Abstract
The purpose of this study was to investigate the intra-rater reliability and agreement of handgrip strength (HGS) measurement using a hydraulic hand dynamometer in patients with chronic obstructive pulmonary disease (COPD). A sample of 19 COPD patients (18 males and 1 female; mean ± SD age, 66.9 ± 6.3 years) was evaluated using a hand dynamometer by the same rater in two different testing sessions with a 7-d interval. During each session, patients were asked to exert three maximal isometric contractions on the dominant hand and the mean value of the 3 efforts (measured in kilogram-force [Kgf]) was used for data analysis. The intraclass correlation coefficient (ICC2,1), the standard error of measurement (SEM), the minimal detectable change (MDC), and Bland–Altman methods were used to estimate the degree of test–retest reliability and the measurement error, respectively. HGS in COPD patients revealed an ICC2,1 score of 0.99, suggesting excellent test–retest reliability. The calculated SEM was relatively small (0.59 Kgf), and the MDC presented a clinically acceptable value of 1.64 Kgf. These findings, in conjunction with the narrow width of the 95% limits of agreements (95% limits of agreement, −2.5–2.1 Kgf) in the Bland–Altman plot, reflected the measurement precision and the narrow variation of the differences during the 2 testing sessions. The results of this study demonstrated an excellent test–retest reliability of HGS measurement, indicating that this method is reliable for repeated monitoring of peripheral muscle strength in patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease which is characterised by chronic inflammation in the airway and lung parenchyma [Citation1]. It stands in the third place of top ten causes of death, according to the most updated World Health Organization report [Citation2]. COPD progressively becomes a multisystem disease, affecting other systems, such as the cardiovascular and the musculoskeletal system [Citation3]. Pathophysiological changes caused by COPD in the musculoskeletal system are associated with morphological and structural alterations of peripheral muscles, such as muscle weakness [Citation4].
Clinicians often use several types of dynamometers to evaluate the treatment effect on muscle strength in patients with COPD. The most common assessment method for muscle strength of upper limbs is isometric handgrip strength (HGS) [Citation5]. Reduced HGS is associated with reduced muscle mass [Citation6–8], increased hyperinflation [Citation9], increased dyspnoea [Citation10], and mortality [Citation11].
A previous study has been conducted aiming to assess the reliability of a hand held dynamometer for measuring muscle strength in a COPD population [Citation12]. Although this type of dynamometer has been frequently used for strength assessment of various muscles in the upper and lower body joints [Citation13,Citation14], it cannot measure HGS. Despite HGS being widely used in clinical practice and research in COPD patients to evaluate general muscle weakness, form inferences in terms of prognosis [Citation11,Citation15,Citation16], and document changes following interventions [Citation17,Citation18], to our knowledge reliability and measurement error of HGS has never been evaluated in patients with COPD. Reliability is not fixing property of measurement tools, but rather is the product of the interplay between several domains, including the measurement tool itself, the rater, the patient population, and the context of the assessment [Citation19,Citation20]. On top of that, reliability parameters are highly dependent on the intrinsic characteristics and variation of the sample and can only have a clinical value when generalised in samples with similar variation [Citation20]. Therefore, the main objective of this study was to evaluate the test–retest reliability of HGS using a hydraulic hand dynamometer in patients with COPD.
Materials and methods
Study design
The study followed published guidelines for Reporting Reliability and Agreement Studies (GRRAS) [Citation21]. A prospective, observational, nonexperimental design was used to explore test–retest reliability of HGS in patients with COPD [Citation22,Citation23]. The patients’ HGS was measured by the same rater on 2 different occasions with an interval of 7 d between the testing sessions, to prevent potential learning or fatigue effects [Citation22–24]. The rater was a qualified physiotherapist with 10 years of experience in the field of cardiopulmonary rehabilitation. The data collector who measured the patients’ HGS was blinded to the purpose and nature of the study design, as well as he did not have access to the baseline data at the second HGS evaluation to prevent potential recorder bias [Citation25]. During HGS trials, patients were blinded to their ΗGS scores to address potential expectation bias [Citation26].
Participants
Participants were stable COPD patients who had been referred for the pulmonary rehabilitation programme at the Pulmonary Clinic of Nicosia General Hospital. Inclusion criteria were the following: 1) diagnosis of COPD confirmed by clinical examination and a pulmonary function test with forced expiratory volume in 1 s <80% predicted and forced expiratory volume in 1 s/forced vital capacity ratio <0.7, as per the Global Initiative for Chronic Obstructive Lung Disease report [Citation1] and 2) stable clinical condition for at least 4 weeks. A physiotherapist took the history and performed physical examination to exclude patients who had: a) musculoskeletal and neurological diseases that could affect strength performance of the upper limbs (i.e. arm or hand tendinopathies, severe arthritis, cervical radiculopathy, stroke) [Citation27–30], b) unstable heart disease, c) malignant disease, or d) who were non-cooperative in measurement and evaluation [Citation31,Citation32].
Before participating in the study, patients gave written informed consent and the study was approved by the Cyprus National Bioethics Committee. A participant information sheet detailed the aim and the procedures of the study and informed patients of their right of privacy, anonymity, and confidentially as well as their right to withdraw from the study at any time without giving a reason, according with the ethical principles of the Declaration of Helsinki.
Instrument
A digital hydraulic hand dynamometer was used to measure the patient’s HGS (Seahan Corporation, Masan, Korea) [Citation33]. According to the American Society of Hand Therapists (ASHT), the instrument has proven reliability and accuracy during repeated gripping trials [Citation34–36]. This device is frequently used in clinical studies among patients in a broad spectrum of health conditions [Citation37–40].
Procedure
Data collection was conducted between December 2018 and June 2019. Patients were asked to attend for 2 testing sessions in a quiet, temperature-controlled laboratory located in the outpatient Pulmonary Clinic of Nicosia General Hospital. Time of testing was standardised, and patients were advised to attend morning sessions between 8:00 and 12:00. Before the HGS assessment patients were asked to grade their dyspnoea and fatigue symptoms, in modified Borg Scale (0 = no symptoms to 10 = maximal dyspnoea/fatigue) [Citation41]. We decided to arbitrarily set a 5/10 cut off point for inclusion in testing as recent guidelines propose that exercise intensity should be set between 4 and 6 in Borg Scale [Citation32,Citation42]. Moreover, evidence suggests that excessive dyspnoea may lead to psychological stress, negative emotions, such as anxiety and scare, which in turn may affect the occurrence of this symptom [Citation43]. If patient symptoms score exceeded 5/10, they were asked to relax for 5 min and then dyspnoea and fatigue were reassessed. If their scores did not change, they were excluded from the procedure. Moreover, patients’ heart rate and oxygen saturation were assessed with a portable oximeter before and after HGS assessment. If a patient presented low saturation (<90%) or if he/she was receiving long term oxygen therapy, low flow rate supplemental oxygen was administered through nasal cannula in order to prevent exercise-induced desaturation during the examination [Citation32].
Patients followed a standardised protocol for ΗGS data collection as recommended by the ASHT [Citation36,Citation44] in both test and retest occasions. Grip strength measurements were applied in the dominant hand in standardised position: seated in a straight back chair with the feet flat on the floor, shoulder at 0° of flexion, abduction, and rotation; elbow flexed at 90°; forearm and wrist rested in a neutral position [Citation45,Citation46]. For the determination of hand dominance, patients were asked to self-evaluate which hand they prefer to use to execute unimanual tasks, such as writing [Citation47–49]. Patients underwent a familiarisation trial as they were instructed to hold the handle and squeeze the hand dynamometer set at the second handle space [Citation50]. Then, patients were advised to exert 3 maximum gripping efforts for 5 s with a 15-s rest between trials to prevent fatigue [Citation36]. During each trial, instructions were given by the rater to each patient as follows: “Are you ready? Squeeze as hard as you can. Harder… harder… harder… harder… harder…. relax.” No other encouragement was given during HGS measurements. After each trial, the blinded rater calculated and recorded the patients’ scores as indicated from the digital screen of the dynamometer and rounded to the nearest 100 g. The mean value of the three efforts (measured in kilogram-force [Kgf]) was used for the analysis of ΗGS. The interval between test and retest was chosen as a time period short enough to guarantee that clinical change had not occurred. Moreover, with aim to ensure the stability of the condition we only included participants who self-rated their condition as unchanged at the second assessment occasion.
Statistical analysis
The Statistical Package SPSS version 20 (SPSS Inc, Chicago, IL) and Medcalc version 19.1 (MedCalc Software Ltd, Ostend, Belgium) were used for analysis. Test–retest reliability of an outcome measure is recommended to be interpreted based on correlation coefficients and measurement error [Citation51]. Test–retest reliability was evaluated by using both Pearson r and a 2-way random effects model Intraclass Correlation Coefficient, type agreement (ICC2,1), because systematic differences are considered to be part of the measurement error [Citation52]. The 95% confidence intervals (CIs) were calculated along with the ICC2,1 value, to make accurate probabilistic statements regarding the precise score of ICC2,1 [Citation53]. The obtained ICC2,1 value was interpreted using the Koo and Li classification [Citation54]. Based on this classification, test–retest reliability is considered as poor for an ICC less than 0.5, moderate for values between 0.5 and 0.75, good for values between 0.75 and 0.9 and excellent for values greater than 0.9 [Citation54].
For absolute reliability, the standard error of measurement (SEM) was calculated (SEMAgreement = SDpooled x √1−ICC), including the systematic differences in order to distinguish them from real changes, e.g. due to time. SEM quantifies measurement error in the same units as the original measurement and can be used to compute the minimal detectable change (MDC95 = 1.96 x √2 × SEM), which corresponds to the minimal amount of within-person change in HGS not attributable to variation in measurement. Bland–Altman methods were used to evaluate absolute agreement and systematic error for test–retest measurements including a scatter plot of differences between applications, with 95% limits of agreement (mean change in HGS of repeated measurements) [Citation55,Citation56]. Paired samples t-test was used to compare the pre and post HGS assessment values. The non-parametric Wilcoxon test was used for intragroup comparison of pre and post HGS assessment values of dyspnoea and fatigue score in modified Borg Scale. Descriptive statistics were used to summarise the characteristics of the participants. The significance level was set at 5% (p < .05).
A difference in reliability of 0.9 and 0.7 at 80% power and a 5% level of significance using two ratings was set [Citation57,Citation58]. Based on two testing sessions, an α value of 0.05, and a power of 0.80, a minimal sample size of 19 patients was identified.
Results
Nineteen patients (18 men and one woman; mean ± SD age, 66.9 ± 6.3 years) with mild to very severe COPD, met the inclusion criteria, and agreed to participate (). All enrolled patients completed the two sessions, and the HGS mean scores (Kgf) were 24.0 ± 5.8 for the first and 24.2 ± 6.1 s occasion. No statistically significant difference was found between pre and post HGS values, t (18)= −0.782, p = .444. Correlation between test and retest was excellent (r = 0.981, p < .001). Based on Koo and Li’s classification, HGS in COPD demonstrated an ICC2,1 of 0.99, suggesting excellent test–retest reliability. The given range of scores of the 95% CI varied from 0.97 to 0.99.
Table 1. Patients characteristics.
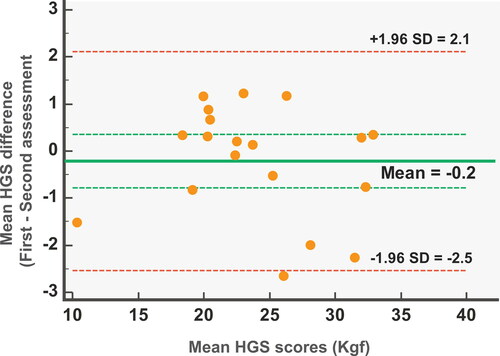
The SEM was relatively small and calculated to be 0.59 Kgf or 2.4% of the grand mean HGS value, while the MDC presented a clinically acceptable value of 1.64 Kgf or 6.8% of the grand mean HGS value. No systematic error was observed by examining the Bland–Altman plot. The mean difference of the two measurements was −0.2 Kgf and the width of the 95% limits of agreements ranged from −2.5 to 2.1 Kgf. Most of the points of ΗGS scores lie between the 95% limits of agreement, with a few outliers, suggesting a normal distribution of the differences (). Dyspnoea and fatigue did not change significantly between HGS assessments (p = .710 and p = .688, respectively). Patient characteristics, symptoms scores and HGS test and retest mean values were provided in a Supplementary Table.
Discussion
The intrarater reliability of HGS using a hydraulic hand dynamometer was found to be almost perfect, the error of the measurement was small, and the MDC was clinically acceptable, supporting the validity and the clinical use of this method of evaluation of patients with COPD.
Peripheral muscle dysfunction is an important systemic consequence of COPD. The dysfunction results from several structural muscle changes such as muscle atrophy, muscle weakness, shift in muscle fibre type, mitochondrial dysfunction, and poor oxidative capacity [Citation4]. Moreover, evidence suggests that muscle strength deteriorates in a time-dependent manner following the progression of the condition, and patients with more severe COPD disease status present with lower strength [Citation9,Citation59]. One of the main goals of pulmonary rehabilitation programmes is the prevention or improvement of muscle dysfunction [Citation31,Citation32]. These programmes include exercise training and have been utilised in COPD populations presenting substantial improvements in muscle strength and function [Citation60,Citation61] and a range of techniques have been used with the aim to capture significant strength differences [Citation5,Citation62].
A number of studies [Citation17,Citation18] have investigated the effect of strength and endurance training on peripheral muscle weakness by using the HGS as an outcome measure. A recent randomised controlled trial evaluated the impact of arm exercise training in HGS in patients with COPD, compared with a control group performing only breathing exercises. The results of the study showed a significant increase in HGS of the exercise group with a mean difference of 2.89 Kgf [Citation17]. Similarly, in another study that compared the effects of resistance training to endurance training, increases of 8 and 30% in predicted HGS were found, respectively [Citation18]. Given the lack of HGS reliability studies in COPD population it is difficult to interpret, in clinically meaningful terms, the statistically significant differences seen in published clinical trials.
With the aim of making clinically relevant comparisons, we need first to compare the HGS of the patients evaluated in this study with normative values of healthy individuals and COPD patients with relatively similar characteristics. The 5th and 95th percentile HGS of a European male individual with similar age (>65 years) and height (160–165 cm) with the patients in this study, range between 20 and 44 kgs on the left hand and 22–46 kgs on the right hand [Citation63]. Despite it is logical the HGS of patients at GOLD III/IV stage to be at the lowest bound of healthy individuals’ normative values [Citation59], the HGS reported herein was substantially lower compared to other studies recruited similar patient population [Citation8,Citation10]. Variability in the measurement setting is inevitable, however in research, standardisation of measurement process, and selection of a homogeneous patient sample assist to prevent bias [Citation36]. These inconsistencies can be attributed mainly to variations on the HGS measurement techniques utilised. HGS in COPD patients has been conducted in sitting [Citation10,Citation15,Citation17,Citation64], in standing [Citation6,Citation65,Citation66], in supine lying [Citation59], while in some cases no information of testing position was reported [Citation7,Citation8]. In addition, HSG has been measured with elbow flexed in 90° [Citation9,Citation15,Citation17,Citation67] or fully extended [Citation6,Citation65], and by using different types of dynamometers, such as Jamar hand held dynamometer [Citation6,Citation7,Citation9,Citation10,Citation15,Citation59,Citation64,Citation67] or rubber bulb dynamometer [Citation68–70]. Finally, another plausible explanation for the HGS value discrepancies between studies is associated with the calculation and reporting of HGS, with some studies [Citation9,Citation65] reporting the mean of three trials as indicated by guidelines [Citation36], while others did not provide information [Citation7,Citation15] or used the maximum HGS value from a range of two to six trials [Citation6,Citation8,Citation10,Citation17,Citation64,Citation66,Citation67]. These significant between study differences render comparisons and firm conclusions unsafe.
In clinical practice, we strongly believe it is important to provide both an estimate of reliability (e.g. ICC) and agreement (e.g. SEM), such that clinicians can make meaningful inferences. We note that previous studies [Citation17,Citation18] assessing strength changes following clinical interventions in patients with COPD assumed HGS measures as valid measures for the condition without established reliability estimates. Reliability is a prerequisite for validity. In practice, the minimum clinically important difference for any measure will be the sum of the “noise” in the measurement (the MDC) and that amount deemed clinically important, for the particular situation. The amount deemed clinically important will be influenced by the specifics of the condition at hand. For example, if it was seen that, say, an improvement of 3 Kgf in HGS was indicative of an improvement in function of patients with COPD, this may be the clinically important amount. If the MDC of the measure was say, 2 Kgf, then the minimum clinically important difference would be the sum of these two, i.e. 5 Kgf. In this study, the excellent reliability and the clinically acceptable measurement error strengthens the clinical applicability of the HGS in patients with COPD. Moreover, the reliability data presented here may be of use in reinterpreting, in clinically meaningful terms, previously documented statistically significant changes in HGS following rehabilitative programmes aimed at strength improvements.
HGS is an easy to use tool for strength assessment which provides excellent reliability results in healthy individuals and populations with several pathologies [Citation34,Citation37,Citation38,Citation50,Citation71–73]. In this study, an excellent test–retest reliability of HGS with an obtained ICC2,1 value of 0.99 was documented. HGS is a reliable outcome measure providing similar and stable results when applied on repeated trials [Citation22] and, therefore, it is recommended for use in patients with COPD. The excellent test–retest reliability was related with low values of SEM and small width of the 95% limits of agreement in the Bland–Altman plot, reflecting the measurement precision and the narrow variation of the differences during the two testing sessions [Citation55,Citation56]. In addition, HGS measurement in patients with COPD seems to be safe, as dyspnoea and fatigue symptoms did not present significant changes during assessment procedure. No other symptoms or signs were detected during evaluation.
We acknowledge that this study has some limitations that should be considered. The population of COPD recruited was not a representative sample in terms of GOLD stages. The current sample included only one female subject and very few subjects with COPD 1–2. The former limits the generalisability of the results and we suggest caution in making clinical inferences in female patients as both sex and GOLD stage may affect HGS values [Citation59]. We suggest that an important area of future research is standardising the HGS measurement process, determining inter-rater reliability of the method, as well as the minimum clinically important difference in HGS in subgroups of COPD patients of different stages of the condition following exercise training as a rehabilitative intervention. Finally, future upper limb interventional trials are essential to elucidate the responsiveness of HGS as an outcome measure.
Conclusions
HGS was found to be a safe and easy-to-use tool with excellent test–retest reliability for patients with COPD, demonstrating its utility and feasibility as an outcome measure in clinical practice. Future studies should evaluate the clinimetric properties including reliability of HGS in female patients and within a broader range of COPD severity.
Declaration of interest
The authors report no conflict of interest.
Acknowledgements
None declared.
References
- Vogelmeier CF , Criner GJ , Martinez FJ , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. DOI:10.1164/rccm.201701-0218PP
- World Health Organization . The top 10 causes of death. 2018. May 24 [2019, October 15]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Tkac J , Man SF , Sin DD . Systemic consequences of COPD. Ther Adv Respir Dis. 2007;1(1):47–59. DOI:10.1177/1753465807082374
- Maltais F , Decramer M , Casaburi R , et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. DOI:10.1164/rccm.201402-0373ST
- Robles PG , Mathur S , Janaudis-Fereira T , et al. Measurement of peripheral muscle strength in individuals with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev. 2011;31(1):11–24. DOI:10.1097/HCR.0b013e3181ebf302
- Marino DM , Marrara KT , Ike D , et al. Study of peripheral muscle strength and severity indexes in individuals with chronic obstructive pulmonary disease. Physiother Res Int. 2010;15(3):135–143. DOI:10.1002/pri.454
- Martinez CH , Diaz AA , Meldrum CA , et al. Handgrip strength in chronic obstructive pulmonary disease. Associations with acute exacerbations and body composition. Ann Am Thorac Soc. 2017;14(11):1638–1645. DOI:10.1513/AnnalsATS.201610-821OC
- Wu ZY , Han YX , Niu ME , et al. Handgrip strength is associated with dyspnoea and functional exercise capacity in male patients with stable COPD. Int J Tuberc Lung Dis. 2019;23(4):428–432. DOI:10.5588/ijtld.18.0269
- Felipe C , Bartolome C , Miguel D , et al. Longitudinal changes in handgrip strength, hyperinflation, and 6-minute walk distance in patients with COPD and a control group. Chest. 2015;148(4):986–994. DOI:10.1378/chest.14-2878
- Kaymaz D , Candemir IC , Ergun P , et al. Relation between upper-limb muscle strength with exercise capacity, quality of life and dyspnea in patients with severe chronic obstructive pulmonary disease. Clin Respir J. 2018;12(3):1257–1263. DOI:10.1111/crj.12659
- Puhan MA , Siebeling L , Zoller M , et al. Simple functional performance tests and mortality in COPD. Eur Respir J. 2013;42(4):956–963. DOI:10.1183/09031936.00131612
- O’Shea SD , Taylor NF , Paratz JD . Measuring muscle strength for people with chronic obstructive pulmonary disease: retest reliability of hand-held dynamometry. Arch Phys Med Rehabil. 2007;88(1):32–36. DOI:10.1016/j.apmr.2006.10.002
- O’Shea SD , Taylor NF , Paratz JD . A predominantly home-based progressive resistance exercise program increases knee extensor strength in the short-term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Aust J Physiother. 2007;53(4):229–237. DOI:10.1016/S0004-9514(07)70003-X
- Janaudis-Ferreira T , Hill K , Goldstein RS , et al. Resistance arm training in patients with COPD: a randomized controlled trial. Chest. 2011;139(1):151–158. DOI:10.1378/chest.10-1292
- Burtin C , Ter Riet G , Puhan MA , et al. Handgrip weakness and mortality risk in COPD: a multicentre analysis. Thorax. 2016;71(1):86–87. DOI:10.1136/thoraxjnl-2015-207451
- Leong DP , Teo KK , Rangarajan S , et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. DOI:10.1016/S0140-6736(14)62000-6
- Calik-Kutukcu E , Arikan H , Saglam M , et al. Arm strength training improves activities of daily living and occupational performance in patients with COPD. Clin Respir J. 2017;11(6):820–832. DOI:10.1111/crj.12422
- Spruit MA , Gosselink R , Troosters T , et al. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J. 2002;19(6):1072–1078. DOI:10.1183/09031936.02.00287102
- Kottner J , Audige L , Brorson S , et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64(1):96–106. DOI:10.1016/j.jclinepi.2010.03.002
- de Vet HC , Terwee CB , Knol DL , et al. When to use agreement versus reliability measures. J Clin Epidemiol. 2006;59(10):1033–1039. DOI:10.1016/j.jclinepi.2005.10.015
- Kottner J , Audige L , Brorson S , et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. Int J Nurs Stud. 2011;48(6):661–671. DOI:10.1016/j.ijnurstu.2011.01.016
- Karanicolas PJ , Bhandari M , Kreder H , et al. Evaluating agreement: conducting a reliability study. J Bone Joint Surg Am. 2009;91(3):99–106.
- Sim J , Wright C . Research in health care: concepts, designs and methods. Cheltenham: Stanley Thornes (Publishers) Ltd.; 2002.
- Streiner DL , Norman GR . Health measurement scales a practical guide to their development and use. Oxford: Oxford University Press; 2008. Available from: 10.1093/acprof:oso/9780199231881.001.0001
- Hicks CM . Research methods for clinical therapists: applied project design and analysis. Edinburgh: Churchill Livingstone; 1999.
- Cardarelli R , Seater MM . Evidence-based medicine, part 4. An introduction to critical appraisal of articles on harm. J Am Osteopath Assoc. 2007;107(8):310–314.
- Chourasia AO , Buhr KA , Rabago DP , et al. Effect of lateral epicondylosis on grip force development. J Hand Ther. 2012;25(1):27–37. DOI:10.1016/j.jht.2011.09.003
- Park S , Park JY . Grip strength in post-stroke hemiplegia. J Phys Ther Sci. 2016;28(2):677–679. DOI:10.1589/jpts.28.677
- Sferra da Silva G , de Almeida Lourenço M , de Assis MR . Hand strength in patients with RA correlates strongly with function but not with activity of disease. Adv Rheumatol. 2018;58(1):20. DOI:10.1186/s42358-018-0020-1
- Mohamed Faisal C , Mathew N , Mathias L . Grip strength and hand function changes in unilateral cervical radiculopathy. Int J Cur Res Rev. 2012;4(21):82–90.
- Bolton CE , Bevan-Smith EF , Blakey JD , et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax. 2013;68(2):ii1–30. DOI:10.1136/thoraxjnl-2013-203808
- Spruit MA , Singh SJ , Garvey C , et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-64–e64. DOI:10.1164/rccm.201309-1634ST
- Bechtol CO . Grip test; the use of a dynamometer with adjustable handle spacings. J Bone Joint Surg Am. 1954;36(4):820–824. DOI:10.2106/00004623-195436040-00013
- Mathiowetz V , Kashman N , Volland G , et al. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69–74.
- Haidar SG , Kumar D , Bassi RS , et al. Average versus maximum grip strength: which is more consistent? J Hand Surg Br. 2004;29(1):82–84. DOI:10.1016/j.jhsb.2003.09.012
- American Society of Hand T . Clinical assessment recommendations. Chicago (401 N. Michigan Ave., 60611-4267). Chicago (IL): The Society; 1992.
- Peolsson A , Hedlund R , Oberg B . Intra- and inter-tester reliability and reference values for hand strength. J Rehabil Med. 2001;33(1):36–41. DOI:10.1080/165019701300006524
- Savva C , Karagiannis C , Rushton A . Test-retest reliability of grip strength measurement in full elbow extension to evaluate maximum grip strength. J Hand Surg Eur Vol. 2013;38(2):183–186. DOI:10.1177/1753193412449804
- Hamilton GF , McDonald C , Chenier TC . Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Orthop Sports Phys Ther. 1992;16(5):215–219. DOI:10.2519/jospt.1992.16.5.215
- Shrout PE . Measurement reliability and agreement in psychiatry. Stat Methods Med Res. 1998;7(3):301–317. DOI:10.1177/096228029800700306
- Kendrick KR , Baxi SC , Smith RM . Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26(3):216–222. DOI:10.1016/S0099-1767(00)90093-X
- Garvey C , Bayles MP , Hamm LF , et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: AN OFFICIAL STATEMENT FROM THE AMERICAN ASSOCIATION OF CARDIOVASCULAR AND PULMONARY REHABILITATION. J Cardiopulm Rehabil Prev. 2016;36(2):75–83. DOI:10.1097/HCR.0000000000000171
- Bailey PH . The dyspnea-anxiety-dyspnea cycle-COPD patients’ stories of breathlessness: “it’s scary /when you can’t breathe”. Qual Health Res. 2004;14(6):760–778. DOI:10.1177/1049732304265973
- Mathiowetz V , Weber K , Volland G , et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222–226. DOI:10.1016/s0363-5023(84)80146-x
- Roberts HC , Denison HJ , Martin HJ , et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. DOI:10.1093/ageing/afr051
- Hogrel JY . Grip strength measured by high precision dynamometry in healthy subjects from 5 to 80 years. BMC Musculoskelet Disord. 2015;16:139. DOI:10.1186/s12891-015-0612-4
- Armour JA , Davison A , McManus IC . Genome-wide association study of handedness excludes simple genetic models. Heredity (Edinb). 2014;112(3):221–225. DOI:10.1038/hdy.2013.93
- Clerke A , Clerke J . A literature review of the effect of handedness on isometric grip strength differences of the left and right hands. Am J Occup Ther. 2001;55(2):206–211. DOI:10.5014/ajot.55.2.206
- Adamo DE , Taufiq A . Establishing hand preference: why does it matter? Hand (N Y). 2011;6(3):295–303. DOI:10.1007/s11552-011-9324-x
- Villafane JH , Valdes K , Buraschi R , et al. Reliability of the handgrip strength test in elderly subjects with Parkinson disease. Hand (N Y). 2016;11(1):54–58. DOI:10.1177/1558944715614852
- Shechtman O , Gestewitz L , Kimble C . Reliability and validity of the DynEx dynamometer. J Hand Ther. 2005;18(3):339–347. DOI:10.1197/j.jht.2005.04.002
- McGraw KO , Wong SP . Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30–46. DOI:10.1037/1082-989X.1.1.30
- Bartlett JW , Frost C . Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31(4):466–475. DOI:10.1002/uog.5256
- Koo TK , Li MY . A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. DOI:10.1016/j.jcm.2016.02.012
- Bland JM , Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. DOI:10.1016/S0140-6736(86)90837-8
- Myles PS , Cui J . Using the Bland-Altman method to measure agreement with repeated measures. Br J Anaesth. 2007;99(3):309–311. DOI:10.1093/bja/aem214
- Donner A , Eliasziw M . Sample size requirements for reliability studies. Stat Med. 1987;6(4):441–448. DOI:10.1002/sim.4780060404
- Walter SD , Eliasziw M , Donner A . Sample size and optimal designs for reliability studies. Statist Med. 1998;17(1):101–110. DOI:10.1002/(SICI)1097-0258(19980115)17:1<101::AID-SIM727>3.0.CO;2-E
- Strandkvist VJ , Backman H , Roding J , et al. Hand grip strength is associated with forced expiratory volume in 1 second among subjects with COPD: report from a population-based cohort study. Int J Chron Obstruct Pulmon Dis. 2016;11:2527–2534. DOI:10.2147/COPD.S114154
- Liao WH , Chen JW , Chen X , et al. Impact of resistance training in subjects with COPD: a systematic review and meta-analysis. Respir Care. 2015;60(8):1130–1145. DOI:10.4187/respcare.03598
- Bernard S , Whittom F , Leblanc P , et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(3):896–901. DOI:10.1164/ajrccm.159.3.9807034
- Nyberg A , Saey D , Maltais F . Why and how limb muscle mass and function should be measured in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(9):1269–1277. DOI:10.1513/AnnalsATS.201505-278PS
- Spruit MA , Sillen MJ , Groenen MT , et al. New normative values for handgrip strength: results from the UK Biobank. J Am Med Dir Assoc. 2013;14(10):775 e5.
- Strandkvist V , Andersson M , Backman H , et al. Hand grip strength is associated with fatigue among men with COPD: epidemiological data from northern Sweden. Physiother Theory Pract. 2020;36(3):408–416. DOI:10.1080/09593985.2018.1486490
- Jeong M , Kang HK , Song P , et al. Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2385–2390. DOI:10.2147/COPD.S140915
- Lee SH , Kim SJ , Han Y , et al. Hand grip strength and chronic obstructive pulmonary disease in Korea: an analysis in KNHANES VI. Int J Chron Obstruct Pulmon Dis. 2017;12:2313–2321. DOI:10.2147/COPD.S142621
- Sirguroh A , Ahmed S . Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Curr Res Rev. 2012;4(19):168–173.
- O’Donnell DE , McGuire M , Samis L , et al. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157(5):1489–1497. DOI:10.1164/ajrccm.157.5.9708010
- Burdet L , de Muralt B , Schutz Y , et al. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156(6):1800–1806. DOI:10.1164/ajrccm.156.6.9704142
- Piitulainen E , Areberg J , LindéN M , et al. Nutritional status and muscle strength in patients with emphysema and severe alpha(1)-antitrypsin deficiency. Chest. 2002;122(4):1240–1246. DOI:10.1378/chest.122.4.1240
- Savva C , Giakas G , Efstathiou M , et al. Test-retest reliability of handgrip strength measurement using a hydraulic hand dynamometer in patients with cervical radiculopathy. J Manipulative Physiol Ther. 2014;37(3):206–210. DOI:10.1016/j.jmpt.2014.02.001
- Savva C , Mougiaris P , Xadjimichael C , et al. Test-retest reliability of handgrip strength as an outcome measure in patients with symptoms of shoulder impingement syndrome. J Manipulat Physiol Ther. 2018;41(3):252–257. DOI:10.1016/j.jmpt.2017.09.005
- Coldham F , Lewis J , Lee H . The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J Hand Ther. 2006;19(3):318–326. DOI:10.1197/j.jht.2006.04.002