Abstract
The 2020 Global Initiative for Obstructive Lung Disease (GOLD) Report highlights the importance of sputum purulence in the decision to prescribe antibiotics for acute exacerbations. The purpose of this systematic review and meta-analysis was to evaluate the strength of literature supporting inclusion of sputum purulence in criteria utilized to evaluate if antimicrobials are indicated in acute COPD exacerbation. A total of 6 observational studies met inclusion criteria for this meta-analysis. Sputum purulence was defined by visual assessment of color, either subjectively by providers and/or patients or by a colored chart, where green or yellow sputum was considered purulent. Four of the studies were primarily conducted in hospitalized patients, one in the emergency department, and one in the primary care setting. Five studies relied upon expectorated sputum and one used bronchoscopy to obtain sputum samples for bacterial cultures. Compared with mucoid sputum, purulent sputum had a significantly higher probability of positive bacterial culture results (RR = 2.14, 95%CI [1.25, 3.67], p = 0.006, moderate quality). For sensitivity analysis, after removal of studies losing 2 or more points from the New Castle-Ottawa scale, the effect value remained statistically significant. This systematic review and meta-analysis showed a moderate level of evidence that purulent sputum during COPD exacerbation, as defined by yellow or green color, is associated with a significantly higher probability of potentially pathogenic bacteria, supporting GOLD report and NICE recommendations.
Introduction
Chronic obstructive pulmonary disease (COPD) exacerbations are common events and a major contributor to disease progression and healthcare utilization [Citation1]. Although a significant portion of COPD exacerbations are non-bacterial and quality of evidence for antibiotic treatment is low to moderate, the 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report recommends treatment with antibacterials based on whether patients meet certain clinical criteria [Citation1]. Although clinical criteria lack specificity, GOLD recommends antibiotic prescribing be based on Anthonisen exacerbation type when two or more cardinal symptoms (dyspnea, increased sputum volume and sputum purulence) are present, or if purulence is found with one other cardinal symptom or the need for noninvasive or invasive ventilation [Citation2]. Clinical resolution is more likely to be achieved when patients present with all three symptoms (Type I exacerbation) or two cardinal symptoms (Type II exacerbation) [Citation2].
The volume and purulence of mucus in the airways increase in COPD exacerbation because of the lung’s inflammatory response to infectious and other stimuli. Inflammatory responses in the respiratory tract secondary to infections include neutrophil and monocyte influx and increases in TNF-alpha, myeloperoxidase, leukocyte elastase, leukotrienes and cytokines [Citation3–6]. When released from neutrophils, leukocyte elastase leads to epithelial tissue damage, goblet cell hyperplasia, and mucus hypersecretion [Citation7]. Neutrophils are activated in the airway by granule protein myeloperoxidase [Citation7], a green heme-containing protein within azurophil granules of neutrophils and monocytes that leads to darker purulent color of sputum during infections. Some pathogens as Pseudomonas aeruginosa may also contribute to darker sputum through producing colored pigments such as pyocyanin [Citation8].
We undertook a systematic review and meta-analyses to determine the strength of evidence of sputum purulence predicting the likelihood of bacterial infection in COPD exacerbation, as no such analysis has been previously published.
Methods
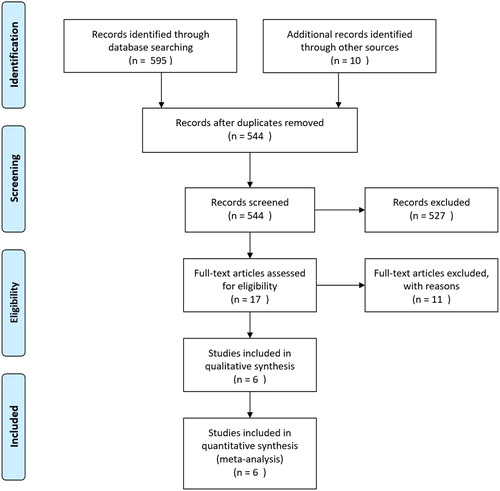
Pubmed, Embase, Cochrane Library, and clinicaltrials.gov were searched until Feb 29, 2020. The search terms were the combination of subject terms and free terms. Detail of search terms for Pubmed and reasons for excluding studies are shown in Online Appendices 1 and 2. References on evaluation of relationship between sputum purulence and culture results in COPD exacerbations were eligible for inclusion in the analysis. Sputum purulence must have been judged by investigator’s or patient’s visual assessment of color by category. No restrictions were applied to patient care settings, definition of positive sputum culture or sputum purulence, follow-up time, study designs, languages, or availability of full texts. The presence of potentially pathogenic bacteria (PPB) on bacterial culture was the only study outcome for sputum purulence studies. Two reviewers (KC and ZY) independently screened titles and abstracts of retrieved citations initially, looked into full-texts, and identified final studies.
Two reviewers (KC and ZY) extracted data from original studies and performed quality appraisal independently. Sputum characteristics (source of sputum, sampling time, definition of purulence, people who judged purulence, etc.) were extracted. The New Castle-Ottawa scale (NOS) was applied to evaluate the quality of included observational studies [Citation9]. Comparability between purulent and mucoid sputum samples was not evaluated because of lack of clinical significance. Quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method. Publication bias was evaluated only if more than 10 studies were included.
Meta-analysis was performed using RevMan 5.1 (Cochran IMS). The Mantel–Heanzel random effects model was used as the statistical model to calculate the risk ratio for pooled outcomes. The Cochran Q χ2 test and I2 statistic were used to assess heterogeneity among studies. p < 0.1 was considered significant because of the low statistical power of the χ2 test for heterogeneity. A sensitivity analysis was performed based on methodological quality of included studies.
Results
A total of six studies were included in this meta-analysis which evaluated sputum purulence as clinical marker of bacterial infection for COPD exacerbations (, ) [Citation10–15]. Studies most often enrolled patients with Type I or II exacerbations (at least two of the three cardinal symptoms) and were done in the acute care setting – four primarily in the hospital, one in the emergency department, and another in primary care.
Table 1. Characteristics and outcome values of included studies.
The quality of studies evaluated by NOS is shown in . Only 2 studies (Allegra et al. and Tsimogianni et al.) reached a full grade when evaluated by NOS [Citation10, Citation15]. Two studies (Soler et al. 2007 and Soler et al. 2012) lost points of reliable exposure because the sputum purulence was judged by patients instead of investigators [Citation12, Citation13]. Patients in one study (Daniels et al.) used antibiotics within past 7 days of sputum collection so they were judged that outcome of interest was present at start of studies [Citation11]. Two studies (Daniels et al. and Stockley et al.) received a lower score because lower respiratory tract origin of sputum samples were not confirmed microscopically [Citation11, Citation14]. Daniels et al. received a lower score because of inadequate follow-up [Citation11].
Table 2. Quality appraisal of included observational studies.
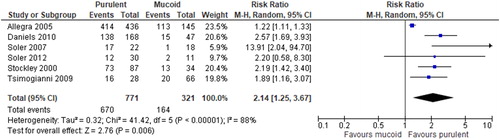
All studies were included in meta-analysis. Compared with mucoid sputum samples, purulent sputum samples have a significantly higher probability of positive culture results (RR = 2.14, 95%CI [1.25, 3.67], p = 0.006) (). Despite the nature of study design as observational study, the result from pooled studies was still rated as moderate quality of evidence by GRADE method () because of the large magnitude of effect that was unlikely to be explained by other bias. After removal of studies losing 2 or more points in NOS [Citation11, Citation12], the effect value remained statistically significant (RR = 1.69, 95%CI [1.09, 2.63], p = 0.005).
Table 3. GRADE summary of findings.
Discussion
COPD exacerbations are common events occurring in more than a quarter of patients every year [Citation2], leading to frequent use of corticosteroids and antibacterials. It is generally assumed that purulent sputum reflects the presence of bacterial infection in exacerbations of chronic lung diseases as COPD, bronchiectasis, and cystic fibrosis; and that these patients would benefit clinically from a course of antibacterials. For COPD, this practice evolved because of studies as Anthonisen et al. demonstrating that antibacterial therapy is beneficial in patients with at least two of three common clinical findings relating to sputum (Type I or Type II exacerbation) [Citation2]. Based on our systematic review and meta-analysis of six studies conducted in different clinical settings, we found there is a moderate level of evidence showing that when sputum is purulent in COPD exacerbation, as judged by a yellow or green color, the likelihood of PPB increases by two-fold. To our knowledge, this is the first meta-analysis addressing this specific clinical measure for COPD exacerbation.
Among studies of COPD exacerbation included in our analysis, all reported that PPB were significantly more likely to be present when sputum is purulent compared to mucoid or clear [Citation10–15]. All but one of these studies [Citation10] found at least a several-fold higher probability of PPB identified on microbiologic culture when sputum was purulent compared to non-purulent sputum [Citation11–15]. In the most rigorous study of COPD exacerbation where sputum specimens were obtained from distal airways by protected brush specimen via bronchoscopy, 78% of purulent sputa and 5% of non-purulent grew bacterial pathogens [Citation12]. Among these patients, for those who presented with dyspnea only, 6% had a positive sputum culture, whereas 77% with a purulent sputum were positive for PPB [Citation12].
While it is known that antibacterials improve clinical outcomes in COPD exacerbation when patients present with at least two of the three Anthonisen cardinal symptoms [Citation2, Citation11, Citation16, Citation17], less clear is the specific contribution of sputum purulence. Although placebo controlled-studies of antibiotics for COPD exacerbations show that a combination of Anthonisen cardinal symptoms is most predictive of bacterial infection [Citation2, Citation11, Citation17], purulence was the single most important contributor to predict presence of PPB [Citation13, Citation17] and clinical response to antibiotic treatment [Citation17]. In an open longitudinal study in the primary care setting in 121 patients with Type I or II COPD exacerbations, patients with yellow or green sputum received antibiotics, whereas those with mucoid sputum did not [Citation14]. Patients with purulent sputum had PPB identified in 84% of instances, while only 38% of those with mucoid sputum had identifiable pathogens. Clinical resolution of symptoms occurred in 32/34 (94.1%) patients with mucoid sputum while receiving no antibiotic treatment, compared to 77/84 (91.7%) patients with purulent sputum who did receive antibiotics. In a 2012 non-randomized study of 73 hospitalized patients with COPD exacerbations, where antibiotic initiation was dependent on whether the sputum was purulent, no significant statistical differences were observed on therapeutic failure criteria (9% non-purulent versus 10% purulent) [Citation13].
In the clinical setting, practitioners rely on visual examination of sputum either directly or by patient report to determine whether antibacterial should be prescribed in COPD exacerbations. Studies included in this meta-analysis have primarily used spontaneously expectorated mucus, one study undertook bronchoscopy using protected specimen brush [Citation18]. Sputum purulence was either determined in the laboratory based on cell types and counts, and/or macroscopic assessment of color by provider and/or patient. In most studies, sputum color was determined by using a reference color chart [Citation10, Citation14, Citation19, Citation20] and in two studies subjectively either by providers and/or patients [Citation11, Citation12] as typically done in the clinical setting. Commonly used categories were: clear, mucoid (white) and purulent (yellow or green) [Citation10, Citation11, Citation14, Citation20]. Using a reference color chart (BronkoTest, Heredilab Inc, Salt Lake City, UT, USA), Stockley et al. demonstrated that a change in color of expectorated sputum during the course of an exacerbation was a sensitive (94.4%) and specific marker (77.0%) for the presence of bacteria and was predictive of a high bacterial load.
Several studies in COPD exacerbations have assessed the reliability of providers and patients to accurately report whether sputum is purulent based on color. While employing bronchoscopy to obtain bacterial cultures, patient and provider report of sputum purulence based on color showed good diagnostic accuracy for the presence of PPB (27-fold odds of having positive sputum culture) [Citation12]. However, two studies indicated that patients were less reliable than providers to accurately report color [Citation10, Citation11]. Concordance in subjectively assessing sputum color between providers and patients was 68% in a study of COPD exacerbations [Citation10]. Another study reported that judgment of sputum color by patients was less reliable than by healthcare providers for the presence of bacteria (sensitivity 90% vs 73%, specificity 52% vs 39%) [Citation11]. In a large study of sputum color and purulence in chronic bronchitis exacerbation that included COPD exacerbations, the consistency of the findings found that sputum color was reliably recognized by physicians [Citation19]. In that study, having green or yellow sputum versus white sputum had a sensitivity for the presence of PPB of 94.7%, and a specificity of 15.0%. Assessment of sputum purulence is most reliable by providers and is preferred.
Limited data is published regarding use of sputum purulence to differentiate between viral and bacterial infections in COPD exacerbation and thus a meta-analysis on this measure was not done. It has been reported that both viral and bacterial infections lead to increased neutrophils in the airways, however threefold higher cell counts are seen with the latter [Citation21]. Bacterial infection in COPD exacerbations is thought to occur through acquiring new organisms, mutations in existing organisms, or increase in bacterial counts of colonizers – these often occur as secondary to viral infections in COPD exacerbation [Citation22]. In a study of 64 COPD patients hospitalized for exacerbation, the frequency of purulent sputa, bacterial cultures, viral PCR, and sputum neutrophil counts were determined [Citation22]. Purulent sputum at exacerbation was more frequent in infective (viral and/or bacteria) exacerbations (30/50) as compared to non-infective exacerbations (3/14) (p < 0.05). Purulent sputum at exacerbation tended to be significantly more frequent in those exacerbations where bacteria were present in sputum samples (22/35) as compared to bacterial negative exacerbation (11/29) (p = 0.086). Sputum neutrophils were elevated to a greater extent with higher bacterial colony counts.
The relationship between sputum purulence and presence of potential pathogenic microorganisms has also been shown during the stable state as well as post exacerbation. In stable ambulatory COPD patients with persistently purulent sputum (dark yellow or green) more than 80% of cases were colonized with pathogenic bacteria as compared to <10% of cases with mucoid (white) sputum. Twenty-seven of the patients had mucoid sputum at presentation with the exacerbation (referred to as mucoid bronchitis) and 42 had purulent sputum (purulent bronchitis). When the two groups were restudied in the stable state (day 56), the majority of samples in both groups were macroscopically mucoid [Citation23]. It is unclear about the role of sputum purulence to guide antibacterial prescription in patients with COPD exacerbations whose sputum is persistently purulent.
Limitations of our meta-analysis include a modest number of studies meeting our criteria (n = 6). We were not able to assess the role of sputum purulence on clinical outcomes as only 2 studies, not included our meta-analysis, looked at measures other than presence of PPB. In particular, additional research is needed where antibiotics treatment is randomized in patients who present with mucoid sputum with dyspnea. Lastly, only one study was exclusively conducted in the outpatients where most exacerbations are treated, thus additional study is needed in this setting.
Conclusion
Our meta-analysis shows that in patients presenting with COPD exacerbation symptoms in whom sputum is determined to be purulent by color assessment as green or yellow, there is significantly higher probability of PPB being present than if the sputum is mucoid (white). This finding has predominantly been investigated in the acute care setting, with less published data available from the outpatient setting. Our meta-analysis, the first published on this specific topic, supports recommendations by GOLD report and NICE for use of sputum purulence to help guide antibiotic treatment in patients presenting with COPD exacerbation.
Supplemental Material
Download PDF (381.7 KB)Disclosure statement
Dr. Drummond reports personal fees from Boehringer-Ingelheim, personal fees from GlaxoSmithKline, personal fees from AstraZeneca, personal fees from Mylan-Theravance, grants from Department of Defense, personal fees from Novavax, personal fees from Parion, personal fees from Midmark, personal fees from Philips, grants from NIH-NHLBI, outside the submitted work. Other authors report no conflicts of interest.
Funding
None.
References
- Global Initiative for Chronic Obstructive Lung Disease Inc. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2020 report. 2020. Available from: http://www.goldcopd.org.
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi:10.7326/0003-4819-106-2-196.
- Gompertz S, O'Brien C, Bayley DL, et al. Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J. 2001;17(6):1112–1119. doi:10.1183/09031936.01.99114901.
- Miravitlles M, Marin A, Monso E, et al. Colour of sputum is a marker for bacterial colonisation in chronic obstructive pulmonary disease. Respir Res. 2010;11(1):58. doi:10.1186/1465-9921-11-58.
- Stockley RA, Bayley D, Hill SL, et al. Assessment of airway neutrophils by sputum colour: correlation with airways inflammation. Thorax. 2001;56(5):366–372. doi:10.1136/thorax.56.5.366.
- Andelid K, Andersson A, Yoshihara S, et al. Systemic signs of neutrophil mobilization during clinically stable periods and during exacerbations in smokers with obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1253–1263. doi:10.2147/COPD.S77274.
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi:10.1016/j.jaci.2016.05.011.
- Palumbo SA. Role of iron and sulfur in pigment and slime formation by Pseudomonas aeruginosa. J Bacteriol. 1972;111(2):430–436.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Allegra L, Blasi F, Diano P, et al. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Respir Med. 2005;99(6):742–747. doi:10.1016/j.rmed.2004.10.020.
- Daniels JM, de Graaff CS, Vlaspolder F, et al. Sputum colour reported by patients is not a reliable marker of the presence of bacteria in acute exacerbations of chronic obstructive pulmonary disease. Clin Microbiol Infect. 2010;16(6):583–588. doi:10.1111/j.1469-0691.2009.02892.x.
- Soler N, Agusti C, Angrill J, et al. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease. Thorax. 2007;62(1):29–35. doi:10.1136/thx.2005.056374.
- Soler N, Esperatti M, Ewig S, et al. Sputum purulence-guided antibiotic use in hospitalised patients with exacerbations of COPD. Eur Respir J. 2012;40(6):1344–1353. doi:10.1183/09031936.00150211.
- Stockley RA, O’Brien C, Pye A, et al. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117(6):1638–1645. doi:10.1378/chest.117.6.1638.
- Tsimogianni AM, Papiris SA, Kanavaki S, et al. Predictors of positive sputum cultures in exacerbations of chronic obstructive pulmonary disease. Respirology. 2009;14(8):1114–1120. doi:10.1111/j.1440-1843.2009.01615.x.
- Llor C, Moragas A, Hernandez S, et al. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(8):716–723. doi:10.1164/rccm.201206-0996OC.
- Miravitlles M, Moragas A, Hernandez S, et al. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest. 2013;144(5):1571–1577. doi:10.1378/chest.13-0518.
- Soler N, Esperatti M, Huerta AH, et al. Sputum purulence guided antibiotic therapy in patients with severe exacerbations of COPD. Paper presented at: American Thoracic Society International Conference; 2010 May 14-19; New Orleans, LA. doi:10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A2380.
- Miravitlles M, Kruesmann F, Haverstock D, et al. Sputum colour and bacteria in chronic bronchitis exacerbations: a pooled analysis. Eur Respir J. 2012;39(6):1354–1360. doi:10.1183/09031936.00042111.
- Brusse-Keizer MG, Grotenhuis AJ, Kerstjens HA, et al. Relation of sputum colour to bacterial load in acute exacerbations of COPD. Respir Med. 2009;103(4):601–606. doi:10.1016/j.rmed.2008.10.012.
- Mallia P, Footitt J, Sotero R, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(11):1117–1124. doi:10.1164/rccm.201205-0806OC.
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi:10.1164/rccm.200506-859OC.
- Johnson AL, Hampson DF, Hampson NB. Sputum color: potential implications for clinical practice. Respir Care. 2008;53(4):450–454.