ABSTRACT
Cyclophilin A (cypA) is overexpressed in many types of carcinomas, including non-small-cell lung cancer (NSCLC). However, the effect of anoxia, a critical feature of the carcinoma cell microenvironment, on cypA expression in NSCLC is unknown. Here, formaldehyde-fixed and paraffin-embedded samples were collected from 60 subjects with NSCLC. The protein expression levels of cypA and hypoxia-inducible factor-1α (HIF-1α) were evaluated using immunohistochemistry. Kaplan–Meier analysis showed that subjects with high cypA expression had remarkably shorter progression-free survival than those with low cypA expression. Furthermore, cypA expression levels were significantly related to HIF-1α expression levels (Spearman’s correlation = 0.34, P < 0.0001). To further assess the effect of cypA, an anoxic carcinoma cell model was established. CypA expression was remarkably upregulated in H1299 and A549 cell lines under hypoxic conditions. Overexpression of cypA restored hypoxia-impaired cell growth and prevented reactive oxygen species (ROS) production and cell death in hypoxic A549 and H1299 cells. However, these phenotypes were not altered by the inactive R55A mutant of cypA. Mechanistic studies demonstrated that cypA can bind to and degrade the tumor suppressor protein TXNIP in H1299 and A549 cells. Restored TXNIP expression in cypA-overexpressed and hypoxic NSCLC cells led to increased ROS levels and apoptotic cell numbers and decreased cell growth compared with cypA-overexpressed and hypoxic NSCLC cells. These findings indicate that anoxia results in an increase in cypA expression in NSCLC. Additionally, cypA served as an oncogene during hypoxia by interacting with TXNIP.
Introduction
Lung cancer (LC), besides being the main cause of cancer-related death worldwide, also has a high incidence rate. The most common LC is non-small-cell lung cancer (NSCLC), accounting for 85% of the cases [Citation1]. NSCLC is often diagnosed in terminal stages, which makes treatment difficult. The tumor microenvironment (TME) plays a significant role in the degree of tumor malignancy and treatment tolerance. Anoxia, which occurs under both pathophysiological and normal conditions, exerts pressure on living bodies and cells. Anoxia is related to a variety of illnesses in humans, such as cancer and diabetes, and poses challenges to mammals living in high-elevation areas [Citation2]. In vitro/in vivo investigations have revealed that neoplastic anoxia is related to an increased possibility of distant metastasis, partial recrudescence, and tolerance to some types of chemotherapy and radiation treatment [Citation3]. Additionally, oxidative stress usually arises from the excessive accumulation of ROS, including singlet oxygen, H2O2, OH−, and superoxide anions [Citation4]. Oxidative stress resulting from increased ROS levels can promote the death of carcinoma cells [Citation5]. To prevent excessive intracellular ROS, cancer cells have evolved strategies to maintain redox balance by improving the potential of oxidation resistance [Citation6,Citation7].
Cyclophilin A (CypA), a typical member of the cyclophilin family, is a highly conserved protein in mammalian cells [Citation8]. Generally, cyclophilin consists of four subtypes (cyclophilin A/B/C/D). Homologous sequence assessment of human CypA suggests a high level of homology between it and cyclophilin B (CypB), C (CypC), and D (CypD; ref. 2) in humans. CypB and CypC are equipped with NH2 terminal sequences targeting them to the endoplasmic reticulum, while CypA is an 18 kDa protein mainly located in the cytoplasm [Citation9]. It belongs to an immunophilin and a cytoplasmic receptor for the immunosuppressant cypA [Citation9]. Additionally, cypA also has peptidyl prolyl cis-trans isomerase (PPIase) activity, which is necessary for protein folding in vivo. Furthermore, the PPIase activity of cyclophilin has recently been shown to participate in various cellular processes, including cellular protein transport [Citation10], mitochondrial function [Citation11], pre-mRNA processing [Citation12], and maintenance of the stability of multi-protein complexes [Citation13].
Previous studies have shown that cypA levels are increased in carcinoma cell strains, including human NSCLC [Citation14]. The effect of cypA on oxidative stress is unknown; however, recent investigations have indicated that cypA, which is secreted by cells during inflammatory stimulation or anoxia/re-aeration, has a protective effect on cardiomyocytes against death from oxidative stress via the Akt/Nox2 pathway [Citation15]. CypA is secreted by vascular smooth muscle cells in response to oxidative stress. It also mediates extracellular signal-regulated kinase (ERK1/2) activation and vascular smooth muscle cell growth through ROS [Citation16]. In addition, cypA protects rat neonatal cardiomyocytes from oxidative stress-induced apoptosis, especially due to ROS generation [Citation17]. These studies indicate that cypA may play an anti-apoptotic role in multiple cell types and may respond to oxidative stress.
In this study, we examined the correlation between cypA and hypoxia inducible factor-1α (HIF-1α) in 60 NSCLC specimens. H1299 and A549 cell lines were exposed to hypoxic conditions to examine the expression and effect of cypA on hypoxic NSCLC cells. Loss and gain of function experiments showed that cypA upregulation inhibited H2O2-induced oxidative damage and apoptosis in H1299 and A549 cells, while silencing of cypA amplified such phenotypic changes. Furthermore, we demonstrated the interaction between cypA and TXNIP. Our data also showed that cypA causes degradation of the TXNIP protein, and the anti-oxidative and anti-apoptotic effects of cypA were partially attributed to modulation of TXNIP levels. Therefore, the present study identified an important role of cypA in oxidative damage and apoptosis in hypoxic NSCLC cells.
Material and methods
Patients samples
Subjects (n = 60; average age, 55.7 years old) were selected from histopathologically and clinically diagnosed NSCLC patients who underwent a primary resection at the First Affiliated Hospital of Xi’an Jiaotong University between Dec 2015 and Jan 2021. Patients who were treated with radiotherapy or chemotherapy before surgery were excluded. Relevant clinical data were collected in . Clinicopathologic classification and stage of tissues were determined according to the current National Cancer Center Network (NCCN), Union for International Cancer Control (UICC) Tumor-Node-Metastasis (TNM) classification. All patients have provided handwritten informed consent. The present research was conducted according to the Declaration of Helsinki following the Approval of Ethics given by the Board of Ethics Review of the First Affiliated Hospital of Xi’an Jiaotong University.
Table 1. Characteristics of 60 Non-small Cell Lung Cancer Patients.
Cellular culturing and transient transfection
NSCLC cell lines H1299 and A549 were transfected with pCMV-flag-cypA, pCMV-flag-cypA R55A, pCMV-flag-empty, and/or pcDNA3.1-HA-TXNIP for TXNIP using Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. shRNAs targeting the control and cypA were provided by RiboBio (Guangzhou, China). Forty-eight hours after transfection, cells were collected. In the case of anoxia treatment, cells were selected using deoxidized RPMI 1640 (Gibco-BRL, Grand Island, NY, USA) rather than nutrient medium prior to induction by anoxia. Then, the cells were placed in an anoxic chamber (Forma Scientific, Marietta, OH, USA) at 37°C for 36 h’s incubation. Before each test, the deoxidized medium was prepared by balancing the medium with the anoxic gas mixture (containing 5% carbon dioxide and 7% hydrogen), and balanced with nitrogen at 37°C. The O2 concentration in the anoxic chamber was maintained at less than 1%, which was measured using an O2 indicator (Forma Scientific). For verapamil treatment, cells with cypA silencing were treated with 1 μM verapamil for 12 h.
RT-qPCR
SYBR Green-based qRT-qPCR was used to assess mRNA expression levels in lung carcinoma cell lines using the 7500 Real-time PCR System (Applied Biosystems, Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA was collected from lung carcinoma cells using the mRNA Isolation kit (Ambion, Austin TX, USA).
Western blot (WB) analysis
Protein extracts were prepared following the standard protocols. Separated cell lysates were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride films (Millipore, Burlington, MA, USA). The films were cultured with specific primary antibodies at 4°C overnight. The protein immune response signals were measured using an ECL detection system (Thermo Fisher Scientific).
Colony formation test
The cells were inoculated into a 24-well plate (1000 cells per well) and incubated overnight to adhere to the surface. At 24 h after treatment or transfection, cell growth was maintained for 9 to 11 days. They were then fixed and stained with aniline violet solution (Sigma-Aldrich, St. Louis, MO, USA) and CH3OH (9:1). Cells were photographed to determine colony-forming units (CFUs), and colonies consisting of 50 cells or more were counted using the colony counter in ImageJ. Untreated cells were used as the control.
ROS production determination
The oxy sensitive fluorogenic probe DCFH-DA was selected for determining intracellular ROS productivity. The cells were inoculated into a 96-well plate (density: 1 × 104 cells per well) and incubated for 24 h. Then, the cells were inoculated with 2ʹ-7ʹdichlorofluorescin diacetate for 30 min at 37°C in the dark. Fluoroscopic images of cells were obtained using an InCell 2000 confocal microscope after washing twice with phosphate-buffered saline. To quantitatively evaluate intracellular ROS productivity, we tested the fluorescence with the software module provided with the InCell Analyzer 2000.
Apoptosis analysis
Apoptosis was analyzed by flow cytometry (FC) using an Annexin V-FITC/PI apoptosis assay kit (Strong Biotech Corp., Taipei, Taiwan) following the manufacturer’s instructions. In brief, the cells were inoculated into a 6-well plate (density: 3 × 105 cells per well) and incubated for 24 h. They were then collected through tryptic digestion, centrifuged at 2000 rpm for 5 min, and then washed twice with ice-cold phosphate-buffered saline. We added 100 µL of Annexin V binding buffer, 5 μL of Annexin V, and 5 μL of propidium iodide, and the specimens were inoculated under ambient temperature for 15 min in the dark. Annexin V binding buffer was added until the total volume reached 1 mL. Then, the cells were transferred to an FC tube and analyzed using an FC analyzer (Cytomics FC 500, Beckman Coulter, Atlanta, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). One-way analysis of variance was used to compare multiple groups, and Student’s t-tests were used to compare two groups. Spearman’s rank correlation coefficient was employed to analyze the correlation between HIF-1α expression and cypA expression in clinical samples. Statistical significance was set at P < 0.05.
Results
CypA expression was associated with HIF-1α expression in NSCLC specimens
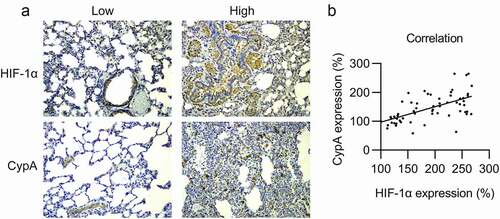
HIF-1α levels were increased in 50.0% (30/60) of NSCLC tumor samples, whereas cypA showed high expression in 58.3% (35/60). It is interesting to note that the distribution of HIF-1α expression levels in cancer biopsies was significantly associated with the distribution of cypA expression levels (), ). Additionally, cypA expression was significantly associated with HIF-1α expression (), Spearman’s correlation = 0.346, P < 0.0001).
Figure 1. Expression of cypA and HIF-1α in NSCLC carcinoma specimens. (A) Increased and decreased expression levels of HIF-1α and cypA on NSCLC specimen sections. (B) Relationship between cypA expression and HIF-1α expression. Spearman’s correlation = 0.346, p <0.0001.
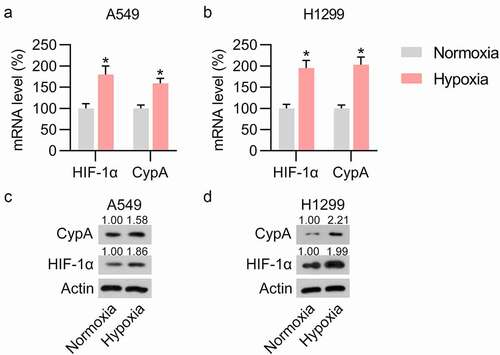
To assess the influence of cypA in LC cells under hypoxic conditions, A549 and H1299 cells were subjected to anoxia. The influence of hypoxia on the mRNA and protein expression levels of HIF-1α was studied. Predictably, anoxia treatment contributed to both mRNA and protein levels of HIF-1α ()), suggesting the successful induction of hypoxia-conditioned LC cells. We then detected the expression of cypA in hypoxia-conditioned A549 and H1299 cells. Both RT-qPCR and WB analyses indicated upregulation of cypA expression in hypoxic cells compared with normoxic groups ()), indicating that cypA could participate in LC cell properties under hypoxic conditions.
Figure 2. Hypoxia treatment induced cypA expression in pulmonary carcinoma cell lines. H1299 and A549 cell lines were cultured under hypoxic or normoxic conditions. (A, B) RT-qPCR revealed upregulated HIF-1α and cypA mRNA levels in H1299 and A549 cells in response to anoxia in comparison with those under normoxia. (C, D) WB showed elevated protein expression levels of cypA and HIF-1α in H1299 and A549 cells in response to anoxia in comparison with those under normoxia. * p <0.05.
Ectopic regulation of cypA in the hypoxic H1299 and A549 cells
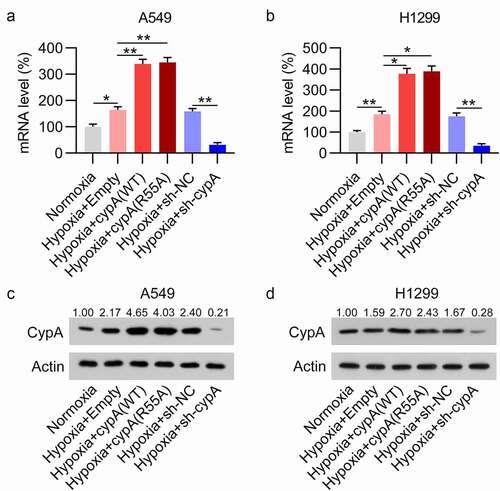
To examine the direct influence of cypA and its enzymatic activity on the phenotypes of NSCLC cells upon hypoxia, overexpression of cypA wild-type (WT) or its catalytically inactive mutant R55A [Citation18], as well as silencing of cypA, were performed in H1299 and A549 cells under hypoxia. Upregulation and downregulation of mRNA and protein expression levels of cypA were observed in H1299 and A549 cells, as assessed by RT-qPCR and WB analyses ()). The H1299 and A549 cells with/without cypA overexpression/knockdown were used in the following experiments.
Figure 3. Artificial regulation of cypA expression in pulmonary carcinoma cells under hypoxic conditions. H1299 and A549 cells were transfected with an ITCH (WT/R55A mutant) overexpression vector or empty vector, and then subjected to hypoxic or normoxic conditions. (A, B) Dysregulated mRNA expression of cypA in H1299 and A549 cells in response to anoxia. (C, D) WB detected cypA protein expression levels in anoxic H1299 and A549 cells transfected with different plasmids. * p <0.05, ** p <0.01.
Overexpression of cypA repressed hypoxia-caused cell injury, ROS generation, and apoptosis of NSCLC cells
Hypoxia induces cell injury, ROS production, and apoptosis in LC cells [Citation19]; therefore, we next evaluated the influence of cypA on these phenotypes in hypoxia-treated H1299 and A549 cells, as assessed by colony formation, DCFH-DA staining, and FC. The colony formation assay showed that hypoxia induction repressed the cell growth rate of H1299 and A549 cells compared with the normoxic group. Overexpression of cypA, rather than its inactive mutant R55A, restored cell growth, whereas depletion of cypA augmented cell injury in hypoxic cells ()).
Figure 4. Effect of cypA on colony formation ability, ROS generation, and apoptosis of hypoxia-conditioned LC cells. H1299 and A549 cells were transfected with an ITCH (WT/R55A mutant) overexpression vector or empty vector, and then subjected to hypoxic or normoxic conditions. (A, B) A colony formation assay was conducted to examine the cell growth rate of H1299 and A549 cells. (C, D) DCFH-DA staining was used to measure ROS production in H1299 and A549 cell lines. (E, F) Apoptosis was measured by FC with Annexin V-FITC/PI staining. * p <0.05, ** p <0.01.
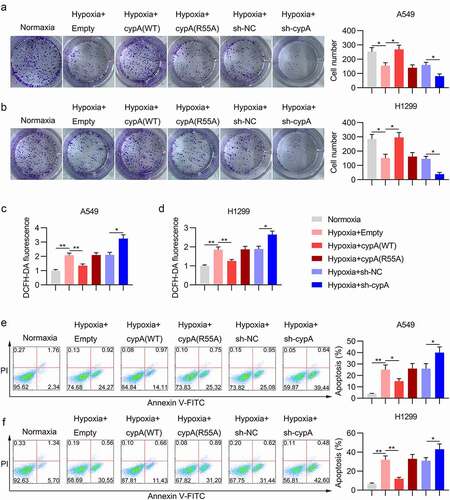
A549 and H1299 cells under hypoxic conditions also showed higher ROS levels than normoxic cells ()). Overexpression of cypA WT, but not cypA R55A, partially reversed the robust generation of ROS in LC cells under hypoxic conditions. In addition, silencing of cypA amplified the ROS levels in the cells ()).
For apoptosis, FC with Annexin V-FITC and propidium iodide staining confirmed that hypoxia triggered the death of A549 and H1299 cells. CypA WT overexpression remarkably reduced the apoptotic cell number of LC cells in the presence of hypoxia compared with the hypoxic and R55A groups; in contrast, cypA depletion contributed to increased apoptosis in hypoxic NSCLC cell lines ()).
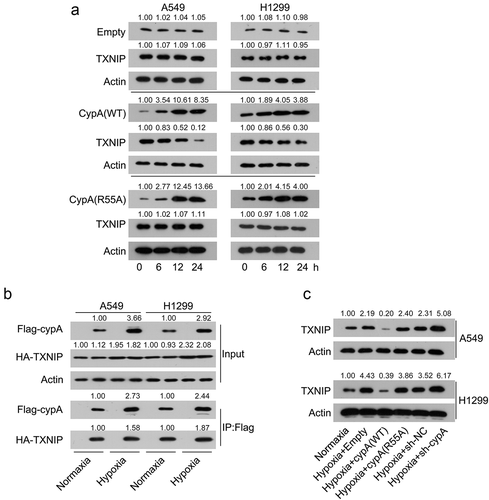
CypA caused degradation of TXNIP in LC cells
Previous studies have indicated that anoxia results in high expression of TXNIP in NSCLC [Citation20]. Additionally, TXNIP is positively associated with metastasis and ROS in multiple cancer types [Citation20,Citation21]. Therefore, we hypothesized that TXNIP might participate in cypA-mediated tumor phenotypes of hypoxic NSCLC cell lines. We transfected cypA WT and R55A mutant into the A549 and H1299 cells and treated the cells with the protein biosynthesis inhibitor cycloheximide (CHX). The data clearly showed that TXNIP protein levels were reduced remarkably more rapidly in the cells with cypA WT overexpression than in those with empty vector or R55A mutant overexpression ()). Next, we assessed the interaction between cypA and TXNIP. Lysates from A549 and H1299 cells co-expressing flag-cypA and HA-TXNIP or the vector controls were used for immunoprecipitation of cypA using FLAG antibodies. WB analysis indicated that cypA co-immunoprecipitated with TXNIP, but not with the empty vector control (). Furthermore, WB showed that cypA silencing upregulated TXNIP protein levels in the cells. Thus, the data suggested that cypA contributes to TXNIP degradation in H1299 cells by binding with TXNIP ().
Figure 5. CypA interacts with and degrades TXNIP. (A) A549 and H1299 cells were co-transfected with cypA WT/R55A mutant. Whole-cell lysis products treated for the indicated period with 100 μg/mL CHX were probed for cypA and TXNIP using WB. (B) A549 and H1299 cells were co-transfected with HA-TXNIP plasmids and flag-cypA. Split products were immunoprecipitated by WB as well as anti-flag antibody using the indicated antibodies. (C) A549 and H1299 cells were transfected with cypA (WT/R55A mutant) overexpression vector or empty vector, and then subjected to hypoxic or normoxic conditions. WB detected TXNIP protein expression levels in hypoxic H1299 and A549 cells transfected with different plasmids.
TXNIP was involved in cypA-repressed ROS production and apoptosis in hypoxic NSCLC cells
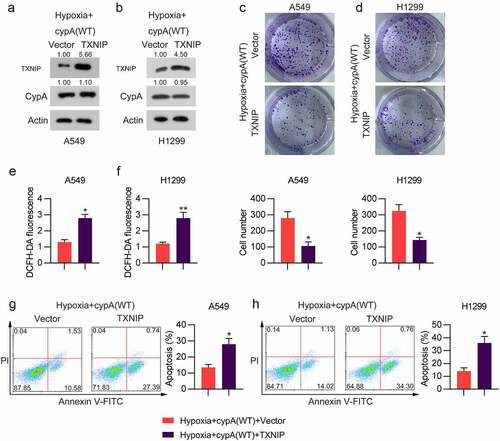
To evaluate the role of the cypA-TXNIP interaction on the hypoxia-induced increase in ROS and apoptosis of NSCLC cells under hypoxic conditions, hypoxic cells were transfected with both cypA and TXNIP overexpression vectors. We observed that TXNIP was significantly restored in cypA-overexpressing hypoxic cells, as assessed by WB ()). Next, we conducted a colony formation assay to detect the effect of TXNIP overexpression on the growth of cypA-overexpressing cells under hypoxic conditions. TXNIP overexpression inhibited cell proliferation in hypoxic H1299 and A549 cells ()). Our data showed that TXNIP upregulation in cypA-overexpressing cells also upregulated ROS generation ()) and apoptosis ()).
Figure 6. Influence of TXNIP overexpression on colony-forming ability, ROS generation, and apoptosis of cypA-overexpressed and hypoxia-conditioned LC cells. H1299 and A549 cells were co-transfected with cypA and TXNIP overexpression vectors for 24 h and then subjected to anoxic conditions. (a, b) WB detected cypA and TXNIP protein levels in hypoxic A549 and H1299 cells transfected with different plasmids. (c, d) The colony-forming assay examined the cell growth rates of H1299 and A549 cells. (e, f) DCFH-DA staining assay was used to examine ROS generation in H1299 and A549 cells. (g, h) Apoptosis was tested by FC with Annexin V-FITC/PI staining. * p < 0.05, ** p < 0.01.
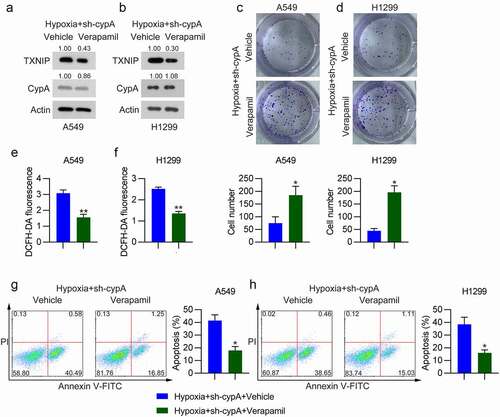
Then, hypoxic cells with cypA silencing were treated with verapamil (RTA, 1 μM) to repress TXNIP expression. WB analysis indicated that verapamil administration downregulated the total protein level of TXNIP ()), compared to that in vehicle-treated cells. The colony formation assay detected an in increase in CFUs of hypoxic H1299 and A549 cells with cypA silencing and verapamil administration compared with the vehicle-treated group ()). Additionally, verapamil administration led to ameliorated ROS production ()) and reduced apoptosis ()) of hypoxic A549 and H1299 cells with cypA silencing. These data clearly demonstrate that TXNIP is partially responsible for cypA-mediated ROS generation and apoptosis in hypoxic LC cells.
Figure 7. Effect of verapamil on colony formation ability, ROS generation, and apoptosis of cypA-silenced hypoxia-conditioned LC cells. H1299 and A549 cells were transfected with siTXNIP for24 h, treated with 1 μM verapamil for 12 h, and then subjected to hypoxic conditions. (A, B) WB detected cypA and TXNIP protein levels in hypoxic H1299 and A549 cells transfected with different plasmids. (C, D) The colony formation assay examined the cell growth rates of H1299 and A549 cells. (E, F) DCFH-DA staining assay was used to measure ROS production in H1299 and A549 cells. (G, H) Apoptosis was tested by FC with Annexin V-FITC/PI staining. * p <0.05, ** p <0.01.
Discussion
NSCLC, as one of the major cancer-related diseases targeted by cellulate as well as immune checkpoints, has remarkably improved subjects’ clinical outcomes [Citation22,Citation23], but the mechanism of NSCLC cells under hypoxic conditions was not well understood. In this investigation, upregulated cypA was found in HIF-1α-upregulated NSCLC samples. Meanwhile, cypA expression was increased in H1299 and A549 cells in response to hypoxia induction. Hypoxia induced ROS production and apoptosis in NSCLC cell lines, which subsequently impaired cell survival; however, these hypoxia-induced consequences in NSCLC cell lines were ameliorated by cypA WT overexpression. Notably, the overexpression of inactive R55A mutant of cypA did not affect these indexes, while silencing of cypA augmented the already upregulated ROS level and apoptosis in A549 and H1299 cells in response to anoxia. Furthermore, we discovered that a key modulator of ROS, TXNIP, was able to bind with cypA and degraded quickly when cypA WT was overexpressed. Loss/gain of function experiments indicated that TXNIP was involved in cypA-mediated inhibition of ROS generation and apoptosis in hypoxia-conditioned LC cells. Therefore, the cypA-TXNIP pathway participates in hypoxia-triggered cell injury in LC cells.
CypA is highly expressed in multiple types of carcinoma cells [Citation24,Citation25], including NSCLC [Citation14], pancreatic cancer [Citation26], hepatocellular carcinoma [Citation27], and breast carcinoma [Citation28]. CypA can aid carcinoma cell proliferation and protect them from death and oxidant stress. According to previous studies, cypA upregulation in cancer is managed by transcription factor HIF-1α, which activates heterogeneous transcription related to vascular endothelial growth, tumor growth, resistance to anoxic environments, metastasis, and other tumor-promoting events. HIF-1α activated by an anoxic environment has the ability to promote gene expression for vascular endothelial growth, low glucose, and oxidant stress. In the case of anoxia, cypA, whose promoter contains the hypoxia response element motif, can be induced by HIF-1α [Citation29]. CypA has been reported to enhance endogenous antioxidant enzymatic activity. For instance, overexpression of cypA markedly reduced ROS levels produced by its interaction with cyclosporin A [Citation30], in which R55A served as a key amino acid in exerting PPIase activity [Citation18]. In this study, the expression level of cypA was positively associated with that of HIF-1α in NSCLC specimens. The in vitro study indicated that hypoxia stimulation led to upregulation of both HIF-1α and cypA in A549 and H1299 cells, confirming that HIF-1α was able to induce cypA expression. Overexpression of cypA WT, but not inactive R55A mutant, significantly improved cellular activity and reduced ROS production and apoptosis in H1299 and A549 cells, while silencing of cypA reversed these phenotypic changes, implying that cypA protects cell survival in hypoxia-induced A549 cells by attenuating oxidative injury through its PPIase activity.
Recently, TXNIP has aroused wide concern in the scope of angiocardiopathy, because it is an apoptosis-promoting protein and a regulator of the thioredoxin system [Citation31]. TXNIP expression levels are tightly controlled in normal cells, and deregulation of TXNIP is involved in carcinoma, cardiopathy, and metabolic disorders [Citation32]. TXNIP is promoted by various stress stimuli, including H2O2, radiation, UV, heat shock, serum deprivation, and growth-inhibiting factors [Citation33]. Many transcription factors (e.g. heat-shock factor, glucocorticosteroid receptor, Forkhead Box O1, and MondoA) have been confirmed to manage TXNIP expression levels in diverse cases [Citation34]. In mammary, gastric, urinary bladder, and colorectal carcinomas, as well as diffuse large B cell lymphoma, depletion of TXNIP expression was found to be related to more invasive diseases, terminal period, and worse prognosis [Citation35]. Conversely, TXNIP is a human gene induced by anoxia in dermal microvascular endothelial cell lines and in the mouse heart [Citation36]. In NSCLC and pancreatic cancer, TXNIP is promoted by anoxia depending on HIF [Citation37], and high TXNIP expression may be a marker of unfavorable prognosis in NSCLC. Recently, TXNIP has also been discovered to undergo ubiquitylation in vivo in a global ubiquitylation analysis of HeLa cells. Lys-122 was tested as a possible ubiquitin attachment site [Citation38]. Here, our data reported that cypA overexpression contributed to the degradation of TXNIP protein in A549 and H1299 cells through PPIase activity of cypA, but whether this degradation occurs through the proteasomal pathway needs further investigation. CypA-inhibited ROS release and apoptosis was recovered with TXNIP overexpression in the H1299 and A549 cells. Cyto-inhibition of TXNIP by verapamil partially abolished the stimulatory role of cypA silencing on cell injury. These data suggest that TXNIP degradation is responsible for the oncogenic effect of cypA on hypoxia-treated NSCLC cells.
In summary, this investigation suggests that the hypoxia-induced cypA-TXNIP pathway plays an important role in hypoxia-associated NSCLC cell damage. On the one hand, these protective effects may be mediated by the degradation of TXNIP protein; on the other hand, the cytoprotective function of cypA seemingly depends upon its PPIase activity. Therefore, the PPIASE activity of cypA could be a potent chemotherapeutic target for cancer therapy by reducing cell survival in the hypoxic microenvironment and improving the treatment of solid breast tumors.
Author contributions
Yang Li and Lan Yang designed experiments, carried out experiments, analyzed experimental results. Yang Li wrote the manuscript, Lan Yang revised the manuscript. Both authors approved the final manuscript.
Ethical approval
The present research was conducted according to the Declaration of Helsinki following the Approval of Ethics given by the Board of Ethics Review of the First Affiliated Hospital of Xi’an Jiaotong University.
Informed consent from participants
All patients have provided handwritten informed consent.
Supplemental Material
Download Zip (74.7 MB)Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
My manuscript has no associated data.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2078615
Additional information
Funding
References
- DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69(6):452–467.
- Cheviron Z, Brumfield R. Genomic insights into adaptation to high-altitude environments. Heredity (Edinb). 2012;108(4):354–361.
- Chen A, Sceneay J, Gödde N, et al. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene. 2018;37(31):4214–4225.
- Ung L, Pattamatta U, Carnt N, et al. Oxidative stress and reactive oxygen species: a review of their role in ocular disease. Clin Sci. 2017;131(24):2865–2883.
- Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23(4):965.
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013 December 01;12(12):931–947.
- Guo Q, Li F, Duan Y, et al. Oxidative stress, nutritional antioxidants and beyond. Sci China Life Sci. 2020;63(6):866–874.
- Nigro P, Pompilio G, Capogrossi M. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4(10):e888–e888.
- Kim H, Oh Y, Kim K, et al. Cyclophilin A regulates JNK/p38-MAPK signaling through its physical interaction with ASK1. Biochem Biophys Res Commun. 2015;464(1):112–117.
- Hsu H-M, Huang Y-H, Aryal S, et al. Endomembrane protein trafficking regulated by a Tv CyP2 Cyclophilin in the Protozoan Parasite, Trichomonas vaginalis. Sci Rep. 2020;10(1):1–18.
- Doti N, Reuther C, Scognamiglio P, et al. Inhibition of the AIF/CypA complex protects against intrinsic death pathways induced by oxidative stress. Cell Death Dis. 2014;5(1):e993–e993.
- Ge Q, Tang Y, Luo W, et al. A cyclophilin OsCYP20–2 Interacts with OsSYF2 to regulate Grain Length by Pre-mRNA splicing. Rice. 2020;13(1):1–10.
- Luan X, Yang W, Bai X, et al. Cyclophilin A is a key positive and negative feedback regulator within interleukin‐6 trans‐signaling pathway. FASEB J. 2021;35(11):e21958.
- Guo Y, Jiang M, Zhao X, et al. Cyclophilin A promotes non‐small cell lung cancer metastasis via p38 MAPK. Thorac Cancer. 2018;9(1):120–128.
- Cheng F, Yuan W, Cao M, et al. Cyclophilin A protects cardiomyocytes against hypoxia/reoxygenation-induced apoptosis via the AKT/Nox2 pathway. Oxid Med Cell Longev. 2019;2019:1–11.
- Tang F-C, Wang H-Y, Ma -M-M, et al. CyPA-CD147-ERK1/2-cyclin D2 signaling pathway is upregulated during rat left ventricular hypertrophy. Sheng li xue bao:[Acta physiologica Sinica].2015;67(4):393–400.
- Cao M, Yuan W, Peng M, et al. Role of CyPA in cardiac hypertrophy and remodeling. Biosci Rep. 2019;39(12). 10.1042/BSR20193190.
- Tanaka Y, Sato Y, Sasaki T. Feline coronavirus replication is affected by both cyclophilin A and cyclophilin B. J Gen Virol. 2017;98(2):190.
- Kim CH, Ko AR, Lee SY, et al. Hypoxia switches glucose depletion-induced necrosis to phosphoinositide 3-kinase/Akt-dependent apoptosis in A549 lung adenocarcinoma cells. Int J Oncol. 2010 Jan;36(1):117–124.
- Li Y, Miao LY, Xiao YL, et al. Hypoxia induced high expression of thioredoxin interacting protein (TXNIP) in non-small cell lung cancer and its prognostic effect. Asian Pac J Cancer Prev. 2015;16(7):2953–2958.
- Zhang C, Wang H, Liu X, et al. Oncogenic microRNA-411 promotes lung carcinogenesis by directly targeting suppressor genes SPRY4 and TXNIP. Oncogene. 2019 Mar;38(11):1892–1904.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454.
- Chu MY, Huang HC, Li EM, et al. CypA: a Potential Target of Tumor Radiotherapy and/or Chemotherapy. Curr Med Chem. 2021;28(19):3787–3802.
- Liao Y, Luo D, Peng K, et al. Cyclophilin A: a key player for etiological agent infection. Appl Microbiol Biotechnol. 2021 Feb;105(4):1365–1377.
- Cong L, Bai Z, Du Y, et al. Citron Rho-Interacting serine/threonine kinase promotes HIF1a-CypA signaling and growth of human pancreatic adenocarcinoma. Biomed Res Int. 2020;2020:9210891.
- Zhang M, Dai C, Zhu H, et al. Cyclophilin A promotes human hepatocellular carcinoma cell metastasis via regulation of MMP3 and MMP9. Mol Cell Biochem. 2011;357(1):387–395.
- Davra V, Saleh T, Geng K, et al. Cyclophilin A inhibitor Debio-025 targets Crk, reduces metastasis, and induces tumor immunogenicity in breast cancer. Mol Cancer Res. 2020;18(8):1189–1201.
- Zhang H, Chen J, Liu F, et al. CypA, a gene downstream of HIF-1α, promotes the development of PDAC. PLoS One. 2014;9(3):e92824.
- Ma Z, Zhang W, Wu Y, et al. Cyclophilin A inhibits A549 cell oxidative stress and apoptosis by modulating the PI3K/Akt/mTOR signaling pathway. Biosci Rep. 2021 Jan 29;41(1). 10.1042/BSR20203219.
- Yoshioka J, Chutkow WA, Lee S, et al. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J Clin Invest. 2012;122(1):267–279.
- Zhou J, Chng W-J. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion. 2013;13(3):163–169.
- Polekhina G, Ascher DB, Kok SF, et al. Structure of the N-terminal domain of human thioredoxin-interacting protein. Acta Crystallogr Sect D Biol Crystallog. 2013;69(3):333–344.
- Yoshihara E. TXNIP/TBP-2: a master regulator for glucose homeostasis. Antioxidants. 2020;9(8):765.
- Qayyum N, Haseeb M, Kim MS, et al. Role of thioredoxin-interacting protein in diseases and its therapeutic outlook. Int J Mol Sci. 2021 Mar 9 22(5):2754.
- Karar J, Dolt KS, Mishra MK, et al. Expression and functional activity of pro-oxidants and antioxidants in murine heart exposed to acute hypobaric hypoxia. FEBS Lett. 2007;581(24):4577–4582.
- Li Y, Miao L-Y, Xiao Y-L, et al. Hypoxia induced high expression of thioredoxin interacting protein (TXNIP) in non-small cell lung cancer and its prognostic effect. Asian Pac J Cancer Prev. 2015;16(7):2953–2958.
- Zhang P, Wang C, Gao K, et al. The ubiquitin ligase itch regulates apoptosis by targeting thioredoxin-interacting protein for ubiquitin-dependent degradation. J Biol Chem. 2010;285(12):8869–8879.
