ABSTRACT
The acrosome reaction (AR) is indispensable for successful spermatozoon-oocyte fusion. Recent studies have indicated that sperm IZUMO1 gradually gathers in the equatorial segment (EQ), which is the initiation site of sperm-egg fusion, after the AR. In addition, by examining the binding process of oocytes and Izumo1-expressing cultured cells to reconstitute the early steps of fertilization, we previously demonstrated that robust IZUMO1-dependent adhesion specifically occurs at the contact site along with the dimerization of IZUMO1. However, when IZUMO1 dimerizes after the AR in living spermatozoon is unknown. Here, we report dynamics of IZUMO1 dimerization during the AR in spermatozoa by combining transgenic mice and time-lapse imaging using a set of bimolecular fluorescence complementation (BiFC) probes. Surprisingly, dimeric IZUMO1 was already formed at the acrosomal cap region before the AR and redistributed into the EQ after the AR. We categorized the translocation of the dimer into two types: Type 1, the near-simultaneous appearance of BiFC signals with IZUMO1-mCherry; and Type 2, the delayed formation of dimer in the EQ. Those findings suggest that, before encountering oocytes, spermatozoa are prepared to boost their affinity with JUNO.
Introduction
Sperm-egg fusion is considered to consist of multiple steps. As a reason for that using gene-modified animals, IZUMO1 and SPACA6 on the sperm side and IZUMO1-receptor JUNO and CD9 on the ovum side have been unveiled as indispensable factors for triggering gamete fusion [Citation1–Citation6], however, the functional relationships among each factor, except for the IZUMO1-JUNO interaction, in which we recently clarified the tertiary structures of human IZUMO1-JUNO complex at atomic-level resolution (2.0–3.2 Å)[Citation7], are still unclear. Furthermore, we have also established an in vitro cell-oocyte binding system, in which cultured cells expressing the Izumo1 gene, such as COS-7 cells, become adhesion-competent towards oocytes. However, because COS-7 cells solely expressing the Izumo1 gene never acquire membrane fusion activity with oocytes [Citation8,Citation9], the detailed molecular mechanisms leading to gamete membrane fusion also remain elusive[Citation10].
The acrosome reaction (AR), which involves exocytosis that allows an oocyte to fuse with a spermatozoon, has been believed to be initiated on the surface of an egg’s zona pellucida (ZP). However, a recent study has revealed that most fertilizing spermatozoa undergo the AR before attaching to the ZP[Citation11]. Surprisingly, most spermatozoa underwent the AR in the upper segments of the oviductal isthmus, which was shown using double transgenic mouse sperm carrying both enhanced green fluorescent protein (EGFP) to the acrosome, which loses fluorescence after the AR, and red fluorescent protein (DsRed2) in midpiece mitochondria [Citation12–Citation14]. In addition, we previously demonstrated that even spermatozoa that had undergone ZP penetration retained the capability to cross the ZP and fertilize eggs[Citation15]. According to Sato and Blandau, the penetration of spermatozoa through the ZP takes an average of 20 min with a range of 15–26 min in vitro after the AR [Citation16,Citation17].
In the present study, we generated a transgenic mouse line to visualize the oligomeric status of IZUMO1 in live spermatozoa using a bimolecular fluorescence complementation (BiFC) tag. We report here the dynamics of dimeric IZUMO1 translocation after the AR.
Results
Production of IZUMO1-BiFC transgenic mouse
To visualize the oligomeric status of IZUMO1 during the AR in living spermatozoa, we generated a transgenic mouse line expressing BiFC-tagged IZUMO1 (IZUMO1-BiFC), in which IZUMO1 fused to one of two BiFC fragments, VN155 or VC, was inserted between a testis-specific Calmegin promotor and rabbit β-globin polyadenylation signal (). Since our previous studies showed that the addition of the mCherry-tag to the carboxy terminal of IZUMO1 did not disturb its function[Citation18] and the cytoplasmic tail of IZUMO1 physiologically plays no role[Citation19], we added the fragment to the carboxy terminal. The BiFC assay is based on the reconstitution of a fluorophore when two complementary non-fluorescent Venus-truncated fragments, such as VN155 and VC, are brought together by a pair of fused proteins in close proximity[Citation20]. We reasoned that this method should be applicable in mouse experiments and enable us to elucidate where dimerization occurs in living spermatozoa.
In the current study, IZUMO1-BiFC expression in spermatozoa was confirmed by Western blotting using anti-IZUMO1 with or without N-glycosidase treatment ()). This result indicates that the expression level was comparable to that of Izumo1-null background IZUMO1 transgenic spermatozoa (IZUMO1-TG) ()). IZUMO1-VN155/VC has a slowly migrating doublet compared to IZUMO1-TG on a SDS gel (), asterisks) and the molecular mass of both IZUMO1-TG and IZUMO1-VN155/VC was slightly reduced by N-glycosidase treatment, as predicted[Citation21]. Control sperm BASIGIN was converted from a doublet of ~ 25 kDa to a fast migrating single band ()). We confirmed that this hybrid protein was biologically functional because after being introduced into an Izumo1-null background, their average litter size was normal, at 9.5 ± 2.14 pups (mean ± s. d., n = 20), compared with that of wild-type mice (general average litter size is 8–10 pups).
Figure 1. Live sperm imaging of oligomerization of IZUMO1 tagged by BiFC. (a) Constructs of transgenes to express mouse Izumo1 under the control of Calmegin promoter. (b) Expression of IZUMO1-BiFC in spermatozoa. The intensity of 60–70 kDa doublet bands (asterisks) of IZUMO1-BiFC (IZUMO1-VN155 and -VC) was comparable to those of His-tagged IZUMO1 TG when a monoclonal antibody against IZUMO1 (Mab18) was used. In either case, treatment with PNGase F (New England Biolabs) caused slight mobility shift. BASIGIN antibody (sc-9757: Santa Cruz Biotechnology) was also used as a loading control. In each lane, 10 μg of sperm protein was used for SDS-PAGE. (c) Fluorescence images of a spermatozoon expressing IZUMO1-mCherry and -BiFC. The relative fluorescence intensity in the arrow of the merged images (acrosome intact and acrosome reacted spermatozoon) in c was analyzed using Fiji. AC and EQ stands for acrosomal cap region and equatorial segment, respectively. Magnified images (3×) of a boxed region after the AR are also shown in Fire LUT of Fiji. BiFC signals were almost absent in the acrosomal cap region. (d) Antibody staining of IZUMO1-BiFC expressing spermatozoon. Non-permeabilized spermatozoon expressing IZUMO1-BiFC was stained with Alexa-546 labeled anti-IZUMO1 monoclonal antibody (Mab18). Three times enlarged images as in d at the bottom. Scale bar, 2 μm.
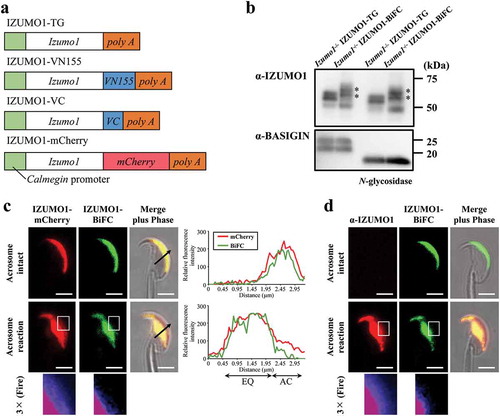
In order to visualize the entire IZUMO1, we introduced the IZUMO1-mCherry transgene by mating with IZUMO1-BiFC mouse line with Izumo1-null background. Acrosome-reacted spermatozoa were judged based on IZUMO1 translocation from the acrosomal cap region to the EQ. To our surprise, even the acrosome intact sperm emitted BiFC-derived fluorescence and evenly distributed throughout the acrosomal cap region (), top). Intriguingly, once the AR was initiated, BiFC-derived fluorescence signals completely covered the EQ, while BiFC signals were absent in the acrosomal cap region (), middle). In ), the right graph is the quantification of the cell image (), arrow). The distributions of the mCherry and BiFC signals were almost identical to each other in the acrosome intact spermatozoa. However, although some IZUMO-mCherry signals still remained in the acrosomal cap region, the BiFC signals were completely absent (), right graph and enlarged images of the boxed regions (middle) at bottom in the Fire LUT). Instead, all BiFC signals had moved to the EQ ()). To further confirm this observation, we stained the spermatozoa from IZUMO1-BiFC with Izumo1-null genetic background using anti-IZUMO1 antibody without permeabilization. Reactivity was only detected after the AR similar distribution to that of IZUMO1-mCherry (), middle and bottom), indicating that partial IZUMO1-VN155 or VC can stay in the acrosomal cap region after the AR. These results suggest that, after the AR, dimeric IZUMO1 cannot remain in the acrosomal cap region of acrosome-reacted spermatozoa.
Time-lapse imaging of dimer IZUMO1 during the acrosome reaction
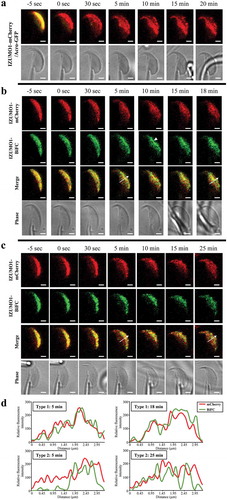
Next, we observed the movement of dimeric IZUMO1 on live spermatozoon during the AR. First, we confirmed whether the AR can be observed under our confocal microscope setup. To this end, we treated the glass surface with poly-L-lysine to attach spermatozoa but not an empty ZP[Citation18]. Consecutive images of IZUMO1-mCherry on spermatozoa during the AR were successfully captured and a representative time-lapse image during the AR is shown both in and Supplementary Video 1. Consistent with our previous report[Citation18], the redistribution of IZUMO1 took place coincidentally with the disappearance of GFP from the acrosome. In addition, slow assembly of IZUMO1 in the EQ of the sperm head was observed over a period of 10 min after the AR, as previously reported[Citation18].
To elucidate how dimerization proceeds after the AR, we examined the time sequences of simultaneously-captured fluorescence images of BiFC and mCherry in IZUMO-BiFC and IZUMO1-mCherry spermatozoa (Izumo1 null genetic background) at 5-sec intervals. By analyzing the time-lapse images, we noticed that the dimer dynamics can be classified into two types after the AR. Type 1: IZUMO1-BiFC spread out as soon as IZUMO1-mCherry moved toward the posterior region of the sperm head. Particularly, the BiFC signals were enhanced in the aggregated area (arrow head). In this type, as early as 5 min after the AR, the IZUMO1-BiFC had almost completely covered the EQ () and Supplementary Video 2). Type 2: Five min after the AR, mCherry signals had occupied the EQ; however, the BiFC signals were not induced ()). The high BiFC area was slowly moved toward the EQ, and was finally merged with the mCherry signals at 25 min after the AR () and Supplementary Video 3). In both cases, all IZUMO1 proteins arrived in the EQ within 25 min of the AR.
Figure 2. Time-lapse of IZUMO1 oligomerization upon the AR. (a) Translocation of IZUMO-mCherry. In Acro-GFP/IZUMO1-mCherry double transgenic mice, mCherry and GFP fluorescence images were captured before and after the AR. Immediately after the AR, no GFP signals were detected, indicating acrosomal exocytosis, and translocation of IZUMO1-mCherry to the EQ was observed. (b) Type 1 movement of IZUMO1-BiFC. Appearance of BiFC fluorescence (green) followed IZUMO1-mCherry (red) almost simultaneously. Arrow head indicates the region where IZUMO1 was clustered. (c) Type 2 movement of IZUMO1-BiFC. Formation of BiFC signals (green) was delayed compared to translocation of IZUMO1-mCherry (red) to the EQ after the AR. Scale bar, 2 μm. (d) Quantification of b and c. Fluorescence intensity was measured at an arrow in b and c. In Type 2 movement, BiFC was largely devoid in the EQ at 5 min, and caught up with mCherry signals over 25 min.
Discussion
Recently, we described a series of novel molecular mechanisms in sperm-egg recognition. JUNO first binds to monomeric IZUMO1. The complex then gradually gathers at the interface of the spermatozoon and induces dimerization. The process follows a tight binding phase where IZUMO1 bends the entire structure towards the sperm membrane side through a thiol-disulfide exchange reaction. The molecule no longer binds to JUNO and instead binds to a putative second oocyte receptor. This event should follow the initiation of force generation to collapse the repulsion between the juxtaposing membranes[Citation9]. By using living spermatozoa, we show here that dimer IZUMO1 is partially formed and slowly propagated to the EQ after the AR.
In our previous study, we described the presence of two types of dimer states in IZUMO1 (“open” and “closed” dimer). The closed dimer IZUMO1 directly participates in membrane fusion after binding with JUNO, most likely through an unidentified receptor on the egg, while the open dimer is induced when JUNO interaction is not involved. We think that the open dimer plays a role in boosting the affinity with JUNO to increase the success rate of fertilization as a preceding step to contact with the egg because the Kd of mouse IZUMO1-JUNO (12.3 μM) corresponds only to approximately 1/100 affinity of that of human IZUMO1-JUNO (91 nM) [Citation1,Citation7]. Thus, this system might be adopted in mouse fertilization to overcome the low affinity in IZUMO1-JUNO interaction.
A previous study reported that it takes an average of 20 min for spermatozoon to cross the ZP[Citation16]. Given that the acquisition of IZUMO1-BiFC type 2 signals in the EQ took 25 min after the AR ()), it is reasonable to assume that mouse spermatozoa may need time to prepare for membrane fusion by increasing dimeric IZUMO1 density in the EQ.
Currently, the molecular mechanism of IZUMO1 movement during the AR is unknown; however, its translocation seems to be dependent on actin polymerization, because latrunculin A, an actin polymerization inhibitor, treated spermatozoa are significantly inhibited translocation of IZUMO1 after the AR [Citation22,Citation23]. This is observed not only in IZUMO1 but also in other sperm membrane proteins such as CD46 and β1 integrin[Citation24]. In addition, spermatozoa from testis-specific serine kinase 6 (TSSK6), which actively participates in actin polymerization in spermatozoa, deficient mice almost completely failed to translocate IZUMO1 after the AR[Citation22]. Given that the cytoplasmic tail of IZUMO1 is dispensable for fertilization[Citation19], the appearance of IZUMO1, along with its dimerization in the EQ, is not regulated by direct binding to F-actin, but by reorganization with other membrane components. Thus, further study is required to identify these essential components.
Further comprehensive understanding of gamete fusion by determining the relationship among all fusion factors, including IZUMO1, JUNO, CD9 and SPACA6, will be made possible by several different lines of experiments, mostly using genetically modified animals. Clarification of the molecular mechanism of fertilization will benefit the clinical treatment of sterility and support the potential development of novel contraceptive strategies.
Materials and methods
Animal
All animal experiments were approved by the Animal Care and Use Committee of Fukushima Medical University, Japan. All mice, including IZUMO1 knockout[Citation2], IZUMO1-His transgenic[Citation2], IZUMO1-mCherry transgenic[Citation18], and Acro-GFP[Citation25] mice, were kindly provided by Osaka University.
Production of IZUMO1-BiFC transgenic mice
A detailed construct design is shown in our previous report[Citation9]. To express them in testis, we designed Izumo1-VN155 or VC, to be inserted between the Calmegin promoter (testis specific promoter) and a rabbit β-globin polyadenylation signal ()). The transgenic mouse line was produced by co-injecting both Izumo1-VN155 and Izumo1-VC, including fragments, into the pronuclei.
Fluorescence imaging
Spermatozoa from the IZUMO1-mCherry and/or IZUMO1-BiFC transgenic mouse lines were incubated for 2 h at 37°C in TYH medium[Citation26]. A 60 × water-immersion objective (NA 1.27) was used to capture confocal images with an A1R microscope (Nikon). A pinhole of 3.0 airy units was set. The images were processed using the built-in software NIS-Elements ver. 4 (Nikon) and Fiji[Citation27].
Time-lapse imaging
Spermatozoa from the IZUMO1-mCherry and Acro-GFP or IZUMO1-BiFC double transgenic mouse lines were incubated for 90 min in TYH medium, and were transferred to the poly-L-lysine coated glass-bottomed dish (glass No.1.5, MatTek) at a final concentration of 5 × 105 cell/ml. Immediately after treatment with 20 μM A23187 to trigger the AR, the dish was set on an inverted microscope stage and kept at 37°C, and fluorescence images were taken every 5 sec for 35 min at a resonant mode of Nikon A1R under simultaneous excitation of 488 and 561 nm lasers. An oil-immersion 60 × objective lens (NA 1.49) was used.
Supplemental Material
Download Zip (20.9 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Bianchi E, Doe B, Goulding D, et al. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487.
- Inoue N, Ikawa M, Isotani A, et al. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238.
- Kaji K, Oda S, Shikano T, et al. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet. 2000;24:279–282.
- Le Naour F, Rubinstein E, Jasmin C, et al. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321.
- Lorenzetti D, Poirier C, Zhao M, et al. A transgenic insertion on mouse chromosome 17 inactivates a novel immunoglobulin superfamily gene potentially involved in sperm-egg fusion. Mamm Genome: off J Int Mamm Genome Soc. 2014;25:141–148.
- Miyado K, Yamada G, Yamada S, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–324.
- Ohto U, Ishida H, Krayukhina E, et al. Structure of IZUMO1-JUNO reveals sperm-oocyte recognition during mammalian fertilization. Nature. 2016;534:566–569.
- Inoue N, Hamada D, Kamikubo H, et al. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development. 2013;140:3221–3229.
- Inoue N, Hagihara Y, Wright D, et al. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat Commun. 2015;6:8858.
- Inoue N. Novel insights into the molecular mechanism of sperm-egg fusion via IZUMO1. J Plant Res. 2017;130:475–478.
- Jin M, Fujiwara E, Kakiuchi Y, et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proceedings of the National Academy of Sciences of the United States of America 2011; 108:4892–4896.
- La Spina FA, Puga Molina LC, Romarowski A, et al. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev Biol. 2016;411:172–182.
- Muro Y, Hasuwa H, Isotani A, et al. Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biol Reprod. 2016;94:80.
- Hino T, Muro Y, Tamura-Nakano M, et al. The behavior and acrosomal status of mouse spermatozoa in vitro, and within the oviduct during fertilization after natural mating. Biol Reprod. 2016;95:50.
- Inoue N, Satouh Y, Ikawa M, et al. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proceedings of the National Academy of Sciences of the United States of America 2011; 108:20008–20011.
- Sato K, Blandau RJ. Time and process of sperm penetration into cumulus‐free mouse eggs fertilized in vitro. Gamete Res. 1979;2:295–304.
- Yanagimachi R. Mammalian fertilization. New York (NY): Raven Press, LTD; 1994.
- Satouh Y, Inoue N, Ikawa M, et al. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci. 2012;125:4985–4990.
- Young SA, Miyata H, Satouh Y, et al. CRISPR/Cas9-mediated mutation revealed cytoplasmic tail is dispensable for IZUMO1 function and male fertility. Reproduction. 2016;152:665–672.
- Kodama Y, Hu CD. Bimolecular fluorescence complementation (BiFC): a 5-year update and future perspectives. Biotechniques. 2012;53:285–298.
- Inoue N, Ikawa M, Okabe M. Putative sperm fusion protein IZUMO and the role of N-glycosylation. Biochem Biophys Res Commun. 2008;377:910–914.
- Sosnik J, Miranda PV, Spiridonov NA, et al. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci. 2009;122:2741–2749.
- Zhou C, Huang L, Shi DS, et al. Effects of latrunculin A on the relocation of sperm IZUMO1 during gamete interaction in mouse. Mol Reprod Dev. 2017;84:1183–1190.
- Frolikova M, Sebkova N, Ded L, et al. Characterization of CD46 and beta1 integrin dynamics during sperm acrosome reaction. Sci Rep. 2016;6:33714.
- Nakanishi T, Ikawa M, Yamada S, et al. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999;449:277–283.
- Toyoda Y, Yokoyama M, Hoshi T. Studies on the fertilization of mouse eggs in vitro I: in vitro fertilization of eggs by fresh epididymal sperm [in Japanese]. Jpn J Anim Reprod. 1971;16:147–151.
- Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682.
