Abstract
In individuals with chronic obstructive pulmonary disease (COPD), the presence of comorbidities is associated with increased mortality risk. We wanted to study the association between bone mineral density (BMD) and mortality among individuals with COPD in a population-based cohort study. Participants were recruited from the second (1995–1997) and third (2006–2008) surveys of the HUNT Study and followed until February 2019. Hip and forearm BMD were included as continuous T-scores or categorized according to WHO criteria (normal, osteopenia, and osteoporosis). Hazard ratios with 95% confidence intervals were estimated by multivariable Cox regression models. In total, 2076 and 3239 participants were identified as having COPD by FEV1/FVC below lower limit of normal (LLN) or <0.70, respectively, according to Global Lung Initiative (GLI) and Global Initiative for Chronic Obstructive Lung Disease (GOLD). The prevalence of osteoporosis was 15.7% vs. 16.6% in the GLI-COPD vs. GOLD-COPD cohorts. Mean follow-up was 12.7 and 11.9 years. Lower T-scores were associated with a 5% (95% confidence interval (CI) 1.01–1.09) increased mortality in the GLI-COPD and GOLD-COPD cohorts, respectively. However, the presence of osteoporosis (T < –2.5), compared to normal BMD, was not associated with mortality in neither GLI-COPD (HR 1.13, 95% CI 0.91–1.41) nor GOLD-COPD cohorts (HR 1.22, 95% CI 0.99–1.51). Thus, a small positive association was found between decreasing BMD T-score and mortality in both GLI-COPD and GOLD-COPD. However, osteoporosis as defined by WHO was not associated with mortality, probably due to loss of power upon categorization.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction and contributes to morbidity and mortality for individuals, and high costs for the society [Citation1]. Increasing life span, lifestyle and environmental factors have contributed to increasing incidence and prevalence of COPD worldwide, with an estimated global prevalence of 11.7% in adults >30 years in 2010 [Citation2] and a prevalence of 14.8% in Norwegians >40 years in 2006–2008 [Citation3].
Comorbidities contribute to symptom burden and prognosis in persons with COPD [Citation4]. A common comorbidity is osteoporosis [Citation5], which is characterized by reduced bone strength and increased fracture risk. Due to methodological differences, the worldwide prevalence of osteoporosis is difficult to determine, but the overall prevalence in the United States among adults 50 years and older was 10.3% in 2010 [Citation6]. Individuals with COPD have a 1.5–2-fold increased risk of osteoporosis [Citation7], and a recent meta-analysis found a pooled global prevalence of osteoporosis in COPD to be 38% (95% CI, 34–43%) [Citation8]. Shared risk factors and an interplay between compounding elements of the two diseases may partly explain the high prevalence [Citation9]. Osteoporosis is an important risk factor for fractures, but the fracture risk in osteoporosis is increased beyond measured bone mineral density (BMD) due to reduced bone quality and altered bone metabolism [Citation7]. Vertebral fractures add a restrictive component to COPD [Citation10], and hip fractures increase the risk for subsequent fractures and mortality [Citation7]. In women, but not in men, low BMD was found to account for a large proportion of post fracture mortality risk [Citation11]. Few studies have addressed potential associations between BMD and all-cause mortality in COPD, but an association between BMD and COPD-specific mortality has been reported by Campos-Obando et al. and Looker [Citation12, Citation13]. Thus, we aimed to examine the association of BMD and osteoporosis with all-cause mortality in individuals with COPD recruited in a population-based study.
Materials and methods
Design
This cohort study includes participants with COPD from the second (HUNT2, 1995–1997) and third survey (HUNT3, 2006–2008) of the population-based HUNT Study in Norway. Comprehensive data on lifestyle and diseases were collected through questionnaires, interviews and clinical measurement [Citation14].
Study population
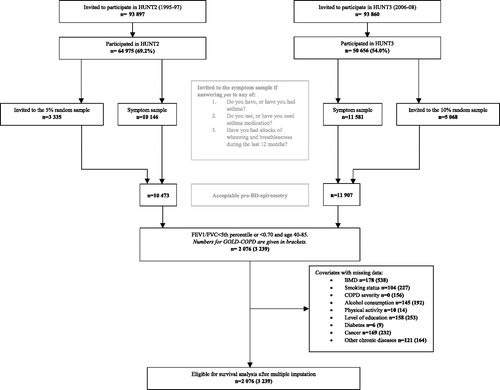
Among 78,962 individuals participating in HUNT2 and/or HUNT3 (69.2% and 54.0% of those invited, respectively), random samples of all participants (5% in HUNT2 and 10% in HUNT3), in addition to participants reporting attacks of wheezing or breathlessness during the last 12 months, use of “asthma” medication or ever having had asthma, COPD, emphysema or chronic bronchitis (symptom sample), were invited to the HUNT Lung Study () [Citation14]. In total, 7282 and 8812 individuals aged 40–85 years had spirometry measured in HUNT2 and HUNT3, respectively. A subset of 1387 participants also performed post-bronchodilator (post-BD) spirometry in HUNT2 20–30 min after inhalation of 1 mg terbutaline. Among those reporting respiratory symptoms 2076 participants had forced expiratory volume in one second divided by forced vital capacity (FEV1/FVC) < fifth percentile (z < –1.645) estimated by the Global Lung Function Initiative (GLI) software (GLI-COPD) [Citation15], and 3239 participants had FEV1/FVC < 0.70 (GOLD-COPD) [Citation4]. Spirometry was performed according to the 1994 ATS recommendations in HUNT2 [Citation16] and the 2005 ATS-ERS recommendations in HUNT3 [Citation17], and quality control of measurements were performed [Citation18, Citation19]. We defined baseline as the earliest participation date in either HUNT2 or HUNT3. Data on covariates were assessed at the time of participation. For those participating in both HUNT2 and HUNT3, only data from HUNT2 were used.
Osteoporosis
In HUNT3 bone densitometry of the total hip was performed by dual-energy X-ray absorptiometry (DXA) (Lunar Prodigy GE Healthcare, Little Chalfont, UK). BMD in g/cm2 was also measured at the distal part of the non-dominant forearm by DTX100 or DTX200 (Osteometer MediTech, Inc., Hawthorne, CA) in both HUNT2 and HUNT3. The distal forearm region was defined as the area 24 mm proximal to the site of 8 mm distance between distal radius and ulna. Methods of calibration and quality control are described elsewhere [Citation20].
In both sexes, BMD was standardized as T-scores, calculated as observed minus mean BMD divided by standard deviation (SD) from a healthy female reference population aged 20–39 years from the HUNT Study [Citation21]. BMD T-score was included in analyses as a continuous variable, but was also categorized according to the WHO criteria into T-score ≥ –1.0 (normal), –1.0 to –2.5 (osteopenia) and ≤ –2.5 (osteoporosis) [Citation21]. In this study we primarily used total hip DXA measurements, but without a measurement at this site, forearm BMD was used as suggested by The International Society for Clinical Densitometry (ISCD) [Citation22].
All-cause mortality
Data on registry status including mortality and migration, was provided by the National Registry. We defined time at risk as from the date of entry into the study until date of death or end of observation (February 1, 2019), whichever came first. There was no loss to follow-up.
Confounders
Based on prior knowledge, potential confounders were identified using directed acyclic graphs (DAGs). This included age at participation, sex, COPD severity, BMI, tobacco smoking, alcohol consumption, physical activity, socioeconomic status and comorbidities including ever cancer, chronic musculoskeletal diseases, chronic diseases affecting activities of daily living (ADL), diabetes mellitus and cardiovascular disorders. Data on these covariates were collected at baseline, either by questionnaires or by clinical measurements. Severity of COPD by GLI criteria was graded using FEV1 in percent of predicted (ppFEV1) calculated by the GLI-2012 software, using cutoff values by z-scores of > −2 (mild), <−2.5 (moderate), <−3 (severe) and < −4 (very severe) [Citation15, Citation23]. Severity of COPD by GOLD criteria was graded using; ppFEV1≥80 (GOLD1/mild), 80 > ppFEV1 ≥ 50 (GOLD2/moderate), 50>ppFEV1≥30 (GOLD3/severe) or 30 > ppFEV1 (GOLD4/very severe) [Citation17]. BMI was calculated in kg/m2 and categorized according to the WHO classification: <18.5 (underweight), 18.5–24.9 (normal range), 25.0–29.9 (overweight) and ≥30.0 (obese) [Citation24]. Tobacco smoking was categorized as: never-, former- or current smoker. The latter two categories were subdivided into <10, 10–20 and >20 smoking pack-years. Alcohol consumption was categorized by weekly consumption: low (<14 alcohol units (U.A.) for men and <10 U.A. for women), intermediate (14–21 U.A. for men and 10–14 U.A. for women) and high intake (>21 U.A. for men and >14 U.A. for women). One U.A. was defined as 13 g of alcohol. Physical activity was categorized according to hours of self-reported physical activity per week: inactive (≤1 h light physical activity and no hard activity), low (>1 h light and <1 h hard), medium (1–2 h of hard regardless of light activity) and high (≥3 h of hard regardless of light activity). Level of education was used as a proxy measure for socioeconomic status and was categorized into low (≤9 years), medium (10–12 years) and high (≥13 years) based on the question: “What is your highest level of education?” from HUNT2. Level of education in HUNT3 was assessed using the occupational classification in HUNT according to the Erikson Goldthorpe Portocarero (EGP) social class scheme, based on the question “What kind of paid work do you do?” [Citation25]. Self-reported cardiovascular disorders included previous myocardial infarction, angina pectoris and ischemic-/hemorrhagic brain infarction. We identified participants with hypertension as those answering yes to prior or current use of antihypertensive medication or having hypertension (defined as ≥140 and/or ≥90 mmHg) upon blood pressure measurements in HUNT2 or HUNT3. Chronic musculoskeletal diseases included rheumatoid arthritis, spondyloarthritis, osteoarthritis and “other long-term skeletal or muscular diseases”. Chronic diseases affecting ADL was assessed by the following question: “Do you suffer from any long-term illness or injury of a physical or psychological nature that impairs your functioning in your everyday life?”. This variable was included to assess the impact of unmeasured comorbidities that were not included separately in the model. All variables on comorbidities were dichotomized to yes/no and included as separate variables in the final models. The final models were: (1) Baseline model with BMD categorized according to T-score adjusted for age and sex; (2) Main model with BMD categorized according to T-score adjusted for all confounders at baseline and (3) Models in (1) and (2) using BMD as a continuous measure.
Statistical analysis
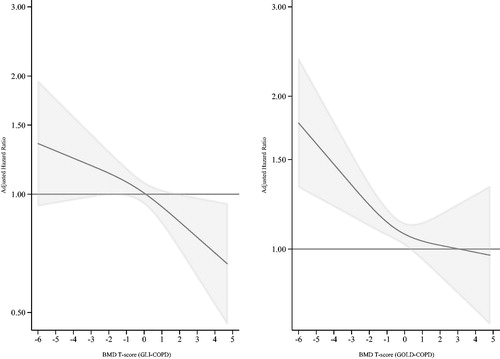
We presented baseline characteristics for categorical variables as proportions and for continuous variables as means with standard deviation using non-imputed data. All models were run separately for the GLI and GOLD cohorts. Proportional hazard assumptions were evaluated by estimation of Schoenfeld residuals and proportional hazards assumption tests. Survival functions were graphed using restricted cubic splines with three knots, showing change in hazard ratio (HR) as a function of BMD T-score (). Number of knots were determined using Akaike’s- and Bayesian information criterion. Statistical interactions between main exposure and confounders were tested, but no significant interactions were identified. Missing data on covariates were completed using multiple imputation with chained equations, M = 20. HRs and 95% confidence intervals (CI) were calculated comparing all-cause mortality in participants with osteopenia and osteoporosis to those with normal BMD. When including BMD T-score as a continuous variable, linearity assumptions of BMD were assessed plotting a Lowess smooth of the Martingale residuals. Furthermore, the fully adjusted models with and without cubic spline terms were compared by Wald- and likelihood ratio tests. None of the tests showed deviation from linearity. In additional sensitivity analyses, cohorts were defined by post-BD spirometry GLI and GOLD cutoff values. Time of follow-up was used as time-scale, defining the starting point as the participation date in either HUNT2 or 3. To assess a potential cohort effect, participation date (HUNT2 or 3) was added in the models. Statistical analyses were performed using Stata 15.1 (StataCorp LCC, College Station, TX, USA).
Ethics
This study was approved by the Regional Committee for Medical and Health Research Ethics (REC) Central Norway (project no. 2014/1114-1). Participants in the HUNT Study have given informed, written consent for research on their data and linkage to specific registries.
Results
Descriptive characteristics
In GLI-COPD 66% of the participants were recruited from HUNT2, and 34% from HUNT3. The corresponding numbers for GOLD-COPD were 54% and 46%, respectively. No cohort effect was found when adjusting for survey recruitment.
GLI LLN criteria identified 2076 participants with COPD with a mean age of 62.1 years, contributing to 26,370 person-years of follow-up (median 12.7, maximum 23.4 years). The severity distribution was as follows: 40.4% had mild, 34.8% moderate, 19.8% severe and 5.1% very severe airway obstruction. In total, 64.2% had a normal BMD T-score, 20.1% had osteopenia and 15.7% had osteoporosis. A higher proportion of females had osteopenia or osteoporosis compared to males. Overall, 24.9% had no comorbidities, 45.6% had one comorbidity, and 29.5% had two or more comorbidities. The distribution of confounders and comorbidities resembled that of GOLD-COPD across T-score categories (). Totally, 3239 participants with a mean age of 64.4 years were included in GOLD-COPD, contributing to 38,406 person-years of follow-up (median 11.9, maximum 23.4 years). There were a slightly higher proportion of men in the sample (56.3%), while there were more women with osteopenia and osteoporosis. The distribution of severity was 30.6% in GOLD1, 52.8% in GOLD2, 14.3% in GOLD3 and 2.4% in GOLD4. With GOLD-COPD definitions there was a slight shift toward a higher prevalence of more severe airway obstruction compared to GLI-COPD. In GOLD-COPD 61.1% had a normal BMD T-score, 22.4% had osteopenia and 16.6% had osteoporosis. Overall, 21.1% had no comorbidities, 44.0% had one comorbidity, and 34.9% had two or more comorbidities.
Table 2. Mortality rates and adjusted HRs with 95% CIs for the association between bone mineral density and all-cause mortality within the COPD cohort of the HUNT Lung Study.
There were more never-smokers among those with osteoporosis compared to those with T-score > –2.5, but a larger proportion of participants with osteoporosis had severe or very severe airway obstruction. Furthermore, participants with osteoporosis had lower BMI, were less physically active and had shorter education compared to the other groups (). There were 282 participants (92.2%) defined as having osteoporosis by forearm BMD in the GLI cohort, and 416 (93.7%) in the GOLD cohort.
Osteoporosis in association with all-cause mortality
During follow-up 1164 (56%) and 1610 (52%) participants in GLI-COPD and GOLD-COPD died, including 258 (12.4%) and 393 (12.1%) among those with osteoporosis. Analyses using BMD T-score as a continuous exposure showed 5% increased mortality per one unit decrease in BMD T-score (HR 1.05, 95% CI 1.01–1.09) in GLI-COPD, and 4% in the GOLD-COPD (HR 1.04, 95% CI 1.00–1.08) (). Adjusted survival graphs illustrate the increasing HRs at decreasing levels of BMD T-score (). Sensitivity analyses using HUNT2 post-BD spirometry values showed similar results as the main analysis (Supplementary File).
In the cohort based on the GLI-COPD definitions for caseness and severity of COPD, the baseline model gave a HR of 1.24 (95% CI 1.04–1.48) for osteopenia, and a HR of 1.23 (95% CI 1.01–1.50) for osteoporosis compared to normal BMD. For the adjusted model, HRs were 1.12 (95% CI 0.92–1.35) for osteopenia and 1.13 (95% CI 0.91–1.41) for osteoporosis (). In both GLI-COPD and GOLD-COPD, smoking, COPD severity and age seemed to be the strongest confounders.
In the corresponding analysis using the GOLD-COPD criteria for caseness and severity of COPD we found a HR of 1.08 (95% CI 0.94–1.24) for participants with osteopenia, and a HR of 1.33 (95% CI 1.15–1.53) for participants with osteoporosis compared to those with normal BMD in the baseline model (). For the adjusted model, HRs were 1.12 (95% CI 0.93–1.34) for osteopenia and 1.22 (95% CI 0.99–1.51) for osteoporosis ().
There was no statistical interaction found between the main exposure, sex and mortality, hence combined models adjusted for sex were chosen. Results from baseline and adjusted models stratified by sex were calculated and showed the same direction and similar magnitude of effect as the combined analyses (data not shown).
Discussion
Key results
This prospective cohort study showed a small but significant positive association between decreasing BMD T-score and mortality in both GLI-COPD and GOLD-COPD, with a clear dose–response relationship between each unit decrease in T-score and mortality. The association remained after adjusting for known confounders, including other chronic diseases that might affect mortality. However, osteoporosis, defined by a T-score below -2.5, was not associated with mortality.
Categorizing T-score according to pre-defined cutoffs, although clinically meaningful, may have led to loss of information, obscuring the real association. The risk gradient of fractures increases continuously as BMD decreases, and in the femoral neck the risk increase is 2.6 times per SD decrease in total hip BMD [Citation26]. Furthermore, categorization could lead to loss of power, leading to wider confidence intervals [Citation26]. Minimizing within-category risk variation by using BMD T-score on a continuous scale could reduce this power loss. To overcome a potential power problem, we did spline analysis of the fully adjusted models, which largely confirmed this assumption as it showed an increase in HR with decreasing BMD (). There were minor differences in mortality between the GOLD-COPD and GLI-COPD cohorts. As these are different models with a potential healthier reference sample in the GOLD cohort, the estimates cannot be directly compared.
Comparison with other studies
Among previous studies on mortality in individuals with COPD and osteoporosis [Citation12, Citation13, Citation27–32], only two studies included BMD measurements in the analyses [Citation12, Citation13]. The remaining studies examined the association between other measures of bone strength and mortality, and included participants with moderate-to-severe airway limitation [Citation27, Citation28, Citation31], several of them on patients hospitalized for either fractures or acute COPD exacerbations [Citation27, Citation29, Citation30]. Assessment of bone quality was done with a range of methods, spanning from CT-measured bone attenuation to information obtained retrospectively from health records.
Campos-Obando et al. included 5779 and 2055 subjects from two study cycles of the Rotterdam study [Citation26]. They found a strong association between BMD and chronic lung disease related mortality, with 170–200% increased mortality in men, and around 70% increased mortality in women [Citation12]. In a similar study from NHANESIII including 3275 participants, Looker reported a 38% increased risk of COPD-specific mortality in participants with osteoporosis [Citation13]. All-cause mortality was not assessed in either of these studies, and both were done on general populations not restricted to persons with COPD, making them less comparable to our study.
Two studies reported twice the mortality in participants with COPD and vertebral compression fractures (VCF) compared to those without such fractures [Citation27, Citation28]. The higher mortality found in these studies compared to the current study likely reflects the inclusion of milder COPD in the present population-based study, with a mean ppFEV1 of 70% (GOLD) compared to 33.5% [Citation27] and 41% [Citation28] in the previous studies. The association between VCF and mortality supports the theory of the negative biomechanical effects of VCF on the thoracic cavity and a resulting restriction of lung movement. De Luise et al. found that in a cohort of 11,985 patients having sustained a hip fracture, having COPD increased the risk of mortality by 70% [Citation29].
In a study comparing 1634 COPD subjects from the ECLIPSE cohort with smoking- and nonsmoking controls, Romme et al. did not find an association between decreased bone attenuation of thoracic vertebrae and all-cause mortality [Citation31]. From the same cohort Miller et al. studied the association between comorbidities and clinical outcome measures in 2164 COPD patients compared to smoking- and nonsmoking controls. They found that a cumulative number of comorbidities, including osteoporosis, increased mortality in individuals with COPD, but did not find osteoporosis itself to contribute to higher mortality [Citation32]. In contrast to the present study, these studies did not include participants with GOLD grade I [Citation32] or excluded participants with inflammatory disease, a recent cancer diagnosis or long-term corticosteroid use [Citation31].
Strengths and limitations
The HUNT Study is a large study with a relatively high participation rate and long follow-up time. The percentage of participation in HUNT2 and HUNT3 for the age group 40–85 years was higher than for other age groups, constituting 78% and 64% [Citation14]. A nonparticipation study after HUNT3 found higher prevalence of several chronic diseases among non-responders, including COPD [Citation33]. For the biological association between COPD and osteoporosis, however, attendance should not affect the estimates.
The distribution among GOLD grades found in this study corresponds well to prevalence reported by other studies [Citation3], indicating that the study sample is representative to a general COPD population. GOLD guidelines recommend the use of post-BD spirometry for the diagnosis of COPD [Citation4]. As we had post-BD spirometry data from only half of the participants in the HUNT2 Lung Study, we included pre-BD spirometry measurements to obtain higher statistical power for the analyses. Use of pre-BD spirometry has been reported to give an overestimation of around 26% in COPD cases compared to use of post-BD results [Citation34]. However, individuals with more severe disease are still identified by pre-BD spirometry, and only persons with mild airway obstruction would potentially be misclassified. Using the presence of respiratory symptoms in addition to spirometry for diagnosis, as we did in the current study, would reduce the number of misclassified participants. Furthermore, sensitivity analyses using post-BD spirometry gave HRs similar to the estimates in the main analysis (Supplementary table), and a clear dose–response relationship was found when using BMD as a continuous measure, even when adjusting for known confounders, indicating that for each SD decrease in BMD, a statistically significant increase in mortality was seen ().
Several studies have shown that the use of fixed-ratio criteria recommended by GOLD for diagnosis of COPD underestimates the prevalence in younger age groups, and overestimates the prevalence in the older age groups [Citation35]. ATS and ERS have therefore recommended that LLN criteria should be used in the diagnosis of COPD [Citation36]. In this study COPD cohorts have been defined according to both diagnostic criteria to increase knowledge of influence by comorbidities for COPD defined by different criteria.
Scandinavian countries have a high prevalence of osteoporosis and osteoporotic fractures [Citation37]. The reason for this is debated but could rely on genetic factors, in addition to lack of sun exposure during the autumn and winter season. This could decrease the external validity of our findings toward other ethnic groups. The WHO international reference standard for osteoporosis is based on total hip T-scores. In this study forearm BMD was used in participants without hip densitometry data. Some studies show that the use of forearm densitometry tends to overestimate the prevalence of osteoporosis [Citation38]. To adjust for this, site of measurement was included in the model, and this did not change the estimates (data not shown).
Due to practical and geographical purposes, participants selected for spirometry in small municipalities had to return to do the measurements some weeks later, resulting in a loss of around 20%. This might lead to selection bias but with small magnitude, as this is a pre-selected group largely constituted by individuals already reporting chronic airway symptoms or a known diagnosis of obstructive lung disease. A relatively high level of nonparticipation in the bone densitometry sub-study was due to lack of personnel resources at the field stations rather than factors related to participants.
Conclusion
Individuals with COPD are at increased risk of osteoporosis due to shared risk factors and disease compounding. Both conditions benefit from secondary preventive strategies such as smoking cessation and increased physical activity, and medical treatment exist for established disease.
We found a small positive association between decreasing BMD T-score and mortality in both GLI-COPD and GOLD-COPD. However, osteoporosis as defined by WHO was not associated with mortality, probably due to loss of power upon categorization. Future studies including patient-centered outcomes could add further value to these findings.
Declaration of interests
The HUNT Lung Study was partly funded by a non-demanding grant provided by AstraZeneca Norway. SAV reports personal fees from Boehringer Ingelheim Norway KS outside the submitted work. LV reports grants, personal fees and non-financial support from Pulmonx, personal fees and non-financial support from Menarini, grants and personal fees from AstraZeneca, personal fees from Chiesi, personal fees from GSK, personal fees from Novartis, personal fees from Boehringer, grants and non-financial support from Fisher&Paykel outside the submitted work. The remaining authors report no conflicts of interest.
Table 1. Descriptive characteristics of the HUNT COPD cohort 40–85 years defined by GLITable Footnotea and GOLDb.
Supplemental Material
Download PDF (288.2 KB)Acknowledgments
The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Center [Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU)], Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
This work was previously presented at the 2018 European Respiratory Society (ERS) International Congress, and a preliminary version of the abstract was published in the ERS Abstract Book [Citation39].
Additional information
Funding
References
- Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. doi:10.1183/09031936.06.00024505.
- Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05-020415.
- Bhatta L, Leivseth L, Mai XM, et al. Prevalence and trend of COPD from 1995–1997 to 2006–2008: The HUNT study, Norway. Respir Med. 2018;138:50–56. doi:10.1016/j.rmed.2018.03.020.
- GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2019 report). 2018. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf
- Sarkar M, Bhardwaj R, Madabhavi I, et al. Osteoporosis in chronic obstructive pulmonary disease. Clin Med Insights Circ Respir Pulm Med. 2015;9:5–21. doi:10.4137/CCRPM.S22803.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi:10.1002/jbmr.2269.
- Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: Links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis. 2016;11:637–648. doi:10.2147/copd.s79638.
- Chen YW, Ramsook AH, Coxson HO, et al. Prevalence and risk factors for osteoporosis in individuals with COPD: A systematic review and meta-analysis. Chest. 2019. doi:10.1016/j.chest.2019.06.036.
- Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. doi:10.1164/rccm.201110-1792OC.
- Harrison RA, Siminoski K, Vethanayagam D, et al. Osteoporosis-related kyphosis and impairments in pulmonary function: A systematic review. J Bone Miner Res. 2007;22(3):447–457. doi:10.1359/jbmr.061202.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009;301(5):513–521. doi:10.1001/jama.2009.50.
- Campos-Obando N, Castano-Betancourt MC, Oei L, et al. Bone mineral density and chronic lung disease mortality: the rotterdam study. J Clin Endocrinol Metab. 2014;99(5):1834–1842. doi:10.1210/jc.2013-3819.
- Looker AC. Relationship between femur neck bone mineral density and prevalent chronic obstructive pulmonary disease (COPD) or COPD mortality in older non-Hispanic white adults from NHANES III. Osteoporos Int. 2014;25(3):1043–1052. doi:10.1007/s00198-013-2601-5.
- Krokstad S, Langhammer A, Hveem K, et al. Cohort profile: The HUNT Study, Norway. Int J Epidemiol. 2013;42(4):968–977. doi:10.1093/ije/dys095.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95 year age range: The global lung function 2012 equations: Report of the Global Lung Function Initiative (GLI), ERS Task Force to establish improved Lung Function Reference Values. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312.
- Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi:10.1164/ajrccm.152.3.7663792.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Hankinson JL, Eschenbacher B, Townsend M, et al. Use of forced vital capacity and forced expiratory volume in 1 second quality criteria for determining a valid test. Eur Respir J. 2015;45(5):1283–1292. doi:10.1183/09031936.00116814.
- Langhammer A, Johannessen A, Holmen TL, et al. Global Lung Function Initiative 2012 reference equations for spirometry in the Norwegian population. Eur Respir J. 2016;48(6):1602–1611. doi:10.1183/13993003.00443-2016.
- Langhammer A, Forsmo S, Lilleeng S, et al. Effect of inhaled corticosteroids on forearm bone mineral density: The HUNT study, Norway. Respir Med. 2007;101(8):1744–1752. doi:10.1016/j.rmed.2007.02.018.
- WHO. WHO Scientific Group on the assessment of osteoporosis at primary health care level 2004 [cited 2017 Nov 21]. Available from: http://www.who.int/chp/topics/Osteoporosis.pdf
- ISCD. Official Positions 2015 ISCD Adults 2015 [updated June 2015; cited 2017 Nov 21]. Available from: https://iscd.app.box.com/v/OP-ISCD-2015-Adult
- Quanjer PH, Pretto JJ, Brazzale DJ, et al. Grading the severity of airways obstruction: New wine in new bottles. Eur Respir J. 2014;43(2):505–512. doi:10.1183/09031936.00086313.
- Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253.
- Krokstad S, Westin S. Health inequalities by socioeconomic status among men in the Nord-Trondelag Health Study, Norway. Scand J Public Health. 2002;30(2):113–124. doi:10.1080/14034940210133753.
- Lorentzon M, Cummings SR. Osteoporosis: The evolution of a diagnosis. J Intern Med. 2015;277(6):650–661. doi:10.1111/joim.12369.
- Pascual-Guardia S, Badenes-Bonet D, Martin-Ontiyuelo C, et al. Hospital admissions and mortality in patients with COPD exacerbations and vertebral body compression fractures. COPD. 2017;12:1837–1845. doi:10.2147/COPD.S129213.
- Kim GW, Joo HJ, Park TS, et al. Vertebral compression fractures may increase mortality in male patients with chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2015;19(5):603–609. doi:10.5588/ijtld.14.0754.
- de Luise C, Brimacombe M, Pedersen L, et al. Chronic obstructive pulmonary disease and mortality following hip fracture: A population-based cohort study. Eur J Epidemiol. 2008;23(2):115–122. doi:10.1007/s10654-007-9211-5.
- Yamauchi Y, Yasunaga H, Sakamoto Y, et al. Mortality associated with bone fractures in COPD patients. COPD. 2016;11:2335–2340. doi:10.2147/COPD.S112142.
- Romme EA, Murchison JT, Edwards LD, et al. CT-measured bone attenuation in patients with chronic obstructive pulmonary disease: Relation to clinical features and outcomes. J Bone Miner Res. 2013;28(6):1369–1377. doi:10.1002/jbmr.1873.
- Miller J, Edwards LD, Agusti A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi:10.1016/j.rmed.2013.05.001.
- Langhammer A, Krokstad S, Romundstad P, et al. The HUNT study: Participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol. 2012;12(1):143. doi:10.1186/1471-2288-12-143.
- Sawalha S, Hedman L, Ronmark E, et al. Pre- and post-bronchodilator airway obstruction are associated with similar clinical characteristics but different prognosis – Report from a population-based study. COPD. 2017;12:1269–1277. doi:10.2147/COPD.S127923.
- Vollmer WM, Gislason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: Results from the BOLD study. Eur Respir J. 2009;34(3):588–597. doi:10.1183/09031936.00164608.
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi:10.1183/09031936.05.00035205.
- Kanis JA, Oden A, McCloskey EV, et al. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239–2256. doi:10.1007/s00198-012-1964-3.
- Pfister AK, Welch CA, Saville PD, et al. Osteoporosis by forearm bone densitometry in postmenopausal women in West Virginia. W V Med J. 2007;103(1):26–28.
- ERJ. Abstract book. ERJ. 2019 Sept;54(Suppl: 63).