ABSTRACT
Bone marrow fibrosis is a reactive process, and a central pathological feature of primary myelofibrosis. Revealing the origin of fibroblastic cells in the bone marrow is crucial, as these cells are considered an ideal, and essential target for anti-fibrotic therapy. In 2 recent studies, Decker et al. (2017) and Schneider et al. (2017), by using state-of-the-art techniques including in vivo lineage-tracing, provide evidence that leptin receptor (LepR)-expressing and Gli1-expressing cells are responsible for fibrotic tissue deposition in the bone marrow. However, what is the relationship between these 2 bone marrow cell populations, and what are their relative contributions to bone marrow fibrosis remain unclear. From a drug development perspective, these works bring new cellular targets for bone marrow fibrosis.
Myelofibrosis was characterized as a myeloproliferative condition around 70 years ago.Citation1 Allogeneic haematopoietic stem cell transplantation is the known therapy for this disease, however this approach involves significant risks for the patients.Citation2 Unfortunately, other treatments do not alter the course of myelofibrosis.Citation3 This chronic disorder is characterized by progressive bone marrow fibrosis. The fibrotic tissue occupies the bone marrow spaces, destabilizing normal hematopoiesis.Citation4 Understanding the origin and the processes that drive fibrous tissue formation in the bone marrow is a central question in myelofibrosis research. Recent studies in several organs have identified perivascular cells as cells with plasticity to differentiate into other cell types,Citation5-21 including extracellular matrix-forming cells.Citation10,14,15,20 Which are the specific cells and the underlying mechanisms that directly contribute to bone marrow fibrosis remain completely unknown. The lack of a detailed knowledge about the cellular contributors mediating bone marrow fibrogenesis restricts the design of effective anti-fibrotic treatments. Nevertheless, in a recent article in Nature Cell Biology, Decker and colleagues propose a possible source for fibrosis-producing cells in primary myelofibrosis.Citation21 The authors used a mouse model of myelofibrosis (transplanted with haematopoietic stem cells overexpressing thrombopoietin) combined with in vivo lineage-tracing technology to track specifically Leptin receptor (LepR)-expressing cells (LepR-Cre/tdTomato mice). Strikingly, their results demonstrated that endogenous bone marrow LepR+ cells are the major source of fibrosis-producing cells in primary myelofibrosis.Citation21 Interestingly, in another recent work in Cell Stem Cell, Schneider and colleagues, using the same murine model of myelofibrosis combined with genetic lineage-tracing technology to track specifically Gli1-expressing cells (Gli1-CreERT2/tdTomato mice), showed that 50% of fibrotic cells in the bone marrow are derived from Gli1+ cells.Citation22 Here, we discuss the findings from these 2 studies, and evaluate recent advances in our understanding of these 2 bone marrow cell populations ().
Figure 1. Participation of Gli1+ and Lepr+ cells in bone marrow fibrosis in myelofibrosis. It is well accepted that the bone marrow hosts various cells with distinct functions in its microenvironment. Gli1+ cells are present around the endosteum and the blood vessels, while LepR+ cells are located mainly around sinusoids. The studies of Decker et al. (2017) and Schneider et al. (2017) now reveal that Gli1+ and LepR+ cells are recruited from endosteal and perivascular regions giving rise to fibrotic cells that contribute to the development of fibrosis in the bone marrow.Citation21,22
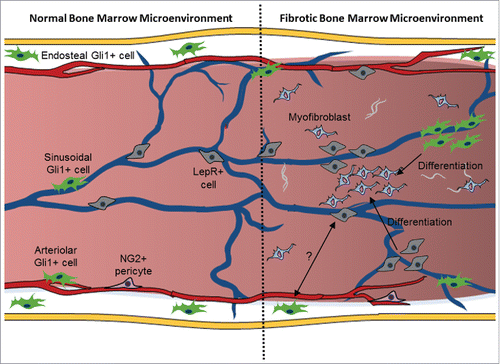
Based on these 2 works, several questions arise about the identity of Gli1+ and LepR+ cells in the bone marrow: Are those different cell populations? Are there Gli1+/LepR+ cells? Do they have a common ancestor? Or are they derived one from the other? Taking the main results from these 2 articles into account, we could simply conclude that probably Gli1+ cells correspond to a subset of LepR+ cells, as Gli1+ cells form only half of fibrotic cells in the bone marrow, while LepR-expressing cells originate the majority of these cells. However, the answer seems not to be so simple. Importantly, Schneider and colleagues did not detect leptin receptor expression in Gli1+ cells.Citation22 Thus, indicating that Gli1+ cells correspond to a cell population distinct from LepR-expressing cells.
The organization of the bone marrow can be best understood by following its vascular layout. There are 2 main types of blood vessels in the bone marrow: sinusoids and arterioles.Citation23,24 Bone marrow sinusoids are interconnected and collectively drain into the central sinus, while arterioles are derived from the branching of arterial vessels spanning the bone marrow cavity. Sinusoids arise directly from arterioles; however their composition differs.Citation25 Sinusoids are lined by a single layer of endothelium, while arterioles are thicker-walled blood vessels.Citation26 The endosteum is a histological structure located between the bone marrow and the bone. All LepR+ cells in the bone marrow are perivascular, located mostly around sinusoids.Citation27 In contrast, Gli1+ cells are heterogeneous on their location within the bone marrow; and the majority of Gli1+ cells reside aligning the bone (in the endosteal niche).Citation22,28 Although a small fraction of Gli1-expressing cells are associated with bone marrow sinusoids and arterioles, these cells do not express leptin receptor.Citation22 Together, these data strongly suggest that LepR-expressing cells differ from Gli1+ cells in the bone marrow.
All the evidence for LepR-expressing cells as the source of fibrotic cells in the bone marrow was derived from genetic lineage tracing experiments using LepR-Cre mouse line, in which expression of a constitutive Cre recombinase is under the control of LepR promoter.Citation29 Thus, LepR-Cre may label multiple cellular lineages from early developmental time points. Consequently, in adult LepR-Cre/tdTomato mice, the labeling includes both cells that express leptin receptor, and cells that derive from LepR-expressing cells. Hence, although Gli1+ cells in the bone marrow do not correspond to LepR-expressing cells, future studies should test whether Gli1+ cells derive from LepR+ cells. The use of LepR-CreER mice, in which Cre is inducible, instead of LepR-Cre will be useful to differentiate between functions of cells that express leptin receptor from cells that derive from LepR-expressing cells.
Interestingly, Decker and colleagues used in their study a mouse model for myelofibrosis that requires a relatively long time for recovery after irradiation followed by stem cells transplantation, and before the analysis of bone marrow fibrosis may be done.Citation21 Since the contribution of LepR+ cells to cells located in the endosteum increases with age,Citation27 future studies will clarify whether, in the LepR-Cre/tdTomato mice with transplanted haematopoietic cells overexpressing thrombopoietin, some of the endosteal Gli1+ cells were already labeled. The use of other mouse models for myelofibrosis that do not require stem cell transplantation, and in which the installation of the disease happens in a shorter period of time, may also clarify whether Gli1+ cells derive from LepR-expressing cells. As Gli1+ cells undergo tremendous expansion in the process of installation of myelofibrosis in the bone marrow,Citation22 it will be interesting to test whether after activation Gli1+ cells can acquire leptin receptor expression; and, thus, be labeled in LepR-Cre/tdTomato mice.
Decker and colleagues used in their genetic-fate-tracing experiments a murine model of thrombopoietin-induced myelofibrosis.Citation21 Several other murine models of myelofibrosis were engineered, including several transgenic mouse models encompassing mutations of JAK2 (JAK2V617F) with various expression levels.Citation30 Future studies should address whether LepR-expressing cells are the main source for fibrotic cells in the bone marrow also in more physiologic myelofibrosis mouse models.
Myofibroblasts are the primary extracellular matrix producing cells during fibrosis.Citation31 What is the nature of cells that originate myofibroblasts in bone marrow fibrosis? One prominent feature of myofibroblasts is the expression of α smooth muscle actin (αSMA).Citation32 Schneider and colleagues used the expression of aSMA to detect myofibroblasts derived from the Gli1 lineage.Citation22 Nevertheless, not all αSMA-expressing cells are myofibroblasts,Citation33 and αSMA-negative fibroblasts also producing extracellular matrix proteins may exist. Thus, the identification of all fibrotic cells with αSMA in the bone marrow may be not so accurate. In contrast, Decker and colleagues used Collagen1a1-GFP reporter mice to detect collagen I-producing cells derived from the LepR lineage.Citation21 However, although collagen I is often cited as the primary extracellular matrix protein expressed by fibroblastic cells in bone marrow fibrosis,Citation34 fibrotic cells also produce a myriad of other extracellular matrix proteins during fibrosis, such as collagens types II, III, IV, V, and VI.Citation35 Additionally, fibroblastic cells produce glycoproteins and proteoglycans, such as fibronectin, laminin, and tenascin.Citation36-39 Yet, myofibroblasts are not necessarily the only source of all these proteins, as, for instance, inflammatory and endothelial cells may produce these proteins as well.Citation46 Increases in the production of these various proteins in the bone marrow may have differing relationships with the disease. Thus, it is important the distinction between increases in the different types of extracellular matrix components in the bone marrow. Albeit the authors of both studies quantified reticulin, its exact cell source remains elusive. Future studies should not only clarify what is the relative contribution of Gli1-expressing and LepR-expressing cells to αSMA-expressing and type I collagen-producing cells, but also to the production of other extracellular matrix components present in the fibrous tissue in the bone marrow in myelofibrosis.
Haematopoietic stem cells (HSC) are the adult stem cells that contain the potential to form all specialized cells present in the blood.Citation25 Although, HSCs are present in some other organs,Citation47,48 they predominantly reside in the bone marrow.Citation23 Several genetically modified mice models have been widely applied to study the complexity of specific HSC niche cell types within the bone marrow.Citation49-50 These studies identified several cell types as potential niche-supporting cells for HSCs, supplying various cytokines and retention factors to regulate HSC activity.Citation24 LepR-Cre-based deletion of stem cell factor negatively affects HSC maintenance in the bone marrow,Citation46 showing that LepR-expressing cells are essential components of the HSC niche. Importantly, genetic depletion of Gli1+ Cells using Gli1CreER/iDTR mice did not affect HSCs in the bone marrow, suggesting that Gli1+ cells are not essential components of the microenvironment needed for HSC maintenance in the bone marrow. Together, these results also strongly suggest that Gli1+ and LepR-expressing cells are distinct cell populations.
Decker and colleagues also showed that LepR-Cre-based deletion of platelet derived growth factor receptor α (PDGFRα) alleviates bone marrow fibrosis.Citation21 Provocatively, although only half of fibrotic cells were derived from the Gli1 lineage, the ablation of Gli1+ cells completely abolished fibrosis in the bone marrow.Citation22 These data suggest that Gli1+ cells and LepR+ cells may interact in the formation of fibrotic tissue, and although Gli1+ cells do not form all fibrotic cells in the bone marrow fibrosis, they are needed for their formation. Future studies, in addition to characterizing better the identity of Gli1+ and LepR+ cells in the bone marrow, will need to determine the relative contributions of Gli1-expressing and LepR+ cells to bone marrow fibrosis in myelofibrosis.
Importantly, Decker et al. (2017) and Schneider et al. (2017) proposed pharmacological targeting of LepR+ and Gli1+ cells with Imatinib and GANT61, respectively. Although both therapies alleviate bone marrow fibrosis, they are not specific exclusively to LepR+ and Gli1+ cells in the bone marrow. Consequently, they may cause several undesired side effects in the patients with myelofibrosis. Imatinib targets and blocks several tyrosine kinases, and has been shown to cause severe bone marrow aplasia in some cases.Citation47 GANT61 blocks both GLI1 and GLI2 proteins.Citation48 As GLI 2 is involved in bone development,Citation49,50 possible side effects for using GANT61 may include bone defects. Thus, the aim of future studies should be to develop a more specific drug that will target specifically only cells involved in bone marrow fibrosis. Since LepR-expressing and Gli1-expressing cells are present in several other organs beside the bone marrow, the effects of blocking or depletion of these cell populations need to be taken into consideration when developing a specific targeted therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Alexander Birbrair is supported by a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Akiva Mintz is supported by the National Institute of Health (1R01CA179072–01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13–121–01-CDD).
References
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372-5. PMID:14820991
- Fleischman AG, Maziarz RT. Hematopoietic stem cell transplantation for myelofibrosis: where are we now? Curr Opin Hematol. 2013;20:130-6. doi:10.1097/MOH.0b013e32835dd862. PMID:23314844
- Tefferi A. How I treat myelofibrosis. Blood. 2011;117:3494-504. doi:10.1182/blood-2010-11-315614. PMID:21200024
- Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annual Rev Med. 2009;60:233-45. doi:10.1146/annurev.med.60.041707.160528
- Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos Santos GS, Mintz A, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol. 2017;427:6-11. doi:10.1016/j.ydbio.2017.05.001. PMID:28479340
- Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavacana N, Assoni AF, Pelatti MV, Birbrair A, de Lima ACP, Singer JM, Rocha FMM, Da Silva GL, Mantovani MS, Macedo-Souza LI, Ferrari MFR, Zatz M. Pericytes extend survival of ALS SOD1 mice and induce the expression of antioxidant enzymes in the murine model and in IPSCs derived neuronal cells from an ALS patient. Stem Cell Rev 2017. doi:10.1007/s12015-017-9752-2. PMID:28710685
- Birbrair A, Delbono O. Pericytes are Essential for Skeletal Muscle Formation. Stem Cell Rev. 2015;11:547-8. doi:10.1007/s12015-015-9588-6. PMID:25896402
- Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, et al. Novel peripherally derived neural-like stem cells as therapeutic carriers for treating glioblastomas. Stem Cells Translational Med. 2017;6:471-81. doi:10.5966/sctm.2016-0007
- Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PloS One. 2011;6:e16816. doi:10.1371/journal.pone.0016816. PMID:21304812
- Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Therapy. 2014;5:122. doi:10.1186/scrt512
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res. 2013;319:45-63. doi:10.1016/j.yexcr.2012.09.008. PMID:22999866
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013;10:67-84. doi:10.1016/j.scr.2012.09.003. PMID:23128780
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298-314. doi:10.1089/scd.2012.0647. PMID:23517218
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Frontiers Aging Neurosci. 2014;6:245. doi:10.3389/fnagi.2014.00245
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond). 2015;128:81-93. doi:10.1042/CS20140278. PMID:25236972
- Birbrair A, Zhang T, Wang Z-M, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol-Cell Physiol. 2014;307:C25-C38. doi:10.1152/ajpcell.00084.2014. PMID:24788248
- Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O. How plastic are pericytes? Stem Cells Dev. 2017;26(14):1013-1019. doi:10.1089/scd.2017.0044. PMID:28490256
- Prazeres PHDM, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, Azevedo PO, Souto L, Almeida GG, Filev R, et al. Macrophages generate pericytes in the developing brain. Cell Mol Neurobiol. 2017. In press.
- Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A. Pericytes make spinal cord breathless after injury. Neuroscientist. 2017. In press. doi: 10.1177/1073858417731522
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013;305:C1098-113. doi:10.1152/ajpcell.00171.2013. PMID:24067916
- Decker M, Martinez-Morentin L, Wang G, Lee Y, Liu Q, Leslie J, Ding L. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol. 2017;19:677-88. doi:10.1038/ncb3530. PMID:28481328
- Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PMH, Bindels EM, Heckl D, Busche G, Fleck D, et al. Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2017;20(6):785-800.e8. doi:10.1016/j.stem.2017.03.008. PMID:28457748
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637-43. doi:10.1038/nature12612. PMID:24107994
- Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma'ayan A, Frenette PS. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19:214-23. doi:10.1038/ncb3475. PMID:28218906
- Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Annals N York Acad Sci. 2016;1370:82-96. doi:10.1111/nyas.13016
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiol. 2005;20:349-56. doi:10.1152/physiol.00025.2005
- Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154-68. doi:10.1016/j.stem.2014.06.008. PMID:24953181
- Sena I, Prazeres P, Santos G, Borges I, Azevedo P, Andreotti J, Almeida V, Paiva A, Guerra D, Lousado L, Souto L, Mintz A, Birbrair A. Identity of gli1+ cells in the bone marrow. Exp Hematol. 2017 Jul 6. pii: S0301-472X(17)30612-4. doi:10.1016/j.exphem.2017.06.349. In press.
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608-13. doi:10.1126/science.1056602. PMID:11283374
- Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, Skoda RC. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931-40. doi:10.1182/blood-2007-08-107748. PMID:18160670
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. doi:10.1002/path.2277. PMID:18161745
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349-63. doi:10.1038/nrm809. PMID:11988769
- Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomechanics. 2010;43:146-55. doi:10.1016/j.jbiomech.2009.09.020
- Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. British J Haematol. 2007; 139:351-62. doi:10.1111/j.1365-2141.2007.06807.x. PMID:17910625
- Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114-25. PMID:7518191
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526-37. doi:10.1038/sj.jid.5700613. PMID:17299435
- Berndt A, Kosmehl H, Katenkamp D, Tauchmann V. Appearance of the myofibroblastic phenotype in Dupuytren's disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix. Pathobiol J Immunopathol Mol Cell Biol. 1994;62:55-8. doi:10.1159/000163879
- Magro G, Fraggetta F, Colombatti A, Lanzafame S. Myofibroblasts and extracellular matrix glycoproteins in palmar fibromatosis. General Diagnostic Pathol. 1997;142:185-90
- Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341-8. PMID:9435560
- Paiva AE, Lousado L, Almeida VM, Andreotti J, Santos GSP, Azevedo PO, Sena IFG, Prazeres PHDM, Borges IT, Azevedo V, et al. Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia. 2017. In press.
- Borges IDT, Sena IFG, de Azevedo PO, Andreotti JP, de Almeida VM, de Paiva AE, Pinheiro Dos Santos GS, de Paula Guerra DA, Dias Moura Prazeres PH, Mesquita LL, Silva LSB, Leonel C, Mintz A, Birbrair A. Lung as a niche for hematopoietic progenitors. Stem Cell Rev Reports. 2017 Jul 1. doi:10.1007/s12015-017-9747-z. In press. PMID:28669077
- Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma'ayan A, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2016;351:176-80. doi:10.1126/science.aad0084. PMID:26634440
- Andreotti JP, Mesquita L, Magno LAV, Birbrair A. Hypothalamic neurons take center stage in the neural stem cell niche. Cell Stem Cell. 2017. In press. doi:10.1016/j.stem.2017.08.005
- Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, Prazeres PHDM, Filev R, Mintz A, Birbrair A. Endothelial cells maintain neural stem cells quiescent in their niche. Neuroscience. 2017. In press.
- Lousado L, Andreotti JP, Paiva AE, Azevedo PO, Prazeres PHDM, Santos GSP, Filev R, Mintz A, Birbrair A. Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death and Dis. 2017. In press.
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457-62. doi:10.1038/nature10783. PMID:22281595
- Srinivas U, Pillai LS, Kumar R, Pati HP, Saxena R. Bone marrow aplasia–a rare complication of imatinib therapy in CML patients. Am J Hematol. 2007;82:314-6. doi:10.1002/ajh.20776. PMID:17013815
- Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104:8455-60. doi:10.1073/pnas.0609699104. PMID:17494766
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063-71. doi:10.1158/0008-5472.CAN-06-0054. PMID:16778178
- Sterling JA, Oyajobi BO, Grubbs B, Padalecki SS, Munoz SA, Gupta A, Story B, Zhao M, Mundy GR. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer Res. 2006;66:7548-53. doi:10.1158/0008-5472.CAN-06-0452. PMID:16885353
