F, Zheng W. Passive smoking and breast cancer risk among non-smoking Chinese women. Int J Cancer 2004; 110:605-9; PMID:15122595; https://doi.org/10.1002/ijc.20168
- Coyle YM. The effect of environment on breast cancer risk. Breast Cancer Res Treat 2004; 84:273-88; PMID:15026625; https://doi.org/10.1023/B:BREA.0000019964.33963.09
- Snedeker SM. Chemical exposures in the workplace: effect on breast cancer risk among women. AAOHN J 2006; 54:270-9; PMID:16800404; https://doi.org/10.1177/216507990605400604
- Bernstein L, Patel AV, Ursin G, Sullivan-Halley J, Press MF, Deapen D, Berlin JA, Daling JR, McDonald JA, Norman SA, et al. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst 2005; 97:1671-9; PMID:16288120; https://doi.org/10.1093/jnci/dji374
- Ganmaa D, Willett WC, Li TY, Feskanich D, van Dam RM, Lopez-Garcia E, Hunter DJ, Holmes MD. Coffee, tea, caffeine and risk of breast cancer: A 22-year follow-up. Int J Cancer 2008; 122:2071-6; PMID:18183588; https://doi.org/10.1002/ijc.23336
- Dumitrescu RG, Shields PG. The etiology of alcohol-induced breast cancer. Alcohol 2005; 35:213-25; PMID:16054983; https://doi.org/10.1016/j.alcohol.2005.04.005
- Emery J, Lucassen A, Murphy M. Common hereditary cancers and implications for primary care. The Lancet 2001; 358:56-63; https://doi.org/10.1016/S0140-6736(00)05257-0
- Sunpaweravong S, Sunpaweravong P. Recent developments in critical genes in the molecular biology of breast cancer. Asian J Surg 2005; 28:71-5; PMID:15691805; https://doi.org/10.1016/S1015-9584(09)60265-7
- Yarden RI, Papa MZ. BRCA1 at the crossroad of multiple cellular pathways: approaches for therapeutic interventions. Mol Cancer Ther 2006; 5:1396-404; PMID:16818497; https://doi.org/10.1158/1535-7163.MCT-05-0471
- Rosen EM, Fan S, Isaacs C. BRCA1 in hormonal carcinogenesis: basic and clinical research. Endocr Relat Cancer 2005; 12:533-48; PMID:16172191; https://doi.org/10.1677/erc.1.00972
- Wu W, Koike A, Takeshita T, Ohta T. The ubiquitin E3 ligase activity of BRCA1 and its biological functions. Cell Div 2008; 3:1-10; PMID:18179693; https://doi.org/10.1186/1747-1028-3-1
- Deng CX. Roles of BRCA1 in centrosome duplication. Oncogene 2002; 21:6222-7; PMID:12214252; https://doi.org/10.1038/sj.onc.1205713
- Yun MH, Hiom K. Understanding the functions of BRCA1 in the DNA-damage response. Biochem Soc Trans 2009; 37:597-604; PMID:19442256; https://doi.org/10.1042/BST0370597
- Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res 2005; 3:531-9; PMID:16254187; https://doi.org/10.1158/1541-7786.MCR-05-0192
- Hartman AR, Ford JM. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet 2002; 32:180-4; PMID:12195423; https://doi.org/10.1038/ng953
- Ye Q, Hu Y-F, Zhong H, Nye AC, Belmont AS, Li R. BRCA1-induced large-scale chromatin unfolding and allele-specific effects of cancer-predisposing mutations. J Cell Biol 2001; 155:911-22; PMID:11739404; https://doi.org/10.1083/jcb.200108049
- Xu B, Kim S-T, Kastan MB. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol Cell Biol 2001; 21:3445-50; PMID:11313470; https://doi.org/10.1128/MCB.21.10.3445-3450.2001
- Thangaraju M, Kaufmann SH, Couch FJ. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J Biol Chem 2000; 275:33487-96; PMID:10938285; https://doi.org/10.1074/jbc.M005824200
- Parvin JD. BRCA1 at a branch point. Proc Natl Acad Sci USA 2001; 98:5952-4; PMID:11371630; https://doi.org/10.1073/pnas.121184998
- Rosen EM, Fan S, Pestell RG, Goldberg ID. BRCA1 gene in breast cancer. J Cell Physiol 2003; 196:19-41; PMID:12767038; https://doi.org/10.1002/jcp.10257
- Baldassarre G, Battista S, Belletti B, Thakur S, Pentimalli F, Trapasso F, Fedele M, Pierantoni G, Croce CM, Fusco A. Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma. Mol Cell Biol 2003; 23:2225-38; PMID:12640109; https://doi.org/10.1128/MCB.23.7.2225-2238.2003
- Thakur S, Nakamura T, Calin G, Russo A, Tamburrino JF, Shimizu M, Baldassarre G, Battista S, Fusco A, Wassell RP, et al. Regulation of BRCA1 transcription by specific single-stranded DNA binding factors. Mol Cell Biol 2003; 23:3774-87; PMID:12748281; https://doi.org/10.1128/MCB.23.11.3774-3787.2003
- Catteau A, Harris WH, Xu CF, Solomon E. Methylation of the BRCA1 promoter region in sporadic breast and ovarian cancer: correlation with disease characteristics. Oncogene 1999; 18:1957-65; PMID:10208417; https://doi.org/10.1038/sj.onc.1202509
- Mueller CR, Roskelley CD. Regulation of BRCA1 expression and its relationship to sporadic breast cancer. Breast Cancer Res 2003; 5:45-52; PMID:12559046; https://doi.org/10.1186/bcr557
- Taylor J, Lymboura M, Pace PE, A'hern RP, Desai AJ, Shousha S, Coombes RC, Ali S. An important role for BRCA1 in breast cancer progression is indicated by its loss in a large proportion of non-familial breast cancers. International Journal of Cancer 1998; 79:334-42; PMID:9699523; https://doi.org/10.1002/(SICI)1097-0215(19980821)79:4<334::AID-IJC5>3.0.CO;2-W
- Girault I, Bieche I, Lidereau R. Role of estrogen receptor alpha transcriptional coregulators in tamoxifen resistance in breast cancer. Maturitas 2006; 54:342-51; PMID:16822624; https://doi.org/10.1016/j.maturitas.2006.06.003
- Jeffy BD, Hockings JK, Kemp MQ, Morgan SS, Hager JA, Beliakoff J, Whitesell LJ, Bowden GT, Romagnolo DF. An estrogen receptor-α/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors: repressive effects of p53 on BRCA-1 transcription. Neoplasia 2005; 7:873-82; PMID:16229810; https://doi.org/10.1593/neo.05256
- Marks JR, Huper G, Vaughn JP, Davis PL, Norris J, McDonnell DP, Wiseman RW, Futreal PA, Iglehart JD. BRCA1 expression is not directly responsive to estrogen. Oncogene 1997; 14:115-21; PMID:9010238; https://doi.org/10.1038/sj.onc.1200808
- Hockings JK, Degner SC, Morgan SS, Kemp MQ, Romagnolo DF. Involvement of a specificity proteins-binding element in regulation of basal and estrogen-induced transcription activity of the BRCA1 gene. Breast Cancer Res 2008; 10:R29. (31 March 2008); PMID:18377656; https://doi.org/10.1186/bcr1987
- Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res 2006; 66:2224-32; PMID:16489025; https://doi.org/10.1158/0008-5472.CAN-05-1619
- Wang W, Schneider-Broussard R, Kumar A, MacLeod M, Johnson D. Regulation of BRCA1 Expression by the Rb-E2F Pathway. J Biol Chem 2000; 275:4532-6; PMID:10660629; https://doi.org/10.1074/jbc.275.6.4532
- Corkery D, Thillainadesan G, Coughlan N, Mohan RD, Isovic M, Tini M, Torchia J. Regulation of the BRCA1 gene by an SRC3/53BP1 complex. BMC Biochem 2011; 12:50-62; PMID:21914189; https://doi.org/10.1186/1471-2091-12-50
- Ohshima K. Pathological features of diseases associated with human T-cell leukemia virus type I. Cancer Sci 2007; 98:772-8; PMID:17388788; https://doi.org/10.1111/j.1349-7006.2007.00456.x
- Azran I, Schavinsky-Khrapunsky Y, Aboud M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 2004; 1:20-43; PMID:15310405; https://doi.org/10.1186/1742-4690-1-20
- Shukrun M, Jabareen A, Abu-akandil A, Chamias R, Aboud M, Huleihel M. HTLV-1 Tax oncoprotein inhibits the estrogen-induced BRCA1 exprestion through the ERalpha non-convetional transcriptional activation. Plos One 2014; 9(2):e89390; PMID:24586743; https://doi.org/10.1371/journal.pone.0089390
- Hino S. Milk-borne transmission of HTLV-I as a major route in the endemic cycle. Acta Paediatr Jpn 1989; 31:428-35; PMID:2514566; https://doi.org/10.1111/j.1442-200X.1989.tb01329.x
- Wiktor SZ, Pate EJ, Rosenberg PS, Barnett M, Palmer P, Medeiros D, Maloney EM, Blattner WA. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J Hum Virol 1997; 1:37-44; PMID:10195229
- Li HC, Biggar RJ, Miley WJ, Maloney EM, Cranston B, Hanchard B, Hisada M. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J Infect Dis 2004; 190:1275-8; PMID:15346338; https://doi.org/10.1086/423941
- Ureta-Vidal A, Angelin-Duclos C, Tortevoye P, Murphy E, Lepère JF, Buigues RP, Jolly N, Joubert M, Carles G, Pouliquen JF, et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers. Int J Cancer 1999; 82:832-6; PMID:10446450; https://doi.org/10.1002/(SICI)1097-0215(19990909)82:6<832::AID-IJC11>3.0.CO;2-P
- Southern SO, Southern PJ. Persistent HTLV-I infection of breast luminal epithelial cells: a role in HTLV transmission? Virology 1998; 241:200-14; PMID:9499795; https://doi.org/10.1006/viro.1997.8978
- LeVasseur RJ, Southern SO, Southern PJ. Mammary epithelial cells support and transfer productive human T-cell lymphotropic virus infections. J Hum Virol 1998; 1:214-23; PMID:10195245
- Southern S, Southern P. Cellular mechanism for milk-borne transmission of HIV and HTLV. Adv Exp Med Biol 2002; 503:183-90; PMID:12026019
- Hecker E, Schmidt R. Phorbolesters–the irritants and cocarcinogens of Croton Tiglium L. Fortschr Chem Org Naturst 1974; 31:377-467; PMID:4609865
- Ito I, Matsuda S, Tokudo H, Nakao Y. Tumor promoting diterpen esters as possible environmental co-factors for ATL. In: R.C. Gallo, ME, L. Gross, ed. Human T-cell leukemia/lymphoma virus and adult T-cell leukemia. New York: Cold Spring Harbor, Cold Spring Harbor, 1984:69-74
- Jones K. Review of Sangre de Drago (Croton lechleri) - A South American tree sap in the treatment of diarrhea, inflammation, insect bites, viral infections, and wounds: Traditional uses to clinical research. J Alternat Complement Med 2003; 9:877-96; https://doi.org/10.1089/107555303771952235
- Matsuda S, Nakao Y, Ohigashi H, Koshimizu K, Ito Y. Plant-derived diterpene esters enhance HTLV-I-induced colony formation of lymphocytes in co-culture. Int J Cancer 1986; 38:859-65; PMID:3025104; https://doi.org/10.1002/ijc.2910380613
- Nakao Y, Matsuda S, Matusi T, Nakagawa T, Fujita T, Uchiyama T, Maeda S, Okamoto Y, Masaoka T, Ito I. Effect of tumor promoters on human T-cell leukemia/lymphoma (HTLV)-structural protein induction in adult T-cell leukemia cells. Cancer Lett 1984; 24:129-39; PMID:6090008; https://doi.org/10.1016/0304-3835(84)90128-9
- Williams JE. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on Una de Gato and Sangre de Grado. Altern Med Rev 2001; 6:567-79
- Kim S, Chun S-Y, Kwon Y-S, Nam K-S. Crosstalk between Wnt signaling and Phorbol ester-mediated PKC signaling in MCF-7 human breast cancer cells. Biomed Pharmacother 2016; 77:114-9; https://doi.org/10.1016/j.biopha.2015.12.008
- Barry OP, Kazanietz MG. Protein kinase C isozymes, novel phorbol ester receptors and cancer chemotherapy. Curr Pharm Design 2001; 7:1725-44; https://doi.org/10.2174/1381612013397041
- Nakao Y, Matsuda S, Matsui T, Koizumi T, Katakami Y, Fujita T, Y I. Inhibitors of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced multinucleated cell formation and HTLV-I p19 antigen expression in HTLV-I-infected T-cell line KH-2Lo. Int J Cancer 1986; 37:911-7; PMID:3011686; https://doi.org/10.1002/ijc.2910370618
- Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat Feb 2004; 83:249-89; https://doi.org/10.1023/B:BREA.0000014042.54925.cc
- Veprik A, Khanin M, Hermoni KL, Danilenko M, Levy Y. Y. S. Polyphenols, isothiocyanates and carotenoid derivatives enhance estrogenic activity in bone cells but inhibit it in breast cancer cells. Am J Physiol Endocrinol Metab 2011: Aug 30. [Epub ahead of print]; PMID:21878663
- Bartholin L, Guindon S, Martel S, Corbo L, Rimokh R. Identification of NF-κB responsive elements in follistatin related gene (FLRG) promoter. Gene Dev 2007; 393:153-62
- Mor-Vaknin N, Torgeman A, Galron D, Lochelt M, Flugel RM, Aboud M. The long terminal repeats of human immunodeficiency virus type-1 and human T-cell leukemia virus type-I are activated by 12-O-tetradecanoylphorbol-13-acetate through different pathways. Virology 1997; 232:337-44; PMID:9191847; https://doi.org/10.1006/viro.1997.8566
- Torgeman A, Mor-Vaknin N, Zelin E, Ben-Aroya Z, Lochelt M, Flugel RM, Aboud M. Sp1-p53 heterocomplex mediates activation of HTLV-I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate that is antagonized by protein kinase C. Virology 2001; 281:10-20; PMID:11222091; https://doi.org/10.1006/viro.2000.0779
- Abou-Kandil A, Eisa N, Jabareen A, Huleihel M. Differential effects of HTLV-1 Tax oncoprotein on the different estrogen-induced-ER α-mediated transcriptional activities. Cell Cycle 2016; 15:1208871; https://doi.org/10.1080/15384101.2016.1208871
- Abou-Kandil A, Chamias R, Huleihel M, Godbey WT, Aboud M. Differential Role of PKC-Induced c-Jun in HTLV-1 LTR Activation by 12-O-Tetradecanoylphorbol-13-acetate in Different Human T-cell Lines. PLoS ONE 2012; 7:e29934; PMID:22299029; https://doi.org/10.1371/journal.pone.0029934
- Demizu Y, Misawa T, Nagakubo T, Kanda Y, Okuhira K, Sekino Y, Naito M, Kurihara M. Structural development of stabilized helical peptides as inhibitors of estrogen receptor (ER)-mediated transcription. Bioorg Med Chem 2015; 23:4132-8; https://doi.org/10.1016/j.bmc.2015.06.067
- Chung M-H, Kim D-H, Na H-K, Kim J-H, Kim H-N, Haegeman G, Surh Y-J. Genistein inhibits phorbol ester-induced NF-κB transcriptional activity and COX-2 expression by blocking the phosphorylation of p65/RelA in human mammary epithelial cells. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2014; 768:74-83; https://doi.org/10.1016/j.mrfmmm.2014.04.003
- Tanaka K, Kawakami T, Tateishi K, Yashiroda H, Chiba T. Control of IκBα proteolysis by the ubiquitin-proteasome pathway. Biochimie 2001; 83:351-6; PMID:11295496; https://doi.org/10.1016/S0300-9084(01)01237-8
- Rauch T, Zhong X, Pfeifer GP, Xingzhi X. 53BP1 is a Positive Regulator of the BRCA1 Promoter. Cell Cycle 2005; 4(8):1078-83; PMID:15970701; https://doi.org/10.4161/cc.4.8.1855
- Arisawa K, Soda M, M A, Fujiwara S UH, M H, Takeda H, Kashino W, Suyama A. Human T-cell lymphotropic virus type-1 infection and risk of cancer: 15.4 year longitudinal study among atomic bomb survivors in Nagasaki, Japan. Cancer Sci 2006; 97:535-9; PMID:16734733; https://doi.org/10.1111/j.1349-7006.2006.00212.x
- Nutt JE, Harris AL, Lunec J. Phorbol ester and bryostatin effects on growth and the expression of oestrogen responsive and TGF-beta 1 genes in breast tumour cells. British Journal of Cancer 1991; 64:671-6; PMID:1911215; https://doi.org/10.1038/bjc.1991.379
- Inadera H. Estrogen-induced genes, WISP-2 and pS2, respond divergently to protein kinase pathway. Biochem Biophys Res Commun 2003; 309:272-8; PMID:12951045; https://doi.org/10.1016/j.bbrc.2003.07.001
- Beck S, Fegert P, Gott P. Factors regulating pS2-reporter gene expression in MCF-7 breast cancer cell line. Int J Oncol 1997; 10(5):1051-5; PMID:21533484
- Cho H, Katzenellenbogen BS. Synergistic activation of estrogen receptor-mediated transcription by estradiol and protein kinase activators. Mol Endocrinol 1993; 7(3):441-52; PMID:7683375; https://doi.org/10.1210/mend.7.3.7683375 10.1210/me.7.3.441
- Martin MB, Garcia-Morales P, Stoica A, Solomon HB, Pierce M, Katz D, Zhang S, Danielsen M, Saceda M. Effects of 12-O-tetradecanoylphorbol-13-acetate on estrogen receptor activity in MCF-7 cells. J Biol Chem 1995; 270(42):25244-51; PMID:7559663; https://doi.org/10.1074/jbc.270.42.25244
- Weigel NL, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med (Berl) 1998; 76(7):469-79; PMID:9660165; https://doi.org/10.1007/s001090050241
- Göttlicher M, Heck S, Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med (Berl) 1998; 76(7):480-9; PMID:9660166; https://doi.org/10.1007/s001090050242
