?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The probable influence of electromagnetic irradiation on cancer treatment has been deduced from the interaction of artificial electromagnetic emissions with biological organisms. Nonetheless, the suspected health effects induced by electromagnetic-based technology imply that such a treatment may contaminate the adjacent healthy cells. Thus, gaining mechanistic insights into the problem is required to avoid athermal health hazards. To tackle that, the current review, based upon in vitro studies into assorted cell lines, depicts the alterations in physiological processes triggered by electromagnetic irradiation via addressing gene regulatory cascades. Furthermore, decisive factors in the hypothesized cause-effect linkage in terms of the cell line-associated, exposure-associated, or endpoint-associated parameters are highlighted. As a result, subcellular structures such as aberrant Ca2+ channels, rich glycocalyx charge, or high water content in cancerous cells, which have attracted a great deal of attention, can explain their higher susceptibility compared with healthy cells under irradiation. Affected by cell components or geometry, the cellular biological window correlates with the metabolic or cell cycle status and determines the irradiation that causes the maximum influence. For instance, correlations between the frequency (or intensity) of irradiation and cell excitability or between the duration of irradiation and cell doubling time are observed. There are unspecified signaling pathways such as the pathway of PPAR- or MAPKs, and also proteins devoid of any investigation such as p14, or S phase-related and G2 phase-related proteins. Other chains, such as the cAMP connection with mitochondrial ATP or ERK signaling, the association of Hsps releases with signaling pathways of MAPKs, or the role of different ion channels in regulating various cell processes, require further investigation.
1 Introduction
Evaluation of the interaction of artificial electromagnetic fields (EMFs) with organisms has led researchers to its potential capability to treat cancerous cells of various tissue types. Therefore, the EMF is rendered as a double-edged sword that functions based on the association between irradiation characteristics and tissue structure. Being a ubiquitous environmental stressing factor, the EMF emitted from many technology-based sources, such as high-voltage transmission lines, induction hobs, or mobile telephony, has reportedly caused functioning disruption in various organisms [Citation1]. Due to its non-ionizing nature and possible athermal health effects, electromagnetic fields without or with co-exposure to chemicals have been investigated for the treatment of cell carcinoma [Citation2,Citation3]. A critical consequence of such deliberate irradiation may be the contamination of healthy cells. Provided that the probable adverse health effects of the electromagnetic field are properly taken into consideration, its numerous technology-based applications will be counted as significant.
The mechanistic elucidation of the EMF coupling into organic tissues is necessary to unravel biological endpoints following the environmental exposure and thus to provide a proper estimation of health effects. Hence, the study question is not as to whether EMF-related biological effects occur or not, but rather as to the natural processes through which such effects are brought about, and the parameters associated with the cell line, EMF exposure, or biological endpoint participating in these effects. The fundamental purpose of studying cells in vitro is to break down an organism into more controllable systems to address mechanistic questions. By contrast, epidemiological studies are devoid of the capability to explain mechanisms underlying alterations in physiological processes due to poor control over influential parameters, difficulty in discriminating between health effects caused by the studied exposure source and other exposure sources, inadequacy of the data acquisition system, or inadequate size of the survey sample. Accordingly, in vitro studies reporting biological effects of EMF exposure, particularly those with emphasis on mechanisms of various key physiological processes which were published during 2015–2021 or earlier (where the information corresponding to the specified period is missing), form the base of the current review aiming at elucidating the mechanistic causal relation between the EMF and assorted cell lines.
The inclusion criterion is defined according to the study question and thus attempts to address the underlying mechanism of different endpoints in terms of gene expression, starting from the most recently published articles. According to the abovementioned explanations, in vitro studies into different cell lines subjected to alternating electromagnetic fields in the frequency range of 0–300 GHz are screened for eligibility. displays an overall view of the non-ionizing electromagnetic spectrum of alternating current, which lies between frequencies 0–300 GHz, along with several artificial sources. The reason to justify the compilation of research studies on different electromagnetic fields over such a broad frequency range is that their outcomes have been attributed to athermal effects leading to alterations in cell physiology. Therefore, alterations in cell physiology that are investigated in a mechanistic approach are the focus of the current review. The inclusion criterion was inspired by SCENIHR’s opinion in 2015, which noted the lack of mechanistic insights that prevents scientific committees from interpreting causality and revising exposure limits [Citation4]. Google Scholar was selected as the search engine utilizing search keywords including electromagnetic, effect, cultured, cell line, and carcinoma.
Figure 1. Non-ionizing electromagnetic field spectrum of alternating current ranged between 0–300 GHz frequencies along with several artificial sources.

Not only does the literature need a comprehensive review of the mechanisms by which electromagnetic fields may disrupt the function of organic tissues, but it is also deficient in mechanistic insights into biological effects that occur in different cell lines. Hence, the current review attempts to provide the research community with a mechanistic report to capitalize on for further research. Furthermore, parameters that may be influential in altering the investigated endpoints are highlighted in terms of characteristics of the cell line, EMF exposure, or diagnostic endpoint. To that end, the current study divides the core of the literature into three parts. Hence, the second section provides a frequency-based overview of the included sources in tabular form. The parameters influencing the causal relationship between EMF exposure and cellular biological effects are discussed in the third section, and the fourth section deals with mechanistic insights into biologic activities. Finally, the review concludes with a summary and several suggestions for future research.
What is noteworthy is that the current study and the perspective findings are based only on cellular studies into cell lines excised from different organisms that can inactivate some natural defenses. Therefore, such a study only attempts to establish the mechanism of action of EMF interaction with the organism at the molecular or cellular level but does not discuss the degree of certainty of a causal relationship.
2 A frequency-based overview
Various cell lines of different morphological characteristics have shown different levels of susceptibility when subjected to electromagnetic fields. Bellow follows a tabulated overview organized into three frequency ranges 0–300 Hz [Citation5–12], 300 Hz-300 kHz [Citation13–17], and 300 kHz-300 GHz [Citation18–21]. It includes the characteristics of emitted, metered, and most importantly absorbed EMFs and also their subsequent implications. Also presented in are the features of the cell line and endpoints under study with the assays used. Moreover, depicts a general diagram of common frequencies applied upon assorted cell lines to assay usual biological endpoints.
Figure 2. Common frequencies applied to investigate assorted cell lines through different endpoints.
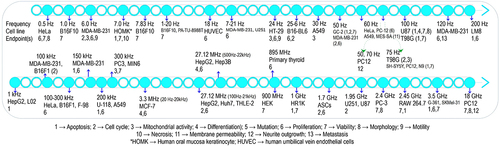
Table 1. EMF characteristics in the extremely low-frequency range (0–300 Hz) utilized to expose assorted cell lines, along with subsequent biological effects.
Table 2. EMF characteristics in the intermediate frequency range (300 Hz-300 kHz) utilized to expose assorted cell lines, along with subsequent biological effects.
Table 3. EMF characteristics in the radio frequency range (300 kHz-300 GHz) utilized to expose assorted cell lines, along with subsequent biological effects.
A glance at the body of related research reveals that EMFs especially at extremely low frequencies have captured the research community’s attention, as represented by and . This attention may suggest the impact of such a frequency range on gene expression at the cellular level in living tissues. For example, the frequency of 50 Hz, mostly investigated with intensities spanning the range of 0.01–20 mT, has commonly been utilized to irradiate different cells. With cancer treatment being the primary goal of the studies, the investigation of healthy cells has been carried out in the shadow of cancerous cells. The special attention of the scientific community to the specific characteristics of EMF such as a certain frequency, duration of exposure, or post exposure to expose particular cells of different tissues or morphology may refer to their higher joint susceptibility in causing biological effects. Therefore, influential parameters of the experiment participating in the results are discussed below in more detail.
3 Causal association
Based on mechanistic in vitro studies, this section attempts to answer the following question: what factors could contribute to the suspected causal association between EMF irradiation exposure and biological effects in various cells? The objective of this section is not to establish the causality of this association but to provide evidence for factors hypothesized to be influential on the suspected causality. shows a combination of such factors related to the cell line, exposure, or biological endpoint.
Figure 3. Various factors influencing the suspected cause-effect linkage between EMF irradiation exposure and biological effects in an in vitro setup.
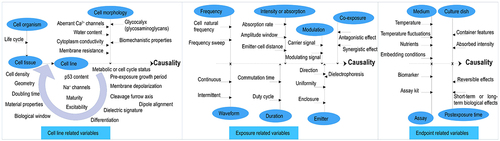
3.1 Cell line-related association: organism, origin tissue, and morphology
A multicellular organism consists of a structural sequence of cellstissues
organs, where cells are the smallest unit of life. The morphological features of a cell can predict the cellular state and whether it is cancerous or healthy. Therefore, cell line-related variables influencing the suspected causality can be defined as organism (e.g. human, mouse, or hamster), tissue (e.g. breast, lung, or melanoma), and morphology (e.g. epithelial, breast carcinoma, or lung carcinoma). The longer an organism’s life cycle, the longer it takes for EMF-contaminated tissues to show up. Regarding the context, several important aspects have been indicated: higher susceptibility in less-differentiated immature cells subjected to 895–2450 MHz exposure [Citation22]; impact of geometry, dimension, and doubling time on cell proliferation under TTFields [Citation13]; reversibility of suspected causal effects depending on doubling time [Citation23]; and role of the cleavage furrow and cell division axis relative to the electric field direction in causality [Citation24].
Regarding cell tissue, the efficacy of membrane depolarization due to the availability of Na+ channels over tissue excitability at frequencies below 1 kHz, nulled bioeffects at frequencies above 1 kHz due to too slow response time of excitable processes, and dielectric losses due to tissue heating at frequencies above many MHz have been implicated [Citation25]. Although this conclusion remarks the effect of membrane depolarization only at low frequencies, mitochondrial membrane depolarization induced due to oxidative stress and calcium release under 900 MHz was reported to cause apoptosis in MDA-MB-231 [Citation26]. When examining the effect of cell density on proliferation in Hela cells, bioinspired EMF inhibited cell proliferation in dishes containing 1000 cells or less but not at higher cell densities [Citation27].
Furthermore, when GC-2 cell line was exposed to EMF at a frequency of 50 Hz, MiR-26b-5p expression showed no change at 1 mT, increased at 2 mT, or decreased at 3 mT [Citation9]. This outcome represents the EMF “biological window” indicating the range of acceptable energy by the cell that is convertible to positive physiological processes. The biological window of the electromagnetic field correlates with the metabolic status of the organism, which is why dissimilar outcomes are obtained when cells are exposed during different metabolic states or cycle phases. Therefore, the role of growth period before exposure or cultural temperature in exposure-induced biological effects is noticeable. As an example of different cellular sensitivities, renal carcinoma cells including 786-O, 769-P, and Caki1 were exposed to a 50-Hz, 4.5-mT field for 30 min per day for 5 days [Citation28]. The examined cells underwent a decrease in viability due to induced cell-cycle arrest by the increased G1 and decreased G2 populations as a result of increased ROS levels, with 786-O indicating the maximum rate of decrease (30%). Additionally, the number of early apoptotic cells increased only in 786-O cells. However, a 27% increase in viability and an increase in ROS level, along with unaffected cell cycle phases, were recorded in healthy HEK293 cells.
As for cell morphology, different subcellular structures leading to different resonance frequencies in normal and cancerous cells have been shown using finite element simulation in MCF7 and MCF10A cells [Citation29]. Hence, material properties and dimensions of cells as crucial parameters decisive in dynamic responses have been proven to mediate biological effects. As an exemplary biomechanistic property, highly expressed levels of glycocalyx in the plasma membrane, consisting of proteoglycans, glycosaminoglycans, and plasma proteins, in cancerous cells but not in normal cells were found to mediate biological effects [Citation6].
Moreover, when exposed to the 50-Hz, 10-mT EMF for 24 h or 72 h, breast cancer cell MDA-MB-231 revealed more significant inhibition in cell growth after 24 h and also higher revival rate after 72-h exposure compared with colon cancer cells SW-480 or the unaffected HCT-116 cell [Citation30]. In another mechanistic study, breast epithelial cell MCF-10A and breast cancer cells, MDA-MB-231, Hs578T, T47D, and MCF-7, as well as colon cancer cell HT-29, were irradiated with EMFs at frequencies spanning 200 MHz-13.6 GHz [Citation31]. As a result, a lower dielectric signature was observed in the normal breast cell compared with the cancer breast cells, and also in the colon cancer cell compared with the breast cancer cells. In other words, higher membrane resistance and lower cytoplasm conductivity were noticed in colon cancer cells versus breast cancer cells. Depending on the cell content, dielectric properties are of a higher level in cancerous cells predominantly due to their increased water content. In addition, T-type Ca2+ channels aberrantly expressed in cancerous cells, for example, in MDA-MB-231 or MCF-7 but not in nonmalignant breast cells [Citation32], or the rich p53 content in the U87 glioblastoma cell but not in the U251 glioblastoma cell [Citation33] could explain the cell susceptibility against the EMF observed during some experiments.
3.2 Exposure-related association
3.2.1 Frequency, intensity or absorption, and modulation
Significantly lower resonance or natural frequencies in cancerous cells versus such frequencies in healthy cells may explain the selective cytotoxicity of EMF in different cell lines, thus revealing the weight of frequency and respective intensity of the exposure. For instance, biological effects that appear as a result of either tissue stimulation or dielectric loss dependent on the applied frequency range were mentioned in the previous subsection. The resonance frequency of cells embedded in water, as an abundant element in cancer cells, was considerably lower than that in agar [Citation29]. In the literature, various EMF frequencies particularly in three ranges 0.5 Hz-100 Hz (with the paramount focus on the power-line frequency, 50 Hz), 100–300 kHz (1–3 V/cm), and 0.9–3.5 GHz have been investigated. The first two ranges received the attention of researchers mainly due to their capability to stimulate cells while the last one due to considerable EMF-based equipment operating within this range. Most studies evaluate cellular responses to a single constant frequency, whereas frequency sweep is a technique for determining the biological effects of EMFs over a frequency range. To cite an example, mouse melanoma cancer cell B16F10 was continuously irradiated for 24 h or 48 h with 0.3-mT square waves of Schumann resonance frequency 7.83 Hz along with sweep intervals, and also with mixed single frequencies 7.83 and 60 Hz [Citation34]. The cell was inhibited by 17% under 7.83 Hz, by 26.4% under 7.830.1 Hz, and without any cell inhibition under 60 Hz. Inhibitory effects decreased as the frequency sweep intervals increased.
Regarding windows of susceptibility to EMFs, the 50-Hz sinusoidal field at 1 mT and 2 mT intensities promoted and suppressed the proliferation rate in HUVECs cells, respectively [Citation35]. These apparently contradictory results were related to the hypothesis of the EMF “amplitude window” or biological window that determines the levels of exposure permitted by each organ. As a representative of distributed field intensity, the specific absorption rate (SAR) refers to the energy per mass absorbed by tissues exposed to the EMF. It is dependent on the dimensions and materials of the cell, the emitted EMF, and the distance between the emitter and the organism, and can be calculated by numerical simulations, for example, using CST Software, or by experimental temperature measurement [Citation36,Citation37].
As another decisive aspect of exposure, modulation is utilized as a ubiquitous real-life data transmission technique and refers to phase-modulated, frequency-modulated, or amplitude-modulated EMF signals. Decrease in the viability of the breast cancer cell MCF-7 under pulsed electromagnetic fields (PEMFs) amplitude-modulated at resonance frequencies 20 Hz-20 kHz with a carrier frequency of 3.3 MHz was dependent on the resonance frequency of specific genes and duration of exposure [Citation38]. Similarly, inhibition in the proliferation rate of HepG2 and Huh7 hepatocellular carcinoma cells under EMFs amplitude-modulated at frequencies 100 Hz-21 kHz with a carrier frequency of 27.12 MHz was related to the downregulation of XCL2 and PLP2 genes [Citation39]. In these studies, no specific conclusion was obtained regarding the evaluation of the modulation parameter. Only when modulated at cell-specific frequencies, the EMF exposure revealed biological effects that were not affected by carrier frequency (50, 147, or 450 MHz) [Citation40]. In addition, the modulation parameter showed no significant impact under intermittent 1800 MHz field without modulation or modulated at GSM Basic or GSM Talk [Citation41].
By contrast, the 14.1-Hz triangular Amplitude Modulated (14.1tAM) signal stimulated the proliferation of U251 cells significantly more than the 7–21 Hz sine Frequency Modulated (7-21sFM) signal, whereas the 7.8-Hz triangular Amplitude Modulated (7.8tAM) signal significantly decreased the proliferation rate. Therefore, importance of the carrier frequencies of 14.1 and 7.8 Hz in inducing opposite biological effects was highlighted [Citation42]. The influence of such extremely low carrier frequencies probably relates to their role in cell excitation which is not the case at high carrier frequencies.
3.2.2 Waveform, continuity, and duration
Waveform represents the shape of a signal versus time, continuity means whether the waveform is continuous or intermittent, and duration represents the period over which a cell line is exposed to EMF irradiation. Among continuous sinusoidal-, square-, or triangular-shaped signals having a frequency ranged from 2 to 60 Hz and an intensity ranged from 2 to 6 mT, the triangular EMF caused the lowest alterations in the viability of U87-MG and 143B cancer cells [Citation43]. The reason can be related to the level of EMF applied over time. In another study, sinusoidal and square EMFs decreased and increased the proliferation of rat osteoblasts, respectively, while the triangular or serrated EMF had no effect [Citation44]. Furthermore, triangular and sinusoidal waves rendered the maximum rate of increase in ALP activity or mineralization potential. Due to the different cell lines and various endpoints used in these two studies, no conclusion can be reached from the appeared biological patterns. By delving into the literature, it appears that the sine wave and square pulse have been widely applied as waveforms. The effects of the narrowing of the pulse width (duty cycle) require further studies due to its higher impact compared to pulse frequency [Citation6]. The duty cycle refers to the ratio of the pulse width to the period of the pulsed wave.
As for continuity, human diploid fibroblast and rat granulosa cells subjected to 1800-MHz continuous or intermittent (5 min on/10 min off) field for 16 h developed DNA strand breaks, while the latter caused considerably more damage than the former [Citation41]. Nonetheless, no significant differences were observed in cell responses under the same continuous or intermittent exposure but for 4 h, thus emphasizing the significance of duration of exposure as a parameter that can affect cells in a certain metabolic state or throughout their entire cell cycle.
3.2.3 Enclosure, direction, and uniformity
Enclosure specifies whether an EMF emitter is placed inside an incubator or in an open area; field direction shows that the electromagnetic field is perpendicular, inclined, or parallel to the culture dish; and uniformity parameter refers to whether or not field intensity is uniform. EMF emitters, including solenoids, copper coils, Helmholtz reconfigured coils, transverse electromagnetic (TEM) cells, radial transmission line (RTL) systems, waveguides, BEMER therapy devices, or spoon-shaped applicators, have largely been used with perpendicularly oriented fields and enclosed inside an incubator. The intensity of irradiated EMF signals in different positions of culture dishes can be nonuniform, and their direction can also be dissimilar. As for the enclosure parameter, there was a significant difference between cellular processes evaluated in a TEM Cell located in an open area and in an incubator [Citation17]. Moreover, cell position-dependent outputs due to non-uniformity in terms of field intensity and probably field direction [Citation34], or the occurrence of dielectrophoresis referring to the movement of charged entities and dipoles toward the field of higher intensity, and dipoles aligned with the field direction [Citation45] can be attributed to uniformity or direction parameter.
Regarding direction parameter, the 50-Hz, 2.49-mT EMF oriented in parallel with culture plates resulted in the highest increase in proliferation rate [Citation46], and AC horizontal (18 Hz) ELF MF affected cells exposed to the DC (60 T) MF by suppressing proliferation and increasing superoxide production [Citation47]. (The ELF MF was perpendicular to the static MF and parallel to the dish culture.) In addition, an investigation of various test points differing in exposure direction (perpendicular, horizontal, or inclined) inside an incubator showed that the inclined direction could render the lowest changes in the endpoints [Citation27].
3.2.4 Co-exposure
The focus of investigations regarding co-exposure is to find co-treatments for medical purposes, and simultaneous exposure to different real-life electromagnetic fields distributed within human habitats has attracted less attention. Exposure to 50-Hz, 1-mT continuous EMF for 12 h followed by 5-fluorouracil (5-FU) treatment for 24 h exhibited a better anti-proliferative effect in the MCF-7 cancer cell, which is explained by the synergistic effect of EMF in leading cells into S phase and 5-FU specifically capable of blocking DNA synthesis [Citation48]. Similarly, when being co-treated with morphine and cisplatin, the 50-Hz, 0.5-mT intermittent sine EMF for 45 min (15 min on/15 min off) altered DNA genes by up-regulating GADD45A (p53 regulated gene), down-regulating non-homologous end joining (NHEJ) associated genes (LIG4, XRCC4, DNA-Pkcs, Ku80), and decreasing viability in SH-SY5Y cells [Citation49]. On the other hand, antagonistic co-exposure of 75-Hz, 2-mT intermittent PEMF with H2O2 in human neuroblastoma cells SK-N-BE(2) prevented H2O2-induced cytotoxicity, abolished the ROS increase, and boosted Mn-dependent superoxide dismutase-based response to H2O2 [Citation50].
Other studies such as co-exposure of Temozolomide (TMZ) and 100-Hz, 100-G continuous square waves in the U87 cell led to a synergistic decrease in Nestin, CD133, and Notch4 genes and an increase in GFAP genes involved in differentiation [Citation51]. Moreover, co-exposure of cisplatin and the 50-Hz, 200-G continuous EMF in the A2780 ovarian cancerous cell increased the caspase activity and p53 expression and decreased matrix metalloproteinases 2 expression [Citation52]. In conjunction with minocycline as an anti-inflammatory agent or lipopolysaccharide as an inflammatory agent, the 7-Hz, 30-mT sine field for 3 days, 4 h per day, was applied to human oral mucosa keratinocyte for evaluating the viability, apoptosis, or necrosis [Citation53].
3.3 Endpoint-related association
3.3.1 Medium and culture dish
Medium is defined as an environment that provides nutritional requirements to maintain the growth and function of the cell, and the culture dish is a container to hold the culture medium. Depending on the assay used, various culture dishes such as multi-well plates or flasks, having various culture areas, with or without stirring tools are utilized. Different containers may introduce additional unknown factors that affect the outcome. For instance, the thickness, shape, or material of the container can differently alter the EMF intensity absorbed by cells. Therefore, the SAR variable has been obtained numerically or experimentally in some studies. However, exposure characteristics in cells in vitro differ from those in cells in vivo irradiated with equivalent exposure.
As for medium nutrients, the impacts of serum deprivation on the cell cycle [Citation33], embedding conditions (embedding in water or agar) on cell vibrations [Citation29], or conditioned medium (medium collected from exposed cell culture) on unexposed cells as indirect exposure [Citation19] indicate the significance of environmental molecules upon the plausible biological effects caused by EMF. Regarding the temperature variable, cells have been exposed mainly at as body temperature or sometimes at room temperature. EMF-caused fluctuations of less than
are supposed to induce non-thermal biological effects.
3.3.2 Assay and postexposure duration
The accuracy of the assay can affect the apparent outcomes. For example, inefficient MTT assay compared to efficient trypan Blue assay for assessing cell viability or cytotoxicity can be cited [Citation21]. Some of the commonly utilized endpoint-related assays are as follows: release of reactive products including reactive oxygen species by DCFH-DA fluorescence, reactive nitrogen species by NO assay, superoxide by enzyme-linked immunosorbent assay, oxidoreductive activity by reduction of tetrazolium, or glutathione (enzymatic antioxidant) level by glutathione assay kit; leakage of inorganic calcium ions (Ca2+) by Fura-2 assay or Fluo-4 AM; protein expression by western blot; gene expression (differentiation) by quantitative RT-PCR; cell cycle by flow cytometry assay; apoptosis by acridine orange/ethidium bromide (AO/EB) staining using flow cytometry; and viability by either MTT assay or AO/PI and DAPI staining.
Postexposure duration represents the period between the end of exposure and the measurement time. Immediate measurement of cell processes reveals short-term biological effects, which can be reversed when the measurement is conducted sometime after exposure. For example, affected miRNA expressions were returned to their normal levels two doubling times after exposure [Citation23].
4 Mechanistic insights
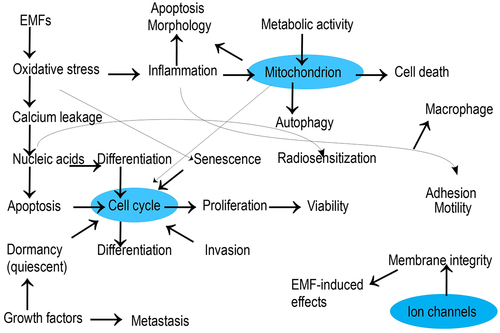
This section attempts to find evidence relevant to the following question: is there any biologically plausible mechanism in the literature to support the suspected causal relationship between EMF exposure and biological effects? The mechanism of biological effects refers to a natural process by which an endpoint is brought about. It can be explained in terms of the cell constituents: organic molecules belonging to four classes of carbohydrates, lipids, proteins, or nucleic acids; inorganic ions, including calcium (Ca2+), potassium (K+), sodium (Na+), magnesium (Mg2+), and phosphate (HPO42–); and water. presents a general correlation of cell processes or endpoints with the electromagnetic field.
shows the significance of cell cycle, mitochondrion, and ion channel activities in regulating cell viability, and a plethora of research works have focused on the proteins or nucleic acids involved in these activities.
4.1 Cell cycle
The cell cycle covers a set of events occurring in the cell to divide it into a pair of daughter cells. Technically speaking, it involves two phases, i.e., interphase with Gap1/(Gap0)/Synthesis/Gap2 (G1/G0/S/G2) sequence and mitotic phase with mitosis/cytokinesis sequence. Mitosis, followed by cytokinesis or division of the cytoplasm, includes prophase, metaphase, anaphase, and telophase. During prophase, chromosomes and the mitotic spindle appear, and the nucleolus disappears. This is followed by the alignment of chromosomes at the equator of the cell during metaphase, chromosome segregation during anaphase, and finally the disappearance of the spindle and the formation of a new nuclear envelope during telophase. Regarding the EMF targeting these phases, continuous TTFields (1–3 V/cm) at 100–300 kHz affected metaphase by disrupting the development of the mitotic spindle and anaphase by translocating intracellular components, leading to apoptosis, prolongation of mitosis, cell destruction in telophase, and rupture of the membrane and membrane blebbing [Citation25]. Analogously, disruption of mitotic spindle formation in glioma cell lines subjected to 100 kHz TTField led to cell cycle arrest. Susceptibility to TTField was observed in cells during telophase/cytokinesis with a narrow mitotic furrow parallel to the field direction, due to increased SAR concentration in the furrow region [Citation13].
Proteins and enzymes can directly or indirectly regulate the cell cycle as promoters or inhibitors. Cyclins and CDKs (cyclin-dependent kinases) related to each cell-cycle phase, E2F, growth factor receptor, and Myc are known as promoters, while inhibitors include pRB, P53, P21, p27, PPAR-, p73, p14, PARP, p38, or miRNAs. shows a schematic representation of such regulators activated in response to electromagnetic field stimuli based on various studies. Interaction between cell cycle processes and EMF through the cell cycle regulators mentioned above is unraveled according to the following investigations, with the primary focus on regulators of the G1 phase divided into early G1 and late G1, the former being dependent on growth factors [Citation54].
Figure 5. Activation of a likely cascade of cell compositions within the cell cycle by the electromagnetic field.
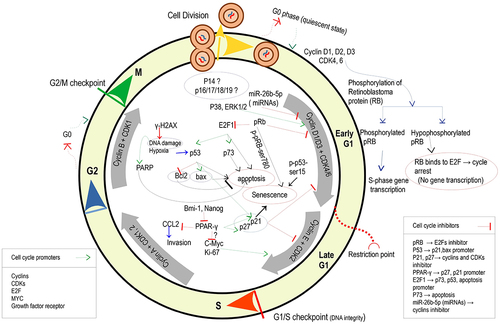
The power of the exposure signal via its characteristics, such as frequency, intensity, duration, and direction or polarization, to promote or inhibit life-sustaining processes suggests the cell-specific nature of biological effects. To cite a few exemplary research works, positive correlations between cyclin-D1 and proliferation and between P53 or Caspase-3 and apoptosis in U87 cells irradiated with 10-Hz, 50-Hz, or 100-Hz EMFs have shown both positive and negative bioeffects depending on the frequency or intensity variable [Citation5]. When investigated under 900-MHz continuous EMF, acute T-lymphoblastoid leukemia cells (CCRF-CEM) underwent induction of DNA breaks (increased pRb/p105 and p21/Waf1) and early activation of both p53-dependent (increased p53, bax, and bcl2) and p53-independent (increased E2F1 and p73) apoptosis at short exposure times (2, 4, or 12 h). However, longer exposure (24 or 48 h) conferred silencing of pro-apoptotic signals and activated genes in both extracellular (increased Ras and Akt1) and intracellular (increased Bcl-2) pro-survival signals [Citation55]. The Ras (GTPase) protein located near the plasma membrane can be activated by EMF irradiation and subsequently activates the MAP kinase signaling, thus leading to the induction of gene expression in the cell cycle.
Exposure to 50-Hz vertical EMF caused a cascade of cell-cycle reactions in breast carcinoma cells: down-regulated stemness markers Bmi-1 and Nanog; increased PPAR-, p21, and p27 protein levels leading to inhibition of cyclin D1, cyclin E, CDK4, and CDK2 proteins and subsequently cell cycle arrest by increasing the G1-phase cells and decreasing the S and G2/M cells with reduced proliferation. It also resulted in decreased CCL2, which led to decreased invasion ability [Citation10]. Further study on PPAR-
association with metastatic molecules such as CCL2 and Matrix metallopeptidase 9 is urged. Furthermore, despite the inhibitory role of PPAR-
on c-Myc expression [Citation56], such a cascade of protein release under the electromagnetic field lacks any investigation. Increased Ki-67 and c-Myc under 3.5-GHz microwave exposure for 24 h were related to increased proliferation and viability [Citation20]. Moreover, followed by cell cycle delay and proliferation decrease, induction of cell senescence under 1.7-GHz LTE EMF was correlated with ROS generation, differentially regulated p21, phosphorylation of p53 at serine 15, and phosphorylation of retinoblastoma (Rb) at serine 780 expression [Citation18].
Regarding disturbance of DNA stability during the cell cycle, intermittent 1.95-GHz UMTS signal (SAR 0.25, 0.5, and 1.00 W/kg; 5 min on/10 min off for 16 h) under serum-free conditions triggered rapidly disappeared DNA damage and induced nucleotide excision repair in the p53 proficient cell line U87, with no effect in the p53 deficient cell U251 and no induction of double-strand breaks in both cells [Citation33].
As for miRNAs, expression of miR-26b-5p in the GC-2 cell line exposed to 50-Hz EMF remained unchanged at 1 mT, increased at 2 mT, and decreased at 3 mT [Citation9]. Besides, when followed by exposure to the 3-mT EMF, miR-26b-5p in GC-2 cells transfected with miR-26b-5p mimic or mimic-nc significantly decreased the G0/G1-phase cells and slightly increased the S-phase cells. Therefore, the expression of miR-26b-5p was negatively correlated with cyclin D2 level, thus disturbing the cell cycle. Similarly, depicted were decreased expressions of miR-21-5p related to cell-cycle progression, miR-17-3p related to mitochondrial antioxidant activity, and miR-421-5p related to DNA repair in human glioblastoma cell T98G subjected to 75-Hz PEMF for 48 h at 2 mT with TMZ co-treatment [Citation57]. In addition, PEMF exposure without TMZ co-treatment only resulted in increased miR-421-5p expression and apoptosis.
Regarding the p38 MAPK pathway in microglial cells, p38 and phosphorylated p38 increased immediately after exposure to EMF (200 kV/m, 200 pulses) but returned to control levels 24 h after exposure [Citation58]. Such effects were followed by increased expression of OX-42 and transient up-regulation of inflammatory cytokine TNF-, anti-inflammatory factor IL-10, and pro-inflammatory factor NO. The role of p38 MAPK signaling in regulating the G1 phase-related and G2 phase-related cyclins and CDKs, mediated by a cascade of different protein expressions including p53, p16, and p19, has been delineated in Thornton and Rincon [Citation59]. Notwithstanding its influential role in protein regulation within the cell cycle, such an EMF-induced signaling pathway demands further research. When exposing the neuroblastoma line NB69 intermittently or continuously to 50-Hz, 100-
T magnetic field, increase of p38 depending on ROS release, promotion of cell proliferation in association with cell cycle via p27 decrease and cyclin D1 increase, and early increase of ERK1/2 or JNK have led to sequentially up-regulated MAPK-p38 and MAPK-ERK1/2 [Citation60]. Similarly, increased levels of P38 and P21 accompanied with 30
apoptosis induction were observed in the mouse breast cancer cell MC4L2 exposed to 1-Hz intermittent EMF at 100 mT [Citation61]. In the end, the expression of p14 as the modulator of p53, p16, p17, p18, p19, and S-related and G2-related proteins in the cell cycle under electromagnetic field exposure is devoid of any molecular research and requires further studies.
The above research works render the EMF technique as a double-edged sword, the role of which to be on either the beneficial or detrimental side is determined by exposure characteristics and tissue’s constitutions.
4.2 Mitochondrial activity
As a biosynthetic and bioenergetic site, mitochondria perform cellular respiration to generate ATP used in various molecular reactions, thereby contributing substantially to energy metabolism through its signaling function. For example, mitochondrial ATP synthase was positively correlated with cell cycle and proliferation [Citation62]. Electromagnetic irradiation may affect mitochondrial functions and cause disturbances in cell composition, the molecular mechanism of which has been under experimental investigation. depicts the role of the electromagnetic field in the regulation of mitochondrial signaling pathways, as derived from various studies.
Figure 6. A likely cascade activation of cell compositions associated with mitochondria stimulated by the electromagnetic field.
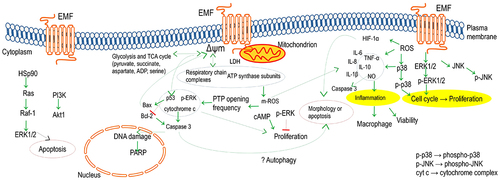
Mitochondrial membrane potential, as the electric potential gradient linking mitochondrial activity with cytoplasm activity, is influenced by membrane permeability. The role of mitochondria-dependent signaling through the respiratory chain, TCA cycle, glycolysis, or mitochondrial reactive oxygen species (m-ROS) in the activation of hypoxic gene expression has been reviewed in Chandel [Citation63]. Investigating HeLa, EJ, HIEC, A549, NIH/3T3, and MRC-5 cells exposed to bio-inspired 0.5-Hz EMF for 48 h (20 min on/20 min off) revealed significant ATP reduction only in HeLa and A549 [Citation27]. In further detail, investigated were endpoints of mitochondrial activity (2-day exposure), proliferation (3-day exposure), and motility (2-day exposure) in MDA-MB-231 and HT-29 cells under 6-Hz and 24-Hz continuous sine waves, respectively [Citation64]. Accordingly, MDA-MB-231 experienced increased expressions of the respiratory chain subunits, i.e. COX2 and COX4, and the ATP synthase subunits, i.e. MT-ATP6 and ATP5B, and HT-29 experienced increased mitochondrial membrane potential. In addition, reduced proliferation and motility were observed in both cells (EMF reversed the effect of transforming growth factor (TGF) on migration). Exposed to an EMF of similar characteristics, the malignant cells of MDA-MB-231 and human pleural mesothelioma MSTO-211 H have shown the following outcomes:
-associated mitochondrial respiration enhanced after 2-day exposure via an increase in mRNA levels of two cytochrome c oxidase or Complex IV subunits (COX2 and COX4), upregulation in two subunits of ATP synthase in nuclear (ATP5B) and mitochondrion transcription (MT-ATP6), and an increase in protein expression of complex I, II, III, and IV and ATP synthase; increased intracellular ROS; and unaffected LDH [Citation65]. However, the nonmalignant cells MCF-10A and Beas-2B remained unaffected. Furthermore, inhibited cell growth modulated by calcium efflux was observed at 6 Hz for MDA-MB-231 and 16 Hz for MSTO-211H, which is reinforced by confirmed decrease in S-phase population. In the same frequency range, BEMER therapy followed by X-ray elevated ROS level, which led to DNA damage and time- and intensity-dependent radiosensitization, while BEMER therapy solo modulated the metabolites of different pathways, particularly of the glycolysis and TCA cycle [Citation11].
Being the master transcription factor regulating hypoxia-induced cellular response, HIF-1 was inhibited by 75-Hz EMF due to the decrease of hypoxia-induced ROS, and this inhibition was associated with the reduced apoptosis and cell death [Citation12]. In addition, release of cytokines TNF-
, IL-1
, IL-6, and IL-8 stimulated by LPS showed a decrease owing to the reduction of LPS-induced ROS. As for cytokines, 2.45-GHz EMF co-exposure with black carbon has resulted in sequential responses through prolonged phagocytosis, NO production, increased oxidative stress, and cytokine expression of TNF-
and IL-1
, which brought up pro-inflammatory activity, increased caspase-3 expression, apoptosis, and also activated macrophage and decreased viability [Citation21].
Originated at the plasma membrane, cAMP signaling, during which ATP-into-cAMP conversion is followed by cAMP-dependent protein kinase A (PKA) activity, controls the mitochondrial metabolic activities. Stimulated by EMF exposure, the cAMP and ERK/MAP kinase signaling pathways have been demonstrated to mediate the proliferation of several cells. To cite a few instances, several malignant cells under Thomas-EMF experienced inhibition of proliferation owing to the promoted ERK phosphorylation or transiently altered cAMP, while being blocked by a MAP-kinase pathway inhibitor or a cMAP inhibitor/activator, respectively [Citation7]. However, nonmalignant cells revealed no changes in cAMP level, expression of phosphorylated ERK, or growth rate. Besides, the ERK phosphorylation level and, thus, the proliferation rate remained unaffected in the active PKA transfected cells. Further mechanistic investigation into the chain of cAMP connection with mitochondrial ATP or ERK signaling is unavailable.
Intracellular expressions of mitochondrial proteins including phospho-ERK, p53, and cytochrome c under 50-Hz EMF were positively correlated with proliferation while transcription of respiratory complexes and ATP subunits remained unchanged, with the mechanism of these correlations being devoid of investigation [Citation8]. The EMF is hypothesized to modulate the frequency of opening in the permeability transition pore (PTP), involved in mitochondrial permeability marked with cytochrome c expression [Citation66]. Regarding this hypothesis, the signaling pathway of expression of Bax (pro-apoptotic factor) and suppression of Bcl-2 (anti-apoptotic factor) leading to the loss of membrane potential in mitochondrion and facilitation of cytochrome c release from mitochondrion into the cytoplasm, which triggered the activation of caspase-3 and, thus, causing an increase in PARP (DNA repair factor), contributed to the mitochondrial-related apoptosis measured 6 h after EMF exposure for 5 min and 15 min in the PC12 cells and also in Wistar rats irradiated with the 2.856-GHz field at SAR 30 mW/cm2 [Citation67].
Heat shock proteins (Hsps), classified into four groups of Hsp90, Hsp70, Hsp60, and such small Hsps as Hsp10 or Hsp27, are counted as anti-apoptosis factors and may be activated by induction of signaling pathways of mitogen-activated protein kinases (MAPKs) including ERKs, c-JNKs, and p38. When exploring this phenomenon in human lens epithelial cells subjected to a 1.8-GHz GSM field at SAR 2, 3, or 4 W/Kg, observed were the activation of ERK1/2 peaking at 30 min post-exposure and JNK1/2 peaking at 2 h post-exposure, with no activation of p38MAPK, as well as the up-regulation of Hsp27 and Hsp70 but not Hsp90 [Citation68]. Similarly, exposed to a 1.95-GHz field at SAR 3.60.2 mW/g, human oropharyngeal epidermoid carcinoma KB cells showed the apoptosis induction in parallel with a decrease in chains of Hsp90 leading to Ras, Raf-1, and ERK1/2 reduction inhibited by an ERK inhibitor or Hsp90 overexpression. Furthermore, observed were increased PI3K, decreased Akt expression, increased JNK-1 activity and Hsp70,27 expression, and decreased p38 kinase activity [Citation69].
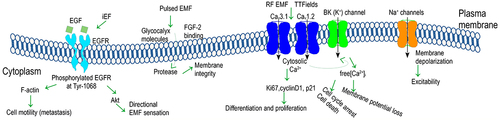
4.3 Ion channels
Being targeted by electromagnetic irradiation, plasma membrane ion channels may undergo alteration in their frequency of opening and closure and also in the magnitude of ions leaking through. These channels can be gated differently, out of which voltage-gated channels open and close depending on membrane potential and ligand-gated channels unlock via binding of ligands to their receptor. Derived from different studies in the literature, depicts plausible impact of the electromagnetic field in the regulation of ion channels.
4.3.1 Voltage-gated channels
Voltage-gated ion channels with ion-selective permeability such as calcium (Ca2+), potassium (K+), and sodium (Na+) channels are excited by alteration in electrical membrane potential. Through voltage-gated ion channels, electromagnetic field exposure has been demonstrated to alter ion concentration within the cytoplasm, mitochondria, or nucleus. As regards different types of Ca2+ channels, namely, 1.1, 1.2, 1.3, 1.4 (L-type),
2.1, 2.2, 2.3, and
3.1, 3.2 (T-type), to regulate cell processes under EMF exposure, the following investigated results have been concluded: identification of cell-specific anti-proliferation and CSC down-regulation effects under amplitude-modulated EMF at 500 Hz-22 kHz with a carrier frequency of 27.12 MHz mediated by increased cytosolic Ca2+ influx through
3.1 into the cell while being blocked by utilizing calcium channel blockers [Citation15]; the occurrence of cell cycle arrest, intrinsic apoptosis, and cell death under TTFields application mediated by the process of Ca2+ influx through
1.2 channels leading to Ca2+ activated BK (K+) channels and thus increased cytosolic free Ca2+ concentration [Citation16]; and identification of an anti-proliferative effect under Thomas EMF mediated by promoted Ca2+ uptake via the T-type Ca2+ channel blocked by such inhibitors as bepridil or mibefradil [Citation70].
The increased ROS levels, the affected spontaneous intracellular Ca2+, and the decreased catalase activity after 30-min exposure (acute exposure), along with the increased catalase activity and basal intracellular Ca2+ after 7-day exposure (chronic exposure) were observed in undifferentiated PC12 rat cells exposed to 50-Hz continuous sine wave of 0.1–1.0 mT of intensity [Citation71]. Moreover, membrane permeability of PC 12 cells increased subjected to 18-GHz exposure (SAR 1.17 kW/kg) and lasted for 9 min after exposure [Citation37]. The enhanced Ca2+ flux was related to cell-specific frequencies of modulated EMF but not their carrier [Citation40].
4.3.2 Ligand-gated channels
Being among transmembrane ion-channel proteins, ligand-gated ion channels once binding to a ligand unlock and allow ions such as Ca2+ to leak into the membrane. As a transmembrane protein, the epidermal growth factor (EGF) receptor is activated by binding specific ligands such as EGF or TGF-. In this context, EGFR activation with a noticeable increase in phosphorylation subjected to the EGF(+)/iEF(-) stimuli in breast cancer cells was hindered by the iEF exposure [Citation14]. The investigated mechanism including the promoted EGFR aggregation/clustering, down-regulated phosphorylated EGFR activation, and the inhibited EGF-promoted F-actin aggregation has led to the hindered cell motility.
Carcinoma cells with heavily charged glycans’ (heparan sulfate, chondroitin sulfate, or sialic acid) expression exposed to pulsed EMFs demonstrated the protease release suggesting altered membrane integrity and subsequent viability [Citation6]. Nevertheless, Heparinase treatment digesting glycocalyx molecules, assessed by the extent of FGF-2 binding to sulfated HS chains, rendered the cell lines insensitive to EMFs.
Summary and future research
Given its suggested function in carcinoma treatment, the electromagnetic field (EMF) as a double-edged sword remains a stressor threatening organisms owing to functioning interference, with its mechanism of action being uncertain. Providing insights into the problem by compiling recent mechanistic advances into a comprehensive review could assist the research community with the current state of knowledge and directions for further research. Therefore, the current review compiles athermal biological effects over a broad electromagnetic spectrum of alternating current ranged between frequencies 0 and 300 GHz that lead to alterations in cell physiology. Nonetheless, the EMF mechanism may differ with respect to cell compositions, thus implying the complexity of the problem due to its tissue-specific and consequently exposure-dependent nature, with the experimental approach including assays and time-related parameters being decisive. Bearing this in mind, a general description of causal association and mechanistic linkage has been provided in the current review.
As regards suspected causality between EMF exposure and health effects, the plausible influential factors have been explained in relation to the cell line, irradiation exposure, or endpoint. Regarding the first category, influences of the following factors have been concluded: life cycle, doubling time, maturity, and metabolic or cell cycle state affecting the exposure or post-exposure duration to render biological effects; material properties, dielectric signature, or geometry affecting cell excitability; and above all cell compositions such as Ca2+ channels, Na+ channels, Glycocalyx level, p53 content, or water content affecting susceptibility to EMF. Consequently, different geometries and subcellular structures, referring to the biological window, leading to different resonance frequencies or dielectric properties, could explain cell susceptibility against the electromagnetic field in assorted cell lines in terms of morphology or tissue, with cancerous cells being more susceptible than healthy ones or cells extracted from some tissues more sensitive than others. The cell-specific biological window (range of acceptable energy which is convertible to positive physiological processes) correlating with the metabolic status influenced by such parameters as pre-exposure growth period or culture temperature can be implicated. As for the second category, the influences of exposure frequency versus cell resonance frequency, cell structure on cell absorption, exposure duration, continuity, uniformity, modulating frequencies, field direction versus cleavage furrow axis, and dielectrophoresis owing to the non-uniformity of the field have been highlighted. Therefore, for a given cell line, the impacts of frequency and respective intensity on tissue stimulation or dielectric loss, duration of exposure compared with doubling time, intermittent exposure, the duty cycle of pulsed waves, and experimental design regarding enclosure, emitter, direction, or uniformity are noticed. Regarding the last category, medium molecules and culture dish, assay kit and biomarker, and post-exposure time are decisive in the appearance of biological effects. There are reported deficiencies regarding each factor in more detail within the text.
The mechanism of stimulatory or inhibitory impact of the electromagnetic field upon organisms’ functioning has been depicted through decisive life-sustaining processes of cell cycle and mitochondria, and ion channels in a chained way. As for the cell cycle, cyclins and CDKs related to the G1 phase as cell cycle promoters affected by different cell cycle inhibitors such as miRNAs, pRB, p21, p27, p53, E2F1, p73, or PPAR- have been shown to regulate proliferation and viability via processes like apoptosis, senescence, invasion, or disruption of mitotic spindle formation. There are proteins with their chain function in the cell cycle devoid of in-detail investigation, among which are p14, p38, PPAR-
chain, or cyclins and CDKs regulations related to the S phase or G2 phase.
Regarding mitochondria-related disturbances, such activities as the TCA cycle, glycolysis, respiratory complexes, ATP synthase subunits, mitochondrial reactive oxygen species, and subsequent gene expression or mitochondrial membrane potential are cited. Investigating proteins such as Hsps, cytokines, caspase-3, cytochrome c, and subsequent cell processes in mitochondria such as apoptosis or macrophage reveals that the association of Hsps releases with signaling pathways of mitogen-activated protein kinases including ERKs, c-JNKs, and p38 or cAMP connection with mitochondrial ATP or ERK signaling require more investigations.
As a gate located on the membrane, voltage- or ligand-gated channels investigated in different studies have been reviewed, with calcium (Ca2+) channels being shown to be more influential. The role of different types of Ca2+ channels, L-type or T-type, for example, and K+ or Na+ channels in the regulation of various cell processes lack adequate investigation.
As for directions for further research, being in the shadow of cancerous cells, normal cells of assorted origin tissues require more detailed studies to finally render a classification of cells in terms of their sensitivity to environmental exposure to the electromagnetic field. Different cell lines in terms of morphology or tissue with more suspected susceptibility to EMF have attracted more investigations. However, such investigations, focusing on one specific frequency and mostly one intensity, are limited in terms of exposure features. With many components participating in the exposure system, it is quite difficult to make an elaborate comparison among works conducted by different researchers. Added to this difficulty are research works with no EMF absorption rate of the cell line versus emitted or metered intensity of EMF. Besides, real-life exposures such as combined EMFs of different features have not been adequately explored.
Acknowledgement
The corresponding author of this work would like to gratefully acknowledge the financial support for her research provided by the NOBELIUM JOINING GUT RESEARCH COMMUNITY through the project No. 11/2020/IDUB/I.1.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Kocaman A, Altun G, Kaplan AA, et al. Genotoxic and carcinogenic effects of non-ionizing electromagnetic fields. Environ Res. 2018;163:71–79.
- Heidari S, Abdi S, Karizi SZ. Evaluation of BCL2 and its regulatory miRs, miR-15-b and miR-16 expression changes under the exposure of extremely low-frequency electromagnetic fields on human gastric cancer cell line. Radiat Prot Dosimetry. 2021;197(2):93–100.
- Xu W, Yan D, Sun J, et al. The activation of cancer cells by a nanosecond-pulsed magnetic field generator. J Phys D Appl Phys. 2020;53(12):125401.
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Potential health effects of Exposure to Electromagnetic Fields (EMF); 27 January 2015.
- Akbarnejad Z, Eskandary H, Vergallo C, et al. Effects of extremely low-frequency pulsed electromagnetic fields (ELF-PEMFs) on glioblastoma cells (U87. Electromagn Biol Med. 2017;36(3):238–247.
- Ashdown CP, Johns SC, Aminov E, et al. Pulsed low-frequency magnetic fields induce tumor membrane disruption and altered cell viability. Biophys J. 2020;118(7):1552–1563.
- Buckner CA, Buckner AL, Koren SA, et al. Exposure to a specific time-varying electromagnetic field inhibits cell proliferation via cAMP and ERK signaling in cancer cells. Bioelectromagnetics. 2018;39(3):217–230.
- Destefanis M, Viano M, Leo C, et al. Extremely low frequency electromagnetic fields affect proliferation and mitochondrial activity of human cancer cell lines. Int J Radiat Biol. 2015;91(12):964–972.
- Liu Y, Liu W, Liu K, et al. Overexpression of miR-26b-5p regulates the cell cycle by targeting CCND2 in GC-2 cells under exposure to extremely low frequency electromagnetic fields. Cell Cycle. 2016;15(3):357–367.
- Oh IR, Raymundo B, Jung SA, et al. Extremely low-frequency electromagnetic field altered PPARγ and CCL2 levels and suppressed CD44+/CD24- breast cancer cells characteristics. Bull Korean Chem Soc. 2020;41(8):812–823.
- Storch K, Dickreuter E, Artati A, et al. Bemer electromagnetic field therapy reduces cancer cell radioresistance by enhanced ros formation and induced DNA damage. PLoS ONE. 2016;11(12):e0167931.
- Vincenzi F, Ravani A, Pasquini S, et al. Pulsed electromagnetic field exposure reduces hypoxia and inflammation damage in neuron-like and microglial cells. J Cell Physiol. 2017;232(5):1200–1208.
- Berkelmann L, Bader A, Meshksar S, et al. Tumour-treating fields (TTFields): investigations on the mechanism of action by electromagnetic exposure of cells in telophase/cytokinesis. Sci Rep. 2019;9(1):1–11.
- Garg AA, Jones TH, Moss SM, et al. Electromagnetic fields alter the motility of metastatic breast cancer cells. Commun Biol. 2019;2(1):1–16.
- Jimenez H, Wang M, Zimmerman JW, et al. Tumour-specific amplitude-modulated radiofrequency electromagnetic fields induce differentiation of hepatocellular carcinoma via targeting Cav3.2 T-type voltage-gated calcium channels and Ca2+ influx. EBioMedicine. 2019;44:209–224.
- Neuhaus E, Zirjacks L, Ganser K, et al. Alternating electric fields (TTFields) activate Cav1.2 channels in human glioblastoma cells. Cancers (Basel). 2019;11(1):110.
- Peng WY, Li KJ, Xie YZ, et al. Development of a TEM-cell-integrated CO2 incubator for cell-based transient electromagnetic field bioeffect study. Electromagn Biol Med. 2020;39(4):290–297.
- Choi J, Min K, Jeon S, et al. Continuous exposure to 1.7 GHz LTE electromagnetic fields increases intracellular reactive oxygen species to decrease human cell proliferation and induce senescence. Sci Rep. 2020;10(1):1–15.
- Jooyan N, Goliaei B, Bigdeli B, et al. Direct and indirect effects of exposure to 900 MHz GSM radiofrequency electromagnetic fields on CHO cell line: evidence of bystander effect by non-ionizing radiation. Environ Res. 2019;174:176–187.
- Mumtaz S, Bhartiya P, Kaushik N, et al. Pulsed high-power microwaves do not impair the functions of skin normal and cancer cells in vitro: a short-term biological evaluation. J Adv Res. 2020;22:47–55.
- Sueiro-Benavides RA, Leiro-Vidal JM, Salas-Sánchez AÁ, et al. Radiofrequency at 2.45 ghzGhz increases toxicity, pro-inflammatory and pre-apoptotic activity caused by black carbon in the RAW 264.7 macrophage cell line. Sci Total Environ. 2021;765:142681.
- Halgamuge MN, Skafidas E, Davis D. A meta-analysis of in vitro exposures to weak radiofrequency radiation exposure from mobile phones (1990–2015. Environ Res. 2020;184:109227.
- Aalami Zavareh F, Abdi S, Entezari M. Up-regulation of miR-144 and miR-375 in the human gastric cancer cell line following the exposure to extremely low-frequency electromagnetic fields. Int J Radiat Biol. 2021;97(9):1324–1332.
- Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–3295.
- Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci. 2013;1291(1):86–95.
- Kahya MC, Nazroğlu M, Çiğ B. Selenium reduces mobile phone (900 MHz)-induced oxidative stress, mitochondrial function, and apoptosis in breast cancer cells. Biol Trace Element Res. 2014;160(2):285–293.
- Liu X, Liu Z, Liu Z, et al. The effects of bio-inspired electromagnetic fields on normal and cancer cells. J Bionic Eng. 2019;16(5):943–953.
- Cios A, Ciepielak M, Stankiewicz W, et al. The influence of the extremely low frequency electromagnetic field on clear cell renal carcinoma. Int J Mol Sci. 2021;22(3):1342.
- Geltmeier A, Rinner B, Bade D, et al. Characterization of dynamic behaviour of MCF7 and MCF10A cells in ultrasonic field using modal and harmonic analyses. PLoS ONE. 2015;10(8):e0134999.
- Filipovic N, Djukic T, Radovic M, et al. Electromagnetic field investigation on different cancer cell lines. Cancer Cell Int. 2014;14(1):1–10.
- Hussein M, Awwad F, Jithin D, et al. Breast cancer cells exhibits specific dielectric signature in vitro using the open-ended coaxial probe technique from 200 MHz to 13.6 GHz. Sci Rep. 2019;9(1):1–8.
- Taylor JT, Huang L, Pottle JE, et al. Selective blockade of T-type Ca2+ channels suppresses human breast cancer cell proliferation. Cancer Lett. 2008;267(1):116–124.
- Al-Serori H, Ferk F, Kundi M, et al. Mobile phone specific electromagnetic fields induce transient DNA damage and nucleotide excision repair in serum-deprived human glioblastoma cells. PLoS ONE. 2018;13(4):e0193677.
- Tang JY, Yeh TW, Huang YT, et al. Effects of extremely low-frequency electromagnetic fields on B16F10 cancer cells. Electromagn Biol Med. 2019;38(2):149–157.
- Delle Monache S, Angelucci A, Sanità P, et al. Inhibition of angiogenesis mediated by extremely low-frequency magnetic fields (ELF-MFs. PLoS ONE. 2013;8(11):e79309.
- Górski R, Nowak-Terpiłowska A, Śledziński P, et al. Morphological and cytophysiological changes in selected lines of normal and cancer human cells under the influence of a radio-frequency electromagnetic field. Ann Agric Environ Med. 2021;28(1):163.
- Perera PGT, Nguyen THP, Dekiwadia C, et al. Exposure to high-frequency electromagnetic field triggers rapid uptake of large nanosphere clusters by pheochromocytoma cells. Int J Nanomed. 2018;13:8429.
- Alcantara DZ, Soliman IJS, Pobre RF, et al. Effects of pulsed electromagnetic fields on breast cancer cell line MCF 7 using absorption spectroscopy. Anticancer Res. 2017;37(7):3453–3459.
- Zimmerman JW, Pennison MJ, Brezovich I, et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer. 2012;106(2):307–313.
- Bawin S, Kaczmarek L, Adey WR. Effects of modulated VHF fields on the central nervous system. Ann N Y Acad Sci. 1975;247(1):74–81.
- Diem E, Schwarz C, Adlkofer F, et al. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res Genet Toxicol Environ Mutagen. 2005;583(2):178–183.
- Makinistian L, Marková E, Belyaev I. A high throughput screening system of coils for elf magnetic fields experiments: proof of concept on the proliferation of cancer cell lines. BMC Cancer. 2019;19(1):1–10.
- Koziorowska A, Romerowicz-Misielak M, Sołek P, et al. Extremely low frequency variable electromagnetic fields affect cancer and noncancerous cells in vitro differently: preliminary study. Electromagn Biol Med. 2018;37(1):35–42.
- Zhou J, Wang JQ, Ge BF, et al. Different electromagnetic field waveforms have different effects on proliferation, differentiation and mineralization of osteoblasts in vitro. Bioelectromagnetics. 2014;35(1):30–38.
- Gutin PH, Wong ET. Noninvasive application of alternating electric fields in glioblastoma: a fourth cancer treatment modality. Am Soc Clin Oncol Educ Book. 2012;32(1):126–131.
- Restrepo AF, Tobar VE, Camargo RJ, et al., . Effects of extremely low frequency electromagnetic fields on in-vitro cellular cultures HeLa and CHO. in ‘2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC)’; 2016; Orlando, FL, USA. IEEE, p. 4193–4196.
- Naarala J, Kesari KK, McClure I, et al. Direction-dependent effects of combined static and ELF magnetic fields on cell proliferation and superoxide radical production. Bio Med Res Int. 2017;2017:1–8.
- Han Q, Chen R, Wang F, et al. Pre-exposure to 50 hz-electromagnetic fields enhanced the antiproliferative efficacy of 5-fluorouracil in breast cancer MCF-7 cells. PLoS ONE. 2018;13(4):e0192888.
- Sanie-Jahromi F, Saadat M. Effects of electromagnetic field, cisplatin and morphine on cytotoxicity and expression levels of DNA repair genes. Mol Biol Rep. 2018;45(5):807–814.
- Falone S, Marchesi N, Osera C, et al. Pulsed electromagnetic field (PEMF) prevents pro-oxidant effects of H2O2 in SK-N-BE (2) human neuroblastoma cells. Int J Radiat Biol. 2016;92(5):281–286.
- Ahmadi-Zeidabadi M, Akbarnejad Z, Esmaeeli M, et al. Impact of extremely low-frequency electromagnetic field (100 Hz, 100 G) exposure on human glioblastoma U87 cells during temozolomide administration. Electromagn Biol Med. 2019;38(3):198–209.
- Baharara J, Hosseini N, Farzin TR. Extremely low frequency electromagnetic field sensitizes cisplatin-resistant human ovarian adenocarcinoma cells via P53 activation. Cytotechnology. 2016;68(4):1403–1413.
- Kaszuba-Zwoińska J, Novak P, Nowak B, et al. Low-frequency electromagnetic field influences human oral mucosa keratinocyte viability in response to lipopolysaccharide or minocycline treatment in cell culture conditions. Biomed Pharmacother. 2021;137:111340.
- Wong E, 2012. ‘Introduction to neoplasia’, http://www.pathophys.org/introneoplasia/.
- Marinelli F, La Sala D, Cicciotti G, et al. Exposure to 900 MHz electromagnetic field induces an unbalance between pro-apoptotic and pro-survival signals in T-lymphoblastoid leukemia CCRF-CEM cells. J Cell Physiol. 2004;198(2):324–332.
- Cerbone A, Toaldo C, Laurora S, et al. 4-Hydroxynonenal and PPARγ ligands affect proliferation, differentiation, and apoptosis in colon cancer cells. Free Radic Biol Med. 2007;42(11):1661–1670.
- Pasi F, Fassina L, Mognaschi ME, et al. Pulsed electromagnetic field with temozolomide can elicit an epigenetic pro-apoptotic effect on glioblastoma T98G cells. Anticancer Res. 2016;36(11):5821–5826.
- Yang LL, Zhou Y, Tian WD, et al. Electromagnetic pulse activated brain microglia via the p38 MAPK pathway. Neurotoxicology. 2016;52:144–149.
- Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5(1):44.
- Martínez MA, Úbeda A, Moreno J, et al. Power frequency magnetic fields affect the p38 mapk-mediated regulation of NB69 cell proliferation implication of free radicals. Int J Mol Sci. 2016;17(4):510.
- Barati M, Fahimi H, Farahmand L, et al. 1hz 100mT electromagnetic field induces apoptosis in breast cancer cells through up-regulation of p38 and p21. Multidiscip Cancer Investig. 2020;4(1):23–29.
- Antico Arciuch VG, Elguero ME, Poderoso JJ, et al. Mitochondrial regulation of cell cycle and proliferation. Antioxidants & Redox Signaling. 2012;16(10):1150–1180.
- Chandel NS. Mitochondria as signaling organelles. BMC Biol. 2014;12(1):1–7.
- Lucia U, Grisolia G, Ponzetto A, et al. Thermomagnetic resonance affects cancer growth and motility. R Soc Open Sci. 2020;7(7):200299.
- Bergandi L, Lucia U, Grisolia G, et al. The extremely low frequency electromagnetic stimulation selective for cancer cells elicits growth arrest through a metabolic shift. Biochim Biophys Acta-Mol Cell Res. 2019;1866(9):1389–1397.
- Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. switching from low-to high-conductance state. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1998;1366(1–2):33–50.
- Zuo H, Lin T, Wang D, et al. Neural cell apoptosis induced by microwave exposure through mitochondria-dependent caspase-3 pathway. Int J Med Sci. 2014;11(5):426.
- Yu Y, Yao K, Wu W, et al. Effects of exposure to 1.8 GHz radiofrequency field on the expression of Hsps and phosphorylation of MAPKs in human lens epithelial cells. Cell Res. 2008;18(12):1233–1235.
- Caraglia M, Marra M, Mancinelli F, et al. Electromagnetic fields at mobile phone frequency induce apoptosis and inactivation of the multi-chaperone complex in human epidermoid cancer cells. J Cell Physiol. 2005;204(2):539–548.
- Buckner CA, Buckner AL, Koren SA, et al. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE. 2015;10(4):e0124136.
- Morabito C, Guarnieri S, Fanò G, et al. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem. 2010;26(6):947–958.