ABSTRACT
Chronic obstructive pulmonary disease (COPD) is characterised by progressive and irreversible airflow limitation associated with chronic inflammation involving cytokines and metalloproteinases (MMPs). MMP-8, MMP-9 and neutrophil elastase (NE) are known to be implicated in COPD but the factors influencing activation and suppression remain unclear. This study aimed to compare MMP-8, MMP-9 and NE in the peripheral blood of COPD patients and controls and to likewise assess exhaled breath condensate (EBC) for these MMPs. Peripheral blood micro(mi)RNA139-5p levels, which may regulate MMPs in COPD, were also measured. Blood and EBC were collected from COPD patients (stable and during exacerbations) and healthy controls. Expression of mRNA for MMP-8, MMP-9, NE and miRNA-139-5p expression in peripheral blood mononuclear cells (PBMCs) was measured using qRT-PCR. MMP-8, MMP-9 and NE protein in plasma as well as MMP-8 and MMP-9 protein in EBC were analysed by enzyme-linked immunoassays. PBMCs from COPD patients showed greater expression of mRNA for MMP-8 (p = 0.0004), MMP-9 (p = 0.0023) and NE (p = 0.0019). PBMC expression of mRNA for NE was significantly higher in COPD exacerbations compared to stable cases (p < 0.05). Expression of mRNA for MMP-9 and NE correlated negatively with spirometry in patients (p < 0.05). Plasma from COPD patients showed greater levels of protein for MMP-8 (p = 0.003), MMP-9 (p = 0.046) and NE (p = 0.018). MMP-8 protein levels were lower in the EBC of COPD patients (p < 0.0001). In PBMCs, enhanced expression of mRNA for MMP-9 and NE is associated with COPD and may correlate with disease severity and exacerbations.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by irreversible and progressive airflow limitation Citation(1, 2). Several theories have been suggested to describe the pathogenesis of COPD, which include chronic inflammation, oxidative stress, proteolytic imbalance and alterations in apoptosis of inflammatory cells.
Several biomarkers of inflammation have been measured in various biological matrices in patients with COPD Citation(3). Matrix metalloproteinase (MMP)-8, MMP-9 and neutrophil elastase (NE) are produced by neutrophils and macrophages. These enzymes are strongly implicated in the inflammatory pathway characterising COPD, with effects not only on proteolysis, but also on perpetuation and regulation of inflammation Citation(4).
MMP-8 and MMP-9 levels are elevated in COPD with increased levels in airway Citation(5), lung tissue Citation(6), bronchoalveolar lavage (BAL) Citation(7, 8) and induced sputum Citation(9). MMP-8 is a collagenase, which can degrade collagens I, II and III, and gelatin Citation(10). MMP-9 (gelatinase B) degrades elastin and is implicated in the lung destruction characteristic of COPD Citation(11). MMP-9 augments the production of cytokines and chemokines, and through the inactivation of protease inhibitors culminates in increased proteolysis, again seen in COPD Citation(12–14). NE is another enzyme associated with COPD, which belongs to the chymotrypsin superfamily of serine proteases Citation(15). In addition to degrading elastase and hydrolysing other proteins, it stimulates mucin production and secretion, which can contribute to airway obstruction in COPD patients Citation(16). NE has also been shown to be elevated in the serum and sputum of COPD patients Citation(17, 18).
The regulation of the pro-inflammatory mediators of COPD is incompletely understood, but microRNAs (miRNAs) are increasingly recognised as modulating the expression of these key agents. miRNAs are small noncoding RNAs, which consist of 20–25 nucleotides that regulate gene expression by either inhibiting mRNA translation or by initiating mRNA degradation Citation(19). MicroRNA-139-5p is a microRNA associated with neoplasia and is predicted to be a regulator of TGF-β1. Expression of miRNA-139-5p is negatively correlated with SMAD3, the main downstream molecule of TGF-β signalling Citation(20), and is also associated with reduced MMP-9 levels Citation(21, 22).
The lung is a difficult organ to sample in human disease, and commonly used techniques are relatively invasive. Exhaled breath condensate (EBC) can be collected by condensing exhaled breath and aerosolised particles from the airway lining fluid and is an inexpensive, non-invasive method to safely sample the airway lining fluid to investigate inflammatory mediators in pulmonary diseases Citation(23–26). Recently, elevated levels of MMP-9 were detected in the EBC of COPD patients Citation(27). Hence, the utility of EBC in measuring MMP-8 and MMP-9 in COPD patients was assessed in this study, given their important role in the pathophysiology of COPD.
This study aimed to measure the expression of MMP-8, MMP-9 and NE mRNA in peripheral blood mononuclear cells (PBMCs) and the corresponding protein in plasma and EBC to establish an association with COPD, and whether these mediators differed during an exacerbation.
Materials and methods
Study design and subjects
This observational, cross-sectional study was approved by the Human Research Ethics Committee of Prince of Wales Hospital (12/119). Informed and written consent was obtained from all participants. Patients with stable COPD as well as patients experiencing an acute exacerbation of COPD (AECOPD) were recruited from clinics and wards, while healthy controls from both the community and hospital were invited to participate. Inclusion criteria were a clinical diagnosis of COPD and/or FEV1/FVC < 70%, but no previous or existing lung malignancy or other significant lung disease. AECOPD was defined as increased breathlessness, wheezing, chest tightness, increased cough and sputum, change of colour or appearance of sputum and fever Citation(28). The control inclusion criteria were a FEV1/FVC ratio of >70%, no significant medical history of lung disease, malignancy or significant lung disease and not a current smoker. Subject details were collected using questionnaire and spirometry was measured using (MINATO auto-spirometer AS-303), according to ATS standards.
EBC was collected using the ECoScreen® condenser (Erich Jaeger GmbH, Hochberg, Germany). Subjects breathed tidally for 10–15 minutes and EBC samples were de-aerated with argon gas to remove CO2 and subsequently stored in Protein LoBind tubes (Eppendorf, Sydney, Australia) at −80°C.
Peripheral blood was collected in acid-citrate-dextrose containing Vacutainer tubes (BD Biosciences, Sydney, Australia). The whole blood was processed on the day of collection and centrifuged at 700× g for 10 minutes. Plasma was then removed and PBMCs were isolated by density gradient centrifugation using Ficoll-Hypaque Lymphoprep (STEMCELL Technologies, Melbourne, Australia). PBMCs were washed with DPBS and subsequently with RPMI-1640 medium supplemented with penicillin-streptomycin and L-glutamine and then resuspended in media containing autologous plasma. Aliquots of cells were cryopreserved following the addition of 20% DMSO(v/v) and stored in vapour-phase nitrogen.
About 1–10 million PBMCs were lysed with TRIzol (Life Technologies, Melbourne, Australia) or TRI Reagent (Sigma-Aldrich, Sydney, Australia) and total RNA was extracted following the manufacturers' protocols. Total RNA concentration and purity was assessed using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Melbourne, Australia).
Analysis of mRNA and miRNA expression
Total PBMC RNA (≥1.0 µg) was used for cDNA synthesis using a Superscript III first-strand synthesis kit (Life Technologies, Melbourne, Australia). Quantitative real-time PCR for MMP-8, MMP-9 and NE was performed using a Sensimix SYBR Hi-ROX kit (Bioline, London, UK) with QuantiTect Primer Assays (Qiagen, Melbourne, Australia) on the Light Cycler 480 (Roche, Mannheim, Germany) under standard conditions with HPRT as the reference control. Quantitative real-time PCR for microRNA-139-5p was performed using TaqMan Universal PCR MasterMix, and TaqMan MicroRNA assays (Life Technologies) according to the manufacturer's instructions with miRNA-16 as the reference control.
MMP-8, MMP-9 and NE in plasma and EBC
MMP-8 was measured in EBC and plasma samples using ELISA kits (Aviscera Bioscience, Melbourne, Australia). A high-sensitivity ELISA (sensitivity of 5 pg/ml; detection range of 15.6–1000 pg/mL) was used to measure MMP-8 in 100 µL of EBC. The intra-assay and inter-assay precision were 4–6% and 8–10%, respectively.
To measure MMP-8 in plasma, a standard ELISA with a sensitivity of 30 pg/ml and a range of 62.5–4000 pg/ml was used. MMP-9 in EBC and plasma samples was measured via a commercially available ELISA with a detection range of 31.2–2000 pg/mL (R&D Systems, Melbourne, Australia). NE was measured in plasma samples via ELISA (range 0.4–25 ng/mL) (Hycult Biotech, Melbourne, Australia).
Statistical analyses
Data analysis was performed using GraphPad Prism 6.0 software (GraphPad, La Jolla, USA), assessed with the D'Agostino & Pearson omnibus normality test and parametric unpaired t-tests or non-parametric Mann-Whitney U-tests used as appropriate. Pearson or Spearman correlation was used to calculate correlation between two data sets as appropriate.
Data are expressed as mean ± SD or median ± (range) and p < 0.05 was considered to be statistically significant.
Results
Subject characteristics
The following subjects were recruited: 23 stable COPD, 7 AECOPD and 23 controls. Not all subjects were able to provide both EBC and blood samples, particularly those who had AECOPD. COPD patients were older, had a significantly lower FEV1 (p < 0.0001), FVC (p = 0.0006) and FEV1/FVC (p < 0.0001) compared to the control group with 90% being either current or ex-smokers compared to 30% who were ex-smokers in the control group ().
Table 1. Clinical characteristics and demographics of COPD patients and healthy controls.
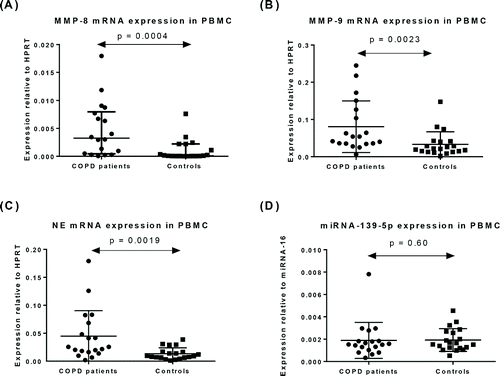
Expression of miR-139-5p and mRNA for MMP-8, MMP-9 and NE in PBMCs
In PBMC from patients with COPD, the level of mRNA was significantly higher for MMP-8 (p = 0.0004; ), MMP-9 (p = 0.0023; ) and NE (p = 0.0019; ) compared to healthy controls. There was no significant difference in PBMC miR-139-5p levels between COPD patients and controls (p = 0.60, )
Figure 1. Expression of mRNA for (A) MMP-8, (B) MMP-9, (C) NE, and (D) miRNA-139-5p in PBMC from patients with COPD (n = 19) and healthy controls (n = 20). Data represented as mean ± SD.
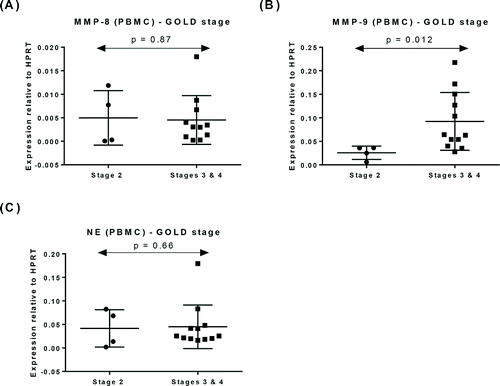
Within COPD patients, the levels of mRNA for NE were significantly higher in patients experiencing an exacerbation (AECOPD) (p = 0.027, ). MMP-8 and MMP-9 mRNA levels were similar in both COPD sub-groups (p = 0.070, , p = 0.64, ), respectively. COPD patients were also analysed according to the GOLD staging system Citation(29). MMP-9 mRNA levels were significantly higher in Stages 3 and 4 patients (p = 0.012, ), however, there was no difference in MMP-8 and NE mRNA levels between the different GOLD stages (p = 0.87, and p = 0.66, ), respectively.
Figure 2. Expression of mRNA for (A) MMP-8, (B) MMP-9 mRNA, and (C) NE in PBMC from patients with stable COPD (n = 12) and patients with exacerbation of COPD (n = 7). Data represented as mean ± SD.
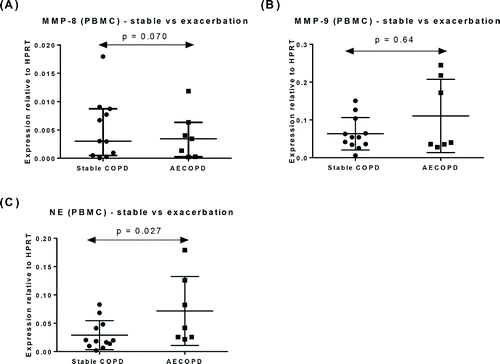
Figure 3. Expression of mRNA for (A) MMP-8, (B) MMP-9, and (C) NE in PBMC from patients with COPD according to the GOLD staging. Data represented as mean ± SD.
MMP-9 mRNA levels were negatively correlated with spirometry results and this correlation was significant for FEV1, FVC and FEV1/FVC (p < 0.05, Supplementary Figure 1). Levels of mRNA for NE were also negatively correlated with FEV1 (p = 0.025, Supplementary Figure 2).
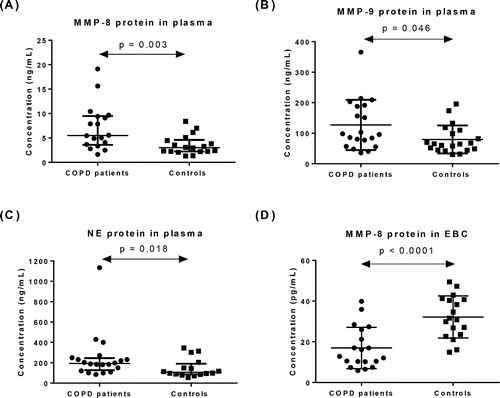
MMP-8, MMP-9 and NE protein in plasma
In plasma from patients with COPD, protein levels were significantly higher for MMP-8 (p = 0.003, ), MMP-9 (p = 0.046, ) and NE (p = 0.018, ) compared to healthy controls. In addition, these markers were not significantly different between stable COPD and AECOPD patients as well as between GOLD stages.
MMP-8 and MMP-9 protein in EBC
MMP-8 protein was detected in all EBC samples. MMP-8 protein levels (pg/mL) were significantly lower in patients (17.0 ± 10.2) compared to controls (32.2 ± 10.3) (p < 0.0001, ). MMP-9 was not detected in any EBC samples (21 controls, 16 COPD subjects).
Discussion
This study has shown elevated levels of PBMC mRNA and plasma protein for MMP-8, MMP-9 and NE in patients with COPD when compared with control subjects.
Given that COPD is a neutrophil-driven disease, it might be expected that PBMC NE expression of mRNA would be significantly higher in COPD patients. In addition, a significant negative correlation between NE and FEV1 in COPD patients was found (Supplementary Figure 2A), which is also seen in BAL fluid in patients with alpha 1-antitrypsin deficiency Citation(30). Furthermore, mRNA for NE in PBMCs was significantly higher in AECOPD compared to stable COPD. One other study has found NE protein to be higher in the sputum during COPD exacerbation, but showed no difference between stable COPD patients and non-smoking controls Citation(31). Serum NE might be useful to identify AECOPD and further studies could investigate this question, although not all AECOPD are related to bacterial infections.
This study also showed that mRNA for MMP-8 in PBMCs as well as MMP-8 protein in plasma was significantly higher in COPD patients. This could suggest that the role of MMP-8 in COPD is more complex than previously thought, perhaps having a systemic pro-inflammatory role.
To date, this study is the first to measure MMP-8 protein levels in EBC. MMP-8 was detectable in all of the EBC samples, but was lower in COPD patients, in contrast to the findings in peripheral blood. Assays on EBC may be measuring a different compartment and reflect a different part of the process in terms of its presence in the airway, as opposed to the inflammatory cells in the peripheral blood. This finding was unexpected as previous studies have shown increased MMP-8 in induced sputum, BAL and lung tissue of COPD patients Citation(6–9). MMP-8-deficient mice display an increased neutrophilic inflammation in the BAL fluid and the peribronchial area, suggesting that MMP-8 has an anti-inflammatory effect and could be responsible for inducing neutrophil apoptosis in the context of allergic asthma Citation(32). Therefore, in COPD, MMP-8 might be down-regulated in the airways of COPD patients leading to decreased apoptosis of neutrophils and hence neutrophil accumulation in the airways, a hallmark of COPD Citation(33). MMP-8 has an anti-inflammatory role during acute experimental lung injury in mice Citation(34) and possibly an imbalance of MMP-8 levels in the EBC of COPD patients may be associated with excessive inflammation. Thus, if EBC is representative of the airway MMP-8 levels, it may have an anti-inflammatory role in the lung but be suppressed in the airway in COPD. It might be thought that although the MMP-8 gene is not known to have a regulatory glucocorticoid response element, the fact that all COPD subjects were on inhaled corticosteroids may mean that there was indirect suppression via the AP-1 site but this was not confirmed in a study of COPD Citation(35).
PBMC MMP-9 mRNA expression was significantly higher in COPD patients compared to non-smoking controls. Furthermore, this MMP-9 mRNA expression was negatively correlated with FEV1, FVC and FEV1/FVC in COPD patients, consistent with increases in serum MMP-9 over time being associated with decreases in FEV1 in COPD patients Citation(36). In addition, this study demonstrated higher PBMC MMP-9 mRNA expression in the latter stages of COPD based on the GOLD staging. The literature has not consistently shown these differences between COPD patients and controls who are current smokers Citation(37, 38). Thus, smoking itself may be a confounding factor for expression of MMP-9 mRNA.
Our study showed a significantly higher PBMC MMP-9 mRNA expression and plasma MMP-9 protein levels in COPD patients. This finding is collaborated with the study of Dickens et al. Citation(39), which showed significantly higher plasma MMP-9 protein levels in COPD patients compared to non-smoking controls. However, Higashimoto et al. Citation(40) found that serum MMP-9 concentration was similar between COPD patients and control subjects. Moreover, Cataldo et al. Citation(41) found no difference in the release of MMP-9 from peripheral blood granulocytes in COPD patients and non-smoking controls. It is thus difficult to interpret the published data as the exact role of MMPs may not be obvious in COPD. Although it has been shown that MMPs can directly degrade the alveolar matrix, they can also serve primarily as signalling molecules Citation(10). Furthermore, the activity of MMPs is tightly regulated from the gene transcription stage to the post-translational stage. In the latter, inactive pro-enzymes can be activated by proteolysis and active enzymes can be inhibited by tissue inhibitors of metalloproteinases (TIMPs) Citation(5). One study showed that despite increased MMP-9 protein, total MMP activity was not higher in sputum from COPD subjects. However, the authors could not find evidence of a reduction in the activation of MMPs or raised levels of MMP inhibitors during stable disease. This finding suggests that MMP activity in COPD may be more complicated than previously thought and multiple factors have to be evaluated when investigating airway inflammation Citation(5).
There were no significant differences in the expression of miR-139-5p in the PBMCs of patients compared to controls. The findings of this study do not support an important role for peripheral blood miR-139-5p in COPD and hence we propose that future studies investigating the role of microRNA in COPD could be more comprehensive.
There are several limitations in this study. The sample size of this study is relatively small. Also, COPD patients were older due to a difficulty in finding healthy age-matched controls. A post hoc analysis of older versus younger control subjects (breakpoint 40 years) showed no significant differences or correlations with age for mRNA or protein expression (data not shown), consistent with other studies of MMP-9 suggesting age is not a major confounder Citation(42). It would be ideal to compare COPD patients with smoking controls but there was also difficulty in recruiting age-matched smoking controls without COPD for this study, and likewise a longitudinal study would be more powerful, showing changes before, during and after exacerbations. It is also acknowledged that not all phenotypic expressions of COPD were analysed, e.g. emphysema versus chronic bronchitis. In addition, this study would have ideally measure NE in EBC but this was not done due to insufficient EBC samples. Furthermore, a more comprehensive study of the COPD pathophysiology requires comparison of EBC MMP-8 with independent biomarkers of respiratory inflammation including EBC biomolecules Citation(43), FENO Citation(44) and electronic nose breathprints Citation(45).
In conclusion, this study is the first to detect MMP-8 protein in the EBC of COPD patients. Expression of mRNA for MMP-9 and NE by PBMCs may be useful for indicating disease severity and exacerbation. Large-scale studies are needed to validate the reproducibility of these findings and the significance of these differences.
Declaration of interest
The authors declare that they have no conflicts of interest.
Supplementary Figure 2
Download TIFF Image (195.1 KB)Supplementary Figure 1
Download TIFF Image (211.3 KB)Supplementary Figures Captions
Download MS Word (11.8 KB)Acknowledgments
The authors acknowledge the kind assistance of the staff at the Department of Respiratory Medicine, Prince of Wales Hospital, for their assistance with recruitment, and to the participants for donating their time and samples.
References
- Vestbo J. COPD: definition and phenotypes. Clin Chest Med 2014; 35(1):1–6.
- Noujeim C, Bou-Khalil P. COPD updates: what's new in pathophysiology and management? Expert Rev Respir Med 2013; 7(4):429–437.
- Malerba M, Montuschi P. Non-invasive biomarkers of lung inflammation in smoking subjects. Curr Med Chem 2012; 19(2):187–196.
- Sng JHJ, Thomas PS. COPD: immunopathogenesis and immunological markers. Adv Res 2014; 3(2):221–235.
- Lowrey GE, Henderson N, Blakey JD, Corne JM, Johnson SR. MMP-9 protein level does not reflect overall MMP activity in the airways of patients with COPD. Respir Med 2008; 102(6):845–851.
- Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. CHEST J 2000; 117(3):684–694.
- Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 1999; 159(6):1985–1991.
- Finlay GA, Russell KJ, McMahon KJ, D'arcy EM, Masterson JB, FitzGerald MX, et al. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax 1997; 52(6):502–506.
- Vernooy JH, Lindeman JH, Jacobs JA, Hanemaaijer R, Wouters EF. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. CHEST J 2004; 126(6):1802–1810.
- Churg A, Zhou S, Wright JL. Matrix metalloproteinases in COPD. Eur Respir J 2012; 39(1):197–209.
- Russell RE, Culpitt SV, DeMatos C, Donnelly L, Smith M, Wiggins J, et al. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2002; 26(5):602–609.
- Aldonyte R, Eriksson S, Piitulainen E, Wallmark A, Janciauskiene S. Analysis of systemic biomarkers in COPD patients. COPD: J Chron Obstruct Pulmon Dis 2004; 1(2):155–164.
- Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol 2001; 69(6):851–859.
- Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, et al. The serpin α1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 2000; 102(5):647–655.
- Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol 2002; 451(1):1–10.
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010; 363(23):2233–2247.
- Pinto-Plata V, Toso J, Lee K, Parks D, Bilello J, Mullerova H, et al. Profiling serum biomarkers in patients with COPD: associations with clinical parameters. Thorax 2007; 62(7):595–601.
- Paone G, Conti V, Vestri A, Leone A, Puglisi G, Benassi F, et al. Analysis of sputum markers in the evaluation of lung inflammation and functional impairment in symptomatic smokers and COPD patients. Dis Markers 2011; 31(2):91–100.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136(2):215–233.
- Butz H, Likó I, Czirják S, Igaz P, Korbonits M, Rácz K, et al. MicroRNA profile indicates downregulation of the TGFβ pathway in sporadic non-functioning pituitary adenomas. Pituitary 2011; 14(2):112–124.
- Liu R, Yang M, Meng Y, Liao J, Sheng J, Pu Y, et al. Tumor-suppressive function of miR-139-5p in esophageal squamous cell carcinoma. PloS one 2013; 8(10):e77068.
- Zhang L, Dong Y, Zhu N, Tsoi H, Zhao Z, Wu CW, et al. microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol Cancer 2014; 13(1):124.
- Bajaj P, Ishmael FT. Exhaled breath condensates as a source for biomarkers for characterization of inflammatory lung diseases. J Anal Sci Methods Instrum 2013; 3(1):17–29.
- Mutlu GM, Garey KW, Robbins RA, Danziger LH, Rubinstein I. Collection and analysis of exhaled breath condensate in humans. Am J Respir Crit Care Med 2001; 164(5):731–737.
- Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol 2002; 110(1):28–34.
- Rosias P. Methodological aspects of exhaled breath condensate collection and analysis. J Breath Res 2012; 6(2):027102.
- Kwiatkowska S, Noweta K, Zieba M, Nowak D, Bialasiewicz P. Enhanced exhalation of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with COPD exacerbation: a prospective study. Respiration 2012; 84(3):231–241.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1256–1276.
- GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2014. Available from: http://www.goldcopd.com
- Rouhani F, Paone G, Smith N, Krein P, Barnes P, Brantly ML. Lung neutrophil burden correlates with increased pro-inflammatory cytokines and decreased lung function in individuals with α1-antitrypsin deficiency. Chest J 2000; 117(5_suppl_1):250S–251S.
- Fujimoto K, Yasuo M, Urushibata K, Hanaoka M, Koizumi T, Kubo K. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J 2005; 25(4):640–646.
- Gueders MM, Balbin M, Rocks N, Foidart J-M, Gosset P, Louis R, et al. Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation. J Immunol 2005; 175(4):2589–2597.
- Rytilä P, Plataki M, Bucchieri F, Uddin M, Nong G, Kinnula VL, et al. Airway neutrophilia in COPD is not associated with increased neutrophil survival. Eur Respir J 2006; 28(6):1163–1169.
- Owen CA, Hu Z, Lopez-Otin C, Shapiro SD. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol 2004; 172(12):7791–7803.
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res 2002; 4(3):157–164.
- Higashimoto Y, Iwata T, Okada M, Satoh H, Fukuda K, Tohda Y. Serum biomarkers as predictors of lung function decline in chronic obstructive pulmonary disease. Respir Med 2009; 103(8):1231–1238.
- Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, et al. The Role of Matrix Metalloproteinase-9 in Cigarette Smoke–induced Emphysema. Am J Respir Crit Care Med 2011; 183(7):876–884.
- Ilumets H, Rytilä P, Demedts I, Brusselle GG, Sovijärvi A, Myllärniemi M, et al. Matrix metalloproteinases-8,-9 and-12 in smokers and patients with stage 0 COPD. Int J Chron Obstruct Pulmon Dis 2007; 2(3):369.
- Dickens JA, Miller BE, Edwards LD, Silverman EK, Lomas DA, Tal-Singer R. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res 2011; 12(1):146.
- Higashimoto Y, Yamagata Y, Iwata T, Okada M, Ishiguchi T, Sato H, et al. Increased serum concentrations of tissue inhibitor of metalloproteinase-1 in COPD patients. Eur Respir J 2005; 25(5):885–890.
- Cataldo D, Munaut C, Noël A, Frankenne F, Bartsch P, Foidart J-M, et al. Matrix metalloproteinases and TIMP‐1 production by peripheral blood granulocytes from COPD patients and asthmatics. Allergy 2001; 56(2):145–151.
- Tayebjee MH, Lip GY, Blann AD, MacFadyen RJ. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and-9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and-2. Thromb Res 2005; 115(3):205–210.
- Santini G, Mores N, Shohreh R, Valente S, Dabrowska M, Trové A, et al. Exhaled and non-exhaled non-invasive markers for assessment of respiratory inflammation in patients with stable COPD and healthy smokers. J Breath Res 2016; 10(1):017102.
- Malerba M, Radaeli A, Olivini A, Damiani G, Ragnoli B, Montuschi P, et al. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed Res Int 2014; 2014:1–7.
- Bofan M, Mores N, Baron M, Dabrowska M, Valente S, Schmid M, et al. Within-day and between-day repeatability of measurements with an electronic nose in patients with COPD. J Breath Res 2013; 7(1):017103.